Abstract
Recent study showed that zinc (Zn) and amino acid transporters may be involved in enhancing Zn absorption from Zn proteinate with moderate chelation strength (Zn-Prot M) in the duodenum of broilers. However, the specific mechanisms by which Zn-Prot M promotes the above Zn absorption are unknown. Therefore, in this study, 3 experiments were conducted to investigate specific and direct effects of Zn-Prot M and Zn sulfate (ZnS) on Zn absorption and expression of related transporters in primary duodenal epithelial cells of broiler embryos so as to preliminarily address possible mechanisms. In experiment 1, cells were treated with 100 μmol Zn/L as ZnS or Zn-Prot M for 20, 40, 60, 80, 100, or 120 min. Experiment 2 consisted of 3 sub-experiments. In experiment 2A, cells were treated with a Zn-unsupplemented basal medium (Control) or the basal medium supplemented with 100 or 200 μmol Zn/L as ZnS or Zn-Prot M for 60 min; in experiment 2B, cells were treated with a Zn-unsupplemented basal medium (Control) or the basal medium supplemented with 200 μmol Zn/L of as the ZnS or Zn-Prot M for 120 min; in experiment 2C, cells were treated with a Zn-unsupplemented basal medium (Control) or the basal medium supplemented with 400 or 800 μmol Zn/L as ZnS or Zn-Prot M for 120 min. In experiment 3, cells were treated with a Zn-unsupplemented basal medium (Control) or the basal medium supplemented with 400 μmol Zn/L as ZnS or Zn-Prot M for 120 min. The results of experiment 1 indicated that the minimum incubation time for saturable Zn absorption was determined to be 50.83 min using the best fit line. The results in experiment 2 demonstrated that a Zn concentration of 400 μmol/L and an incubation time of 120 min were suitable to increase the absorption of Zn from Zn-Prot M compared to ZnS. In experiment 3, Zn absorption across cell monolayers was significantly increased by Zn addition (P < 0.05), and was significantly greater with Zn-Prot M than with ZnS (P < 0.05). Compared to the control, Zn addition significantly decreased Zn transporter 10 and peptide-transporter 1 mRNA expression levels and increased y + L-type amino transporter 2 (y + LAT2) protein abundance (P < 0.05). Moreover, protein expression levels of zrt/irt-like protein 3 (ZIP3), zrt–irt-like protein 5 (ZIP5), and y + LAT2 were significantly greater for Zn-Prot M than for ZnS (P < 0.05). These findings suggest that Zn-Prot M promote Zn absorption by increasing ZIP3, ZIP5 and y + LAT2 protein expression levels in primary duodenal epithelial cells.
Keywords: amino acid transporters, broilers, duodenal epithelial cells, zinc absorption, zinc proteinate, zinc transporters
Zinc proteinate with moderate chelation strength increased the zinc absorption in primary duodenal epithelial cells of broiler embryos by promoting the protein expression levels of ZIP3, ZIP5, and y + LAT2 compared to zinc sulfate.
Introduction
Zinc (Zn) is a trace element that is essential for animal and human nutrition and biochemical functions (Saper and Rash, 2009; Muhamed and Vadstrup, 2014). This element is required for the catalytic activity of more than 300 enzymes (Vallee and Auld, 1990; Li et al., 2021). Moreover, dietary Zn deficiency has been found to cause growth retardation, oxidative stress, and impaired immune responses in chickens (Ogbuewu and Mbajiorgu, 2023). Zinc content in commonly used feedstuffs is low, and chickens poorly utilize Zn because of the presence of phytic acid and other anti-nutritional factors in plant feedstuffs (Yu et al., 2008). Therefore, Zn supplementation in poultry diets is generally used to meet their Zn demands and prevent Zn deficiency.
Inorganic Zn sulfate (ZnS) is the most commonly used Zn source in poultry diets, however, some unique organic Zn sources have received increased attention and have been incorporated into poultry diets due to their higher bioavailability (Chen et al., 2022; Khan et al., 2024). Min et al. (2019) reported that the methionine hydroxy analog chelated Zn was more readily available in laying hens than ZnS, using tibial Zn content and tibial strength as the responsive criteria. Chen et al. (2022) discovered that Zn-methionine had a higher bioavailability than ZnS, as reflected by pancreatic metallothionein content and mRNA expression in broilers. Yu et al. (2017) demonstrated that the organic Zn sources exhibited greater absorptivity than ZnS in the ligated duodenal segment, and that the relative absorptivity of organic Zn sources in broilers was strongly associated with the chelation strengths (measured by quotient of formation [Qf] values) between Zn and its ligands. Moreover, Huang et al. (2009) observed the highest bioavailability of Zn proteinate with a moderate Qf value (Zn-Prot M) in broilers, compared to ZnS and other organic Zn sources with either weak or strong Qf values. However, the reasons for the highest Zn bioavailability of Zn-Prot M in broilers remain unclear. Although in vivo studies on the absorption of Zn as Zn-Prot M have been performed in the small intestine of broilers (Yu et al., 2017; Hu et al., 2022, 2023), further in vitro investigations are required to elucidate the precise molecular mechanisms of the above Zn absorption.
Zn transporters include the Zn transporter families (ZnTs) and zrt/irt-like protein families (ZIPs), which are required to maintain cellular Zn homeostasis (Yin et al., 2023). About 14 ZIPs and 10 ZnTs have been identified and named in human cells (Kambe, 2013). Furthermore, ZIPs transport Zn into the cytoplasm from internal organelles and vesicles. In contrast, ZnTs mediate Zn efflux into the organelles and vesicles (Sekler et al., 2007; Kimura and Kambe, 2016). Studies on broilers have suggested that ZnTs and ZIPs may play a role in the transport of organic Zn in the small intestine (He et al., 2019; Hu et al., 2022, 2023). He et al. (2019) identified that broilers fed a Zn-amino acid complex exhibited an increased mRNA expression of jejunal ZnT1. Our recent in vivo studies demonstrated that Zn-Prot M promoted the protein abundances of small intestinal ZIP3, ZnT4, ZIP5, ZnT7, ZnT9, or ZnT10 in broilers compared to ZnS (Hu et al., 2022, 2023). Furthermore, Zn-Prot M enhanced the protein abundances of the small intestinal y + L-type amino transporter 2 (y + LAT2), b0,+-type amino acid transporter (rBAT), and peptide-transporter 1 (PepT1) in broilers (Hu et al., 2022, 2023). These studies indicated that ZnT, ZIP, peptide, and amino acid transporters could potentially be involved in the transport of Zn as organic Zn sources in the small intestine of broilers. Nevertheless, it is recognized that the aforementioned in vivo studies in broilers might be influenced by dietary, environmental, and physiological factors other than Zn. Therefore, additional in vitro investigations are required to determine the specific and direct impacts of ZnS and Zn-Prot M on the expression of the carriers mentioned above using a primary cultured model of duodenal epithelial cells from broiler embryos, which was previously established in our laboratory (Zhang et al., 2018).
Based on the above in vivo studies performed in our laboratory and by other researchers, we hypothesized that organic Zn-Prot M would increase Zn absorption potentially by upregulating the expression of Zn, amino acid and peptide transporters in the primary duodenal epithelial cells. Therefore, the objective of the present study was to determine the effect of supplemental Zn-Prot M and ZnS on Zn absorption and gene expression of ZnT, ZIP, peptide, and amino acid transporters in the primary cultured duodenal epithelial cells of chick embryos.
Materials and Methods
The experiment protocols were reviewed and approved by the Animal Ethics Committee of Yangzhou University (approval number: SYXK (Su) 2021-0027).
Isolation and cultivation of duodenal epithelial cells
Duodenal epithelial cells were isolated from 18-d-old chick embryos (Zhang et al., 2018), and a total of 2,000 chicken embryos were used to isolate the cells in this study. The isolated epithelial cells were seeded into 6-well Transwell plates (Cat. No. 3450, Corning Incorporated, Corning, NY, USA) at an initial density of 4.5 × 106 cells/well, and then cultured in a growth medium containing Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) basal medium (Gibco, Invitrogen Corporation, Carlsbad, CA, USA), 100 µg/mL of heparin sodium, 20 ng/mL of epidermal growth factor, 1% penicillin, 1% insulin–transferrin–selenium, and 3% fetal bovine serum, and kept in a humidified incubator for 48 h of cultivation at 37 °C with 5% CO2. To assess the integrity of the duodenal epithelial cell monolayers at 48 h of cultivation, the trans-epithelial electrical resistance (TEER) and phenol red transmittance were measured using the techniques detailed by Cao et al. (2020). A commercial lactate dehydrogenase (LDH) kit (Nanjing Jiancheng Bioengineering Institute, China) was used to measure the LDH activity in the culture medium to assess the cells’ integrity at 48 h after seeding. Only when the duodenal epithelial cell monolayers reached a TEER > 300 Ω cm2, phenol red transmittance < 5%, and LDH activity < 400 U/L, such an absorption model is successful (Zhang et al., 2018). The results indicated that the TEER value and phenol red transmittance of the cell monolayers were 434 ± 10 Ω·cm2 and 2.42 ± 0.35 % (n = 6) at 48 h, respectively. The LDH activity in the culture medium was 315 ± 16 U/L (n = 6) at 48 h. Therefore, the established primary cultured duodenal epithelial cell monolayer model of chick embryos at 48 h in the present study was acceptable to be used in the subsequent Zn absorption experiments.
Preparation of Zn solutions
A Zn stock medium containing 1,000 μmol Zn/L in the form of ZnS (ZnSO4.7H2O) or Zn-Prot M (feed grade, Qf = 51.6, comprising 17.09% of Zn by analysis) was freshly prepared. The ZnS and Zn-Prot M used in this study were identical to those used in our previous study (Hu et al., 2022). The Zn media were prepared by mixing the DMEM/F12 basal medium buffer with the Zn stock medium to obtain Zn concentrations of 100, 200, 400, and 800 μmol/L, respectively.
A completely randomized design was adopted, and there were 3 to 8 replicate wells depending on the different indices for each treatment in the following experiments. According to power and sample size analysis performed using an online Power and Sample Size Calculator (https://www.stat.ubc.ca/~rollin/stats/ssize/b2.html), as well as a previous publication (Huang et al., 2024), 3 replicate wells were sufficient to obtain reliable TEER value, phenol red transmittance, and LDH enzyme activity data for statistical analyses, and 6 replicate wells were sufficient to obtain reliable data of Zn absorption and molecular indices for statistical analyses.
Time responses of Zn absorption across the duodenal epithelial cell monolayers (experiment 1)
Experiment 1 was conducted to determine the appropriate cell incubation time. A 2 (Zn treatment) × 6 (incubation time) factorial arrangement of treatments was used, with ZnS and Zn-Prot M as the 2 Zn treatments as well as 6 incubation timepoints of 20, 40, 60, 80, 100, and 120 min. Yu et al. (2008) reported that the time points for maximum Zn absorption were 49, 46, and 60 min in the ligated duodenum, jejunum, and ileum of broilers, respectively. Therefore, in order to ensure the adequate Zn absorption in the primary cultured duodenal epithelial cells, the aforementioned 6 incubation time points were selected. In addition, the supplemental Zn level of 100 μmol/L was adopted according to a previous study (Shao et al., 2017). Moreover, to assess the integrity of cell monolayers after Zn treatment at 120 min of incubation, a basal medium control with no Zn was included. At 48 h of cultivation after seeding, the cells were gently washed thrice with DMEM/F12 basal medium at 37 °C. Subsequently, 1.5 mL of growth media supplemented with 100 μmol Zn/L in the form of either ZnS (containing 98 μmol/L of Zn by analysis) or Zn-Prot M (containing 101 μmol/L of Zn by analysis) were supplemented into the apical chamber (donor chamber) of one well in each 6-well Transwell plate. Moreover, 2.6 mL of DMEM/F12 basal media (containing 1.82 μmol/L of Zn by analysis) were added to its basolateral chamber (the receiver chamber). After Zn treatments, all cells were incubated in a humidified culture incubator for 20, 40, 60, 80, 100, or 120 min according to the above-mentioned incubation timepoint design. At each incubation time point, 2.2 mL of incubation media were sampled from the basolateral chamber of each well to analyze Zn concentration (except for the control). At 120 min, the integrity of the cell monolayers was assessed as described above.
Effect of Zn source and added Zn level on Zn absorption in duodenal epithelial cells (experiment 2)
Experiment 2 was a serial trial consisting of 3 sub-experiments 2A to C to determine the appropriate added Zn level by investigating the effects of supplemental Zn-Prot M and ZnS on Zn absorption under different levels of added Zn in the primary cultured duodenal epithelial cells based on the appropriate cell incubation time (50.82 min), which was determined in experiment 1. In experiment 2A, a 1 (control) + 2 (Zn source) × 2 (added Zn level) factorial arrangement of treatments was used. The 2 Zn sources were ZnS and Zn-Prot M, and the 2 added Zn levels were 100 and 200 μmol/L based on the previous study (Shao et al., 2017), while the Zn-unsupplemented cells were set as the control. The Zn incubation time was set to 60 min, according to the results of experiment 1.
According to the results of experiment 2A, there was no difference in Zn absorption between the 2 Zn sources at either 100 or 200 μmol Zn/L, possibly due to the shorter incubation time (60 min) in this trial. Therefore, experiment 2B was conducted to evaluate the effects of supplemental Zn-Prot M and ZnS on Zn absorption across the primary cultured duodenal epithelial cells, when the incubation time was extended to 120 min at 200 μmol Zn/L. Therefore, 3 treatments were designed as the control with no Zn addition, ZnS and Zn-Prot M treatments, respectively.
According to the results of experiment 2B, there was still no difference in Zn absorption between the 2 Zn sources, possibly due to the lower added Zn level (200 μmol/L). Therefore, experiment 2C was conducted to further investigate the effects of supplemental Zn-Prot M and ZnS on Zn absorption across the duodenal epithelial cells at increased supplemental Zn levels of 400 and 800 μmol/L and the incubation time of 120 min. A 1 (control) + 2 (Zn source) × 2 (added Zn level) factorial arrangement of treatments was used. The 2 Zn sources were ZnS and Zn-Prot M, and the 2 added Zn levels were 400 and 800 μmol/L, while the Zn-unsupplemented cells were set as the control.
At 48 h of cultivation after seeding, the cells were gently washed thrice with DMEM/F12 basal medium at 37 °C. Then 1.5 mL of growth basal media with no Zn addition (control, containing 2.97 μmol/L of Zn by analysis) or the growth basal media supplemented with 100, 200, 400, or 800 μmol Zn/L in the form of ZnS (containing 98, 195, 395, or 778 μmol/L of Zn by analysis) or Zn-Prot M (containing 101, 195, 397, or 783 μmol/L of Zn by analysis) were added to the apical chamber of one well in each 6-well Transwell plate. Furthermore, 2.6 mL of DMEM/F12 basal media (containing 1.82 μmol/L of Zn by analysis) were added to its basolateral chamber according to the experimental designs described. After Zn treatment, all cells were incubated for 60 or 120 min. At 60 or 120 min, 2.2 mL of the incubation media were sampled from the basolateral chamber of each well for the analysis of Zn concentration, and also the integrity of the cell monolayers was assessed as described above.
Effect of supplemental Zn source on Zn absorption and gene expression of related transporters in duodenal epithelial cells (experiment 3)
According to the results of experiment 2C, Zn-Prot M significantly enhanced Zn absorption compared with ZnS at either 400 or 800 μmol Zn/L. However, 800 μmol Zn/L was excessive for the duodenal epithelial cells at 120 min of incubation. Therefore, in experiment 3, we used the added Zn level of 400 μmol/L and the cell incubation time of 120 min. Three treatments were designed, including the control with no Zn addition, ZnS, and the Zn-Prot M treatments with 6 replicate wells per treatment.
At 48 h of cultivation after seeding, the cells were gently washed thrice with DMEM/F12 basal medium at 37 °C. Then 1.5 mL of growth basal media with no Zn addition (control, containing 2.97 μmol/L of Zn by analysis) or the growth basal media supplemented with 400 μmol Znl/L from ZnS (containing 397 μmol/L of Zn by analysis) or Zn-Prot M (containing 397 μmol/L of Zn by analysis) were added to the apical chamber of one well in each 6-well Transwell plate, along with the addition of 2.6 mL DMEM/F12 basal media (containing 1.82 μmol/L of Zn by analysis) to its basolateral chamber. After Zn treatment, all cells were incubated for 120 min. After incubation for 120 min, 2.2 mL of the incubation media were sampled from the basolateral chamber of each well for analysis of Zn concentration, and the duodenal epithelial cells were collected to examine the mRNA and protein expression levels of ZnT, ZIP, peptide, and amino acid transporters. The integrity of the cell monolayers was assessed as described above.
Measurement of Zn contents and calculation of Zn absorption amount
An Agilent Technologies 5110 plasma emission spectrometer (Agilent Technologies, Santa Clara, CA, USA) was used to analyze the Zn content in the culture media before and after incubation, according to the method described by Li et al. (2013). The amount of Zn absorption was calculated as follows:
In the equation, the 2.6 mL is the liquid volume in the receiver chamber of the Zn treatment, and the 4.67 cm2 is the area of the bottom of the receiver chamber in each well of a 6-well Transwell plate.
Real-time qPCR (RT-qPCR)
Total RNA was extracted from the duodenal epithelial cells using TRIzol reagent (Vazyme Biotech Co., Ltd, Nanjing, China) (Cao et al., 2020). The synthesis of cDNA was performed using 1,000 ng of total RNA with a commercially available kit obtained from Vazyme Biotech Co., Ltd. AceQ SYBR Green Master Mix (Vazyme Biotech Co., Ltd.) was used for the RT-qPCR procedure. The relative mRNA expression level was determined using the 2−ΔΔCT method, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S ribosomal RNA (18S rRNA) serving as the internal controls (Livak and Schmittgen, 2001). All primers and their respective PCR fragment lengths are listed in Table 1.
Table 1.
Primer sequences used for real-time qPCR
| Genes | GenBank ID | Primer sequences (5ʹ to 3ʹ) | Product length (bp) |
|---|---|---|---|
| ZnT1 | XM_421021.5 | F: AGAGCCTGGGTTTGGATTCG | 229 |
| R: AGCCCATGCATGAACACTGA | |||
| ZnT4 | XM_423325 | F: GCCATCTTGACGGACGTAGT | 190 |
| R: CAAGGTACACCAGGACACCC | |||
| ZnT5 | NM_001031419.2 | F: ATGGAGGAAAAGTACAGCAGCC | 118 |
| R: TCAGAAACTTGGCGAAGCAC | |||
| ZnT6 | XM_045691225.1 | F: CTACGGTTCTGGCACAGCTT | 126 |
| R: GTCGCCCCGTGTGTATCTC | |||
| ZnT7 | NM_001008788.1 | F: TGCTGCCCCTCTCCATTAAG | 114 |
| R: AGAGGTTGCGGGATGTCTTG | |||
| ZnT9 | XM_015285471.1 | F: ACATGTTTTTCCGTGCAGCC | 265 |
| R: CGGAACACAACCTTTACCAGC | |||
| ZnT10 | XM_015283897.1 | F: GAGCTGTAGAGATGGGTCGC | 184 |
| R: ACACCGAGCAAACCGATGAT | |||
| ZIP3 | XM_015299966.2 | F: CATACATCCAGGAGGCAGAGG | 246 |
| R: CCTGGATGATCTTGACGGGG | |||
| ZIP5 | XM_025145573.1 | F: CCAAGATGAAACGCACGCAA | 284 |
| R: TAAGCTGCGACCAAGTCCTG | |||
| B 0 AT1 | XM_419056.6 | F: CTTGGGTGAGGTAGGTGGGA | 167 |
| R: GATGCGGGTGCTCTCATGTA | |||
| LAT1 | NM_001030579.2 | F: GGAAAGGCCCATCAAGGTGA | 248 |
| R: ACGATTCTTGGGGAACCAC | |||
| rBAT | XM_004935370.3 | F: CCTAGGAGGAGAGGCACGAA | 223 |
| R: TCCTGCATAGGGCTGCAATG | |||
| EAAT3 | XM_424930.6 | F: CGTCCAGGCCTGTTTTCAAC | 171 |
| R: TCGGAATACATGCCCACGAT | |||
| PepT1 | NM_204365.1 | F: AAAACAGGTTTCGGCATCGC | 167 |
| R: CTGCTGGTCAAAAAGTGCCC | |||
| GAPDH | NM_204305.1 | F: CTTTGGCATTGTGGAGGGTC | 128 |
| R: ACGCTGGGATGATGTTCTGG | |||
| 18S rRNA | NM_001182073.1 | F: ATAACGAACGAGACTCTGGCA | 147 |
| R: CGGACATCTAAGGGCATCACA |
ZnT1, zinc transporter 1; ZnT4, zinc transporter 4; ZnT5, zinc transporter 5; ZnT6, zinc transporter 6; ZnT7, zinc transporter 7; ZnT9, zinc transporter 9; ZnT10, zinc transporter 10; ZIP3, zrt–irt-like protein 3; ZIP5, zrt–irt-like protein 5; B0AT1, b-0-system neutral amino acid co-transporter; LAT1, L-type amino acid transporter 1; rBAT, b0,+-type amino acid transporter; EAAT3, excitatory amino acid transporter 3; PepT1, peptide-transporter 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 18S rRNA, 18S ribosome RNA; F, forward; R, reverse.
Total protein extraction and western blotting
Epithelial cells were homogenized in radioimmunoprecipitation assay buffer supplemented with 1% (v/v) phenylmethylsulfonyl fluoride using a homogenizer (Beijing Hede Technology Co., Ltd, China). The cell lysate was centrifuged at 10,000 × g for 10 min. The supernatant was collected, and the protein concentrations were determined. The cell lysates with a protein content of 40 μg were separated using either 7.5% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The primary antibodies for ZnT1 (ab214356), ZnT10 (ab229954), ZIP3 (ab254868), ZIP5 (ab105194), and B0AT1 (ab180516) were purchased from Abcam (Cambridge, MA, USA). Primary antibodies against ZnT5 (25604-1-AP), ZnT7 (13966-1-AP), and y + LAT2 (13823-1-AP) were purchased from Proteintech Group, Inc. (Chicago, IL, USA). The primary antibodies for ZnT9 (BS62531), LAT1 (DF8065), and rBAT (DF7379) were purchased from Bioworld Technology, Inc. (Louis Park, MN, USA) or Affinity Biosciences (Cincinnati, OH, USA). In addition, the primary antibodies for ZnT4 (BA3484) and PepT1 (A03672-1) were purchased from Boster Biological Technology Co., Ltd. (Wuhan, China). All antibodies used in the present study were either mouse- or human-derived antibodies. The protein bands were scanned using an ECL system (Tanon Technology Co. Ltd., Shanghai, China), and the intensity of the protein bands was quantified using Tanon Gis 1D software.
Statistical analyses
TEER value, phenol red transmittance, and LDH enzyme activity in experiment 1, and all data in experiments 2B and 3 were analyzed by one-way ANOVA using the generalized linear model (GLM) procedure of SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Data on the Zn absorption amount in experiment 1 were analyzed by 2-way ANOVA using the GLM procedure, and the statistical model included Zn treatment, incubation time, and their interactions. Orthogonal comparisons were applied for linear and quadratic responses of the dependent variables to independent variables. Regression analyses of quadratic, asymptotic, and broken-line models were performed, and the best-fitted model was selected to estimate the suitable incubation time (the break point from the broken-line model). Data in experiments 2A and C were analyzed using a single degree of freedom contrast to compare all supplemental Zn treatments with the control. Data excluding the control were analyzed by 2-way ANOVA using the GLM procedure, and the statistical model included Zn source, added Zn level and their interaction. Differences among the means were tested using the least significant difference method. The replicate well served as the experimental unit, and the statistical significance was set at P < 0.05.
Results
Effects of Zn treatment and incubation time on Zn absorption in duodenal epithelial cells (experiment 1)
Zinc treatment did not affect the TEER value (>300 Ω∙cm2), phenol red transmittance (<5%) of the cells, and LDH activity (<400 U/L) in the culture medium after incubation for 120 min (P > 0.34) (Table 2), indicating that the integrity of the duodenal epithelial cells was not affected by Zn treatment, and thus the following results of Zn absorption in this experiment are reliable.
Table 2.
Effect of supplemental Zn on TEER value and phenol red transmittance of primary cultured duodenal epithelial cells and LDH activity in the culture medium at 120 min of incubation1 (experiment 1)
| Zn treatment | TEER value, Ω.cm2 | Phenol red transmittance, % | LDH enzyme activity, U/L |
|---|---|---|---|
| Control | 424 | 2.83 | 141 |
| ZnS | 420 | 3.04 | 149 |
| Zn-Prot M | 418 | 3.05 | 139 |
| Pooled SE | 3 | 0.11 | 8 |
| P value | 0.3869 | 0.3438 | 0.6461 |
1Data represent the means of 5 to 8 replicates (n = 5 to 8).
LDH, lactate dehydrogenase; TEER, the trans-epithelial electrical resistance; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6).
As shown in Table 3, Zn treatment and its interaction with the incubation time did not affect the amount of Zn absorption in the primary cultured duodenal epithelial cells (P > 0.27), but the incubation time significantly affected it (P < 0.001). Cells at either 40, 60, 80, 100, or 120 min incubation had a higher amount of Zn absorption than those at 20 min (P < 0.05), but no differences were observed among the 40, 60, 80, 100, and 120 min of incubation time points (P > 0.05). Moreover, the amount of Zn absorption increased linearly and quadratically with the increase in incubation time (P < 0.03).
Table 3.
Effects of Zn treatment (added Zn level of 100 μmol/L) and incubation time on Zn absorption in primary cultured duodenal epithelial cells (experiment 1)
| Zn treatment | Incubation time, min | Amount of Zn absorption, nmol/cm2 |
|---|---|---|
| ZnS1 | 20 | 1.35 |
| 40 | 2.27 | |
| 60 | 2.18 | |
| 80 | 2.47 | |
| 100 | 2.37 | |
| 120 | 2.75 | |
| Zn-Prot M1 | 20 | 1.47 |
| 40 | 2.10 | |
| 60 | 3.29 | |
| 80 | 2.46 | |
| 100 | 2.73 | |
| 120 | 2.63 | |
| Pooled SE | 0.31 | |
| Zn treatment2 | ZnS | 2.23 |
| Zn-Prot M | 2.45 | |
| Pooled SE | 0.13 | |
| Incubation time3 | 20 | 1.41b |
| 40 | 2.19a | |
| 60 | 2.73a | |
| 80 | 2.46a | |
| 100 | 2.55a | |
| 120 | 2.69a | |
| Pooled SE | 0.22 | |
| P value | Zn treatment | 0.2758 |
| Incubation time | 0.0009 | |
| Interaction | 0.4775 | |
| Linear | 0.0004 | |
| Quadratic | 0.0206 |
1Data represent the means of 6 to 8 replicates (n = 6 to 8).
2Data represent the means of 40 to 44 replicates (n = 40 to 44).
3Data represent the means of 14 to 16 replicates (n = 14 to 16).
a,bMeans with different superscripts within the same column differ significantly (P < 0.05).
ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6).
The appropriate incubation time for Zn absorption by the primary cultured duodenal epithelial cells was estimated based on the best-fitted broken-line model [Y = 0.6296 + 0.039X (0 < X ≤ 50.82); Y = 0.6347 + 0.0389X (50.82 < X ≤ 120)]. Therefore, to maintain adequate Zn absorption across the cell monolayers, the minimum incubation time for the duodenal epithelial cells was 50.82 min.
Effect of Zn source and added Zn level on Zn absorption in the duodenal epithelial cells at 60 min of incubation (experiment 2A)
The TEER value and phenol red transmittance of the cells, as well as LDH activity in the culture media were unaffected by Zn treatment at 60 min of incubation compared to the control (P > 0.05) (Table 4). The above-mentioned indices were not affected by the Zn source, added Zn level, and their interaction (P > 0.16), indicating that the integrity of the duodenal epithelial cells was not affected by the Zn source and added Zn level, and thus the following results of Zn absorption in this experiment are reliable.
Table 4.
Effects of Zn source and added Zn level on TEER value, phenol red transmittance and Zn absorption of primary cultured duodenal epithelial cells and LDH activity in the culture medium at 60 min of incubation (experiment 2A)
| Zn source | Added Zn level, μmol/L | TEER value, Ω.cm2 | Phenol red transmittance, % | LDH enzyme activity, U/L | Amount of Zn absorption, nmol/cm2 |
|---|---|---|---|---|---|
| Control1 | 0 | 416 | 2.44 | 134 | 0.77* |
| ZnS1 | 100 | 427 | 2.53 | 139 | 2.87 |
| 200 | 409 | 2.62 | 156 | 4.00 | |
| Zn-Prot M1 | 100 | 442 | 2.42 | 162 | 2.58 |
| 200 | 431 | 2.55 | 133 | 3.98 | |
| Pooled SE | 12.16 | 0.09 | 28 | 0.50 | |
| Zn source2 | ZnS | 418 | 2.58 | 148 | 3.44 |
| Zn-Prot M | 437 | 2.49 | 147 | 3.28 | |
| Pooled SE | 8.60 | 0.07 | 20 | 0.35 | |
| Added Zn level3 | 100 | 434 | 2.47 | 150 | 2.73b |
| 200 | 420 | 2.59 | 145 | 3.99a | |
| Pooled SE | 8.60 | 0.07 | 20 | 0.35 | |
| P value | Zn source | 0.2825 | 0.4576 | 0.9919 | 0.7528 |
| Added Zn level | 0.1630 | 0.3662 | 0.8398 | 0.0194 | |
| Interaction | 0.8044 | 0.9726 | 0.4471 | 0.7856 |
1Data represent the means of 3 to 6 replicates (n = 3 to 6).
2Data represent the means of 6 to 12 replicates (n = 6 to 12).
3Data represent the means of 6 to 12 replicates (n = 6 to 12).
*Different from all Zn supplemental groups (P < 0.05).
a,bMeans with different superscripts within the same column differ significantly (P < 0.05).
LDH, lactate dehydrogenase; TEER, the trans-epithelial electrical resistance; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6).
Compared to the control, the addition of Zn significantly increased the absorption of Zn in the duodenal epithelial cells (P < 0.05) (Table 4). The amount of Zn absorption was significantly influenced by the added Zn level (P < 0.02), but not by Zn source or its interaction with the added Zn level (P > 0.75). The Zn absorption was significantly greater when the cells were exposed to a concentration of 200 μmol Zn/L than 100 μmol Zn/L (P < 0.05).
Effects of Zn source (added Zn level of 200 μmol/L) on Zn absorption in duodenal epithelial cells at 120 min of incubation (experiment 2B)
The integrity of duodenal epithelial cells was not affected by Zn treatment, as evidenced by the unaffected TEER value, phenol red transmittance, and LDH activity at 120 min of incubation (P > 0.30) (Table 5), and thus the following results of Zn absorption in this experiment are reliable.
Table 5.
Effect of Zn source (added Zn level of 200 μmol/L) on TEER value, phenol red transmittance and Zn absorption of primary cultured duodenal epithelial cells and LDH activity in the culture medium at 120 min of incubation1 (experiment 2B)
| Zn source | TEER value, Ω.cm2 | Phenol red transmittance, % | LDH enzyme activity, U/L | Amount of Zn absorption, nmol/cm2 |
|---|---|---|---|---|
| Control | 422 | 2.26 | 100 | 0.36b |
| ZnS | 417 | 2.41 | 144 | 4.88a |
| Zn-Prot M | 430 | 2.25 | 115 | 4.35a |
| Pooled SE | 10 | 0.07 | 21 | 0.28 |
| P value | 0.7082 | 0.3034 | 0.3681 | <0.0001 |
1Data represent the means of 3 to 6 replicates (n = 3 to 6).
a,bMeans with different superscripts within the same column differ significantly (P < 0.05).
LDH, lactate dehydrogenase; TEER, the trans-epithelial electrical resistance; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6).
Zinc source significantly influenced the Zn absorption amount in the duodenal epithelial cells (P < 0.0001) (Table 5). Compared to the control, 2 Zn sources significantly increased the amount of Zn absorption by 1,256% (ZnS) and 1,108% (Zn-Prot M) (P < 0.05), respectively with no significant difference between the 2 Zn sources (P > 0.05). However, it was expected that Zn-Prot M should promote Zn absorption compared to ZnS based on Zn biologically relevant (Hu et al., 2022).
Effect of Zn source and added Zn level on Zn absorption in the duodenal epithelial cells of broiler embryos at 120 min of incubation (experiment 2C)
The TEER value and phenol red transmittance of the cells, as well as LDH activity in the culture media at 120 min of incubation were unaffected by Zn treatment when compared to the control (P > 0.05) (Table 6). The TEER value and LDH activity were not affected by Zn source, added Zn level, or their interaction (P > 0.34). The added Zn level significantly affected the phenol red transmittance (P < 0.006), whereas the Zn source and its interaction with the added Zn level did not affect it (P > 0.11). The phenol red transmittance was significantly greater at a concentration of 800 μmol Zn/L compared to that at 400 μmol Zn/L (P < 0.05), indicating that the supplementation of 800 μmol Zn/L decreased the tight junctions between the epithelial cells, and induced a certain degree of damage on the barrier function of the epithelial cell monolayers. Therefore, the following results of Zn absorption at 400 μmol Zn/L, regardless of Zn source, are reliable in this experiment.
Table 6.
Effects of Zn source and added Zn level on TEER value, phenol red transmittance and Zn absorption of primary cultured duodenal epithelial cells and LDH activity in the culture medium at 120 min of incubation (experiment 2C)
| Zn source | Added Zn level, μmol/L | TEER value, Ω.cm2 | Phenol red transmittance, % | LDH enzyme activity, U/L | Amount of Zn absorption, nmol/cm2 |
|---|---|---|---|---|---|
| Control1 | 0 | 413 | 2.11 | 142 | 0.26* |
| ZnS1 | 400 | 406 | 2.18 | 143 | 6.95 |
| 800 | 405 | 2.49 | 157 | 11.99 | |
| Zn-Prot M1 | 400 | 413 | 2.13 | 161 | 9.19 |
| 800 | 400 | 2.31 | 125 | 12.82 | |
| Pooled SE | 12 | 0.06 | 25 | 0.64 | |
| Zn source2 | ZnS | 406 | 2.33 | 150 | 9.47b |
| Zn-Prot M | 406 | 2.22 | 143 | 11.01a | |
| Pooled SE | 8 | 0.05 | 18 | 0.45 | |
| Added Zn level3 | 400 | 409 | 2.16b | 152 | 8.17b |
| 800 | 402 | 2.40a | 141 | 12.44a | |
| Pooled SE | 8 | 0.05 | 18 | 0.48 | |
| P value | Zn source | 0.5885 | 0.1147 | 0.7852 | 0.0345 |
| Added Zn level | 0.9516 | 0.0052 | 0.6833 | <0.0001 | |
| Interaction | 0.6727 | 0.3061 | 0.3475 | 0.3063 |
1Data represent the means of 3 to 6 replicates (n = 3 to 6).
2Data represent the means of 6 to 12 replicates (n = 6 to 12).
3Data represent the means of 6 to 12 replicates (n = 6 to 12).
*Different from all Zn supplemental groups (P < 0.05).
a,bMeans with different superscripts within the same category of the same column differ significantly (P < 0.05).
LDH, lactate dehydrogenase; TEER, the trans-epithelial electrical resistance; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6).
Zinc supplementation resulted in a significant increase in the Zn absorption amount in the duodenal epithelial cells compared to the control (P < 0.05) (Table 6). The Zn source and added Zn level significantly affected the Zn absorption amount (P < 0.04), whereas their interaction did not affect it (P > 0.30). Zn-Prot M significantly enhanced the amount of Zn absorption compared with ZnS (P < 0.05). The absorption of Zn was significantly greater when cells were exposed to a concentration of 800 μmol Zn/L than 400 μmol Zn/L (P < 0.05).
Effect of Zn source (added Zn level of 400 μmol/L) on Zn absorption in duodenal epithelial cells at 120 min of incubation (experiment 3)
Zinc source did not affect the TEER value and phenol red transmittance of the cells, and LDH activity in the culture medium at 120 min of incubation (P > 0.55) (Table 7), indicating that the integrity of duodenal epithelial cells was not affected by the Zn source, and thus the following results of Zn absorption and other indices in the present experiment are reliable.
Table 7.
Effect of Zn source (added Zn level of 400 μmol/L) on TEER value, phenol red transmittance and Zn absorption of primary cultured duodenal epithelial cells and LDH activity in the culture medium at 120 min of incubation1 (experiment 3)
| Zn source | TEER value, Ω.cm2 | Phenol red transmittance, % | LDH enzyme activity, U/L | Amount of Zn absorption, nmol/cm2 |
|---|---|---|---|---|
| Control | 430 | 1.63 | 153 | 0.27c |
| ZnS | 416 | 1.74 | 141 | 2.62b |
| Zn-Prot M | 423 | 1.67 | 153 | 5.01a |
| Pooled SE | 8 | 0.08 | 33 | 0.34 |
| P value | 0.5557 | 0.6299 | 0.9327 | <0.0001 |
1Data represent the means of 3 to 6 replicates (n = 3 to 6).
a,b,cMeans with different superscripts within the same column differ significantly (P < 0.05).
LDH, lactate dehydrogenase; TEER, the trans-epithelial electrical resistance; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6).
Zinc source significantly influenced the Zn absorption amount in the duodenal epithelial cells (P < 0.0001) (Table 7). Compared with the control group, the 2 Zn sources exhibited a significant increase in the amount of Zn absorption (P < 0.05). Furthermore, when compared with ZnS, Zn-Prot M demonstrated a significant increase in Zn absorption by 91% (P < 0.05).
Effect of Zn source (added Zn level of 400 μmol/L) on mRNA expression levels of ZnT, ZIP, small peptide, and amino acid transporters in the duodenal epithelial cells at 120 min of incubation (experiment 3)
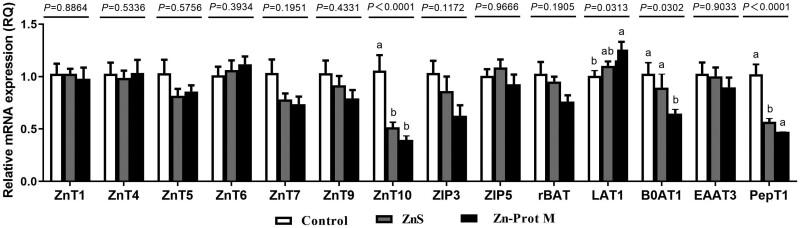
Zinc source did not affect the mRNA expression levels of ZnT1, ZnT4, ZnT5, ZnT6, ZnT7, ZnT9, ZIP3, ZIP5, rBAT, and EAAT3 in the duodenal epithelial cells (P > 0.11) (Figure 1). However, the Zn source significantly influenced the expression levels of ZnT10, PepT1, LAT1, and B0AT1 (P < 0.04) (Figure 1). Compared with the control, the mRNA expression levels of ZnT10 and PepT1 were significantly reduced by 2 Zn sources (P < 0.05). Zn-Prot M significantly increased LAT1 mRNA expression compared to the control (P < 0.05), while Zn-Prot M significantly decreased the mRNA expression of B0AT1 compared to the control and ZnS (P < 0.05).
Figure 1.
Effect of Zn source (added Zn level of 400 μmol/L) on mRNA expression levels of ZnT, ZIP, amino acid, and small peptide transporters in primary cultured duodenal epithelial cells at 120 min of incubation (experiment 3). Values are means ± SE (n = 4 to 6). Lacking the same letters (a, b) means significant differences (P < 0.05). ZnT1, zinc transporter 1; ZnT4, zinc transporter 4; ZnT5, zinc transporter 5; ZnT6, zinc transporter 6; ZnT7, zinc transporter 7; ZnT9, zinc transporter 9; ZnT10, zinc transporter 10; ZIP3, zrt–irt-like protein 3; ZIP5, zrt–irt-like protein 5; B0AT1, b-0-system neutral amino acid co-transporter; LAT1, L-type amino acid transporter 1; rBAT, b0,+-type amino acid transporter; EAAT3, excitatory amino acid transporter 3; PepT1, peptide-transporter 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6). The mRNA expression levels were calculated as the relative quantities of the target gene mRNA to the geometric mean of 18S rRNA and GAPDH mRNA using the 2−ΔΔCT method.
Effect of Zn source (added Zn level of 400 μmol/L) on the protein expression levels of ZnT, ZIP, small peptide, and amino acid transporters in the duodenal epithelial cells at 120 min of incubation (experiment 3)
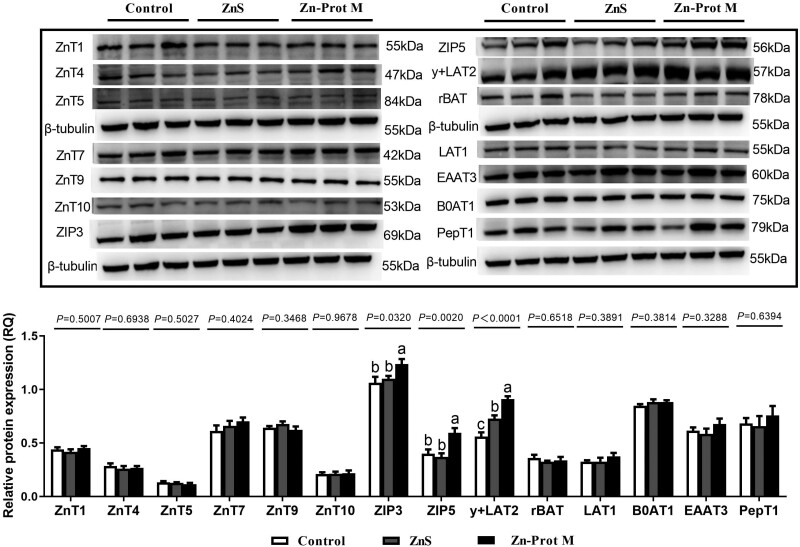
Zinc source did not affect the protein abundances of ZnT1, ZnT4, ZnT5, ZnT7, ZnT9, ZnT10, rBAT, LAT1, B0AT1, EAAT3, and PepT1 (P > 0.32) (Figure 2). However, the protein abundances of ZIP3, ZIP5, and y + LAT2 in the duodenal epithelial cells were significantly affected by Zn source (P < 0.04) (Figure 2). Compared to the control, 2 Zn sources demonstrated a significant increase in protein abundance of y + LAT2 (P < 0.05). Furthermore, when compared to ZnS, Zn-Prot M exhibited a significant increase (P < 0.05). The protein abundances of ZIP3 and ZIP5 were significantly greater for Zn-Prot M compared to the control and ZnS (P < 0.05), with no significant difference between the control and ZnS (P > 0.05).
Figure 2.
Effect of Zn source (added Zn level of 400 μmol/L) on protein expression levels of ZnT, ZIP, amino acid, and small peptide transporters in primary cultured duodenal epithelial cells at 120 min of incubation (experiment 3). Values are means ± SE (n = 5 to 6). Lacking the same letters (a, b, c) means significant differences (P < 0.05). ZnT1, zinc transporter 1; ZnT4, zinc transporter 4; ZnT5, zinc transporter 5; ZnT6, zinc transporter 6; ZnT7, zinc transporter 7; ZnT9, zinc transporter 9; ZnT10, zinc transporter 10; ZIP3, zrt–irt-like protein 3; ZIP5, zrt–irt-like protein 5; B0AT1, b-0-system neutral amino acid co-transporter; LAT1, L-type amino acid transporter 1; rBAT, b0,+-type amino acid transporter; EAAT3, excitatory amino acid transporter 3; PepT1, peptide-transporter 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ZnS, zinc sulfate; Zn-Prot M, zinc proteinate with moderate chelation strength (Qf = 51.6). The protein expression levels were calculated as the relative quantities of the target gene protein band intensity to the β-tubulin protein band intensity.
Discussion
In this study, Zn-Prot M notably enhanced the absorption of Zn and the protein abundances of ZIP3, ZIP5, and y + LAT2 in the primary cultured duodenal epithelial cells compared to the control and ZnS, suggesting that Zn-Prot M enhanced the Zn transport possibly by upregulating the protein expression levels of ZIP3, ZIP5, and y + LAT2, which has supported our hypothesis. These findings have not been previously reported, and are presented as a new perspective on the molecular modes of Zn absorption as the organic Zn-Prot M in the duodenum of broilers.
In the current study, Zn-Prot M increased Zn absorption in cells compared to ZnS, which is in accordance with our previous findings in broilers where Zn-Prot M displayed greater Zn absorption than ZnS in both ligated duodenal loops and the small intestine (Yu et al., 2017; Hu et al., 2022). The absorption of Zn in the in situ ligated duodenum of broilers is a saturable carrier-mediated process (Yu et al., 2017), and is primarily regulated by ZnTs and ZIPs (Tang et al., 2015; Wu et al., 2020; Hu et al., 2022, 2023; Rao et al., 2023). A previous study demonstrated that broilers fed a Zn-amino acid complex had higher mRNA expression level of jejunal ZnT1 (Huang and Gitschier, 1997). According to our recent researches (Hu et al., 2022, 2023), the increased transport of Zn as Zn-Prot M may be linked to elevated protein expression levels of ZnT1, ZnT4, ZnT7, ZnT10, ZIP3, and ZIP5 in the small intestine of broilers. Similarly, in the current study, it was found that Zn-Prot M upregulated the protein abundances of ZIP3 and ZIP5 in the primary cultured duodenal epithelial cells compared to the control and ZnS. However, no significant changes were observed in the protein abundances of ZnTs. Factors other than Zn source, such as feed consumption, environmental, and physiological factors, might be responsible for the disparities mentioned above in findings between in vivo and in vitro studies. For example, feed consumption change as affected by Zn may regulate the protein expression levels of ZnTs in the small intestine of broilers possibly by affecting the intake of Zn and other nutrients. Dufner-Beattie et al. (2006) reported that mice become more vulnerable to Zn deficiency in the absence of the ZIP3 gene, indicating that ZIP3 participated in the absorption of Zn. Geiser et al. (2013) demonstrated that ZIP5 was involved in the control of Zn excretion in mice. Therefore, the results of this study suggest that ZIP3 and ZIP5 could potentially be involved in the transport of Zn as the organic Zn-Prot M in the primary duodenal epithelial cells of boilers.
According to previous studies, the absorption of organic trace elements might be influenced by amino acid and peptide transporters (Gao et al., 2014; Hu et al., 2022, 2023). Gao et al. (2014) reported that Cu could be transported as a Cu-methionine complex in the Caco-2 cells, which was facilitated by amino acids transporters. Our recent studies revealed that broilers fed a Zn-Prot M diet exhibited increased protein expression levels of y + LAT2 and PepT1 in the duodenum, jejunum, or ileum of broilers compared to those fed a ZnS diet, suggesting that these amino acid and peptide transporters might play an important role in the absorption of Zn from the organic Zn-Prot M (Hu et al., 2022, 2023). Similar results were observed in our current study regarding y + LAT2 protein expression level in the cells, suggesting that the absorption of Zn from the organic Zn-Prot M in primary duodenal epithelial cells might also be enhanced by the neutral amino acid transporter y + LAT2.
In the present study, we also observed the discordant mRNA and protein expression levels of certain Zn and amino acid transporters in the primary duodenal epithelial cells. For example, the mRNA expression of B0AT1 was significantly decreased by Zn-Prot M compared to the control and ZnS, whereas no significant change was detected in its corresponding protein expression. The protein expression levels of ZIP3 and ZIP5 were significantly greater in the Zn-Prot M than in the control and ZnS. However, no changes were observed in the mRNA expression levels of these proteins. This might reflect negative feedbacks for the expression of their mRNA or protein, or the presence of other regulatory factors that are not currently understood (Chen et al., 2002).
The findings from the present study suggest that ZIP3, ZIP5, and y + LAT2 might play an important role in the transport of Zn from the organic Zn-Prot M in the primary duodenal epithelial cells of chick embryos. Nevertheless, further studies are needed to be conducted to validate the functions of the aforementioned carriers in promoting the transport of Zn as Zn-Prot M in primary epithelial cells using gene silencing and overexpression techniques.
Conclusions
Zn-Prot M increased Zn absorption in the primary duodenal epithelial cells of broiler embryos compared to ZnS possibly by elevating the protein expression levels of ZIP3, ZIP5, and y + LAT2 at a Zn concentration of 400 μmol/L.
Acknowledgments
This work was supported by the Jiangsu Shuang Chuang Ren Cai Program (JSSCRC2021541), the National Natural Science Foundation of China (31972583), the Key International Cooperation Program of the National Natural Science Foundation of China (32120103011), the Jiangsu Shuang Chuang Tuan Dui Program (JSSCTD202147), the Initiation Funds of Yangzhou University for Distinguished Scientists, and the Qing Lan Project of Yangzhou University.
Glossary
Abbreviations
- B0AT1
b-0-system neutral amino acid co-transporter
- DMEM/F12
dulbecco’s modified eagle medium/nutrient mixture F-12
- EAAT3
excitatory amino acid transporter 3
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- LAT1
l-type amino acid transporter 1
- LDH
lactate dehydrogenase
- PepT1
peptide-transporter 1
- rBAT
b0,+-type amino acid transporter
- TEER
trans-epithelial electrical resistance
- y + LAT2
y + L-type amino transporter 2
- Zn
zinc
- ZnS
zinc sulfate
- ZnTs
zinc transporter families
- ZIPs
zrt/irt-like protein families
- ZnT1
zinc transporter 1
- ZnT4
zinc transporter 4
- ZnT5
zinc transporter 5
- ZnT6
zinc transporter 6
- ZnT7
zinc transporter 7
- ZnT9
zinc transporter 9
- ZnT10
zinc transporter 10
- ZIP3
zrt–irt-like protein 3
- ZIP5
zrt–irt-like protein 5
- Zn-Prot M
zinc proteinate with a moderate Qf value
- 18S rRNA
18S ribosome RNA
Contributor Information
Yun Hu, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Wei Wu, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Liang Huang, Mineral Nutrition Research Division, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, People’s Republic of China.
Liyang Zhang, Mineral Nutrition Research Division, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, People’s Republic of China.
Chunyu Cao, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Weiyun Zhang, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Yangyang Hu, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Xiaoyan Cui, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Tingting Li, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Shengchen Wang, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Xugang Luo, Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, People’s Republic of China.
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Literature Cited
- Cao, S., Zhang S., Liu G., Zhang L., Lu L., Zhang R., Liao X., and Luo X... 2020. Kinetics of phosphorus absorption and expressions of related transporters in primary cultured duodenal epithelial cells of chick embryos. J. Anim. Physiol. Anim. Nutr. 104:237–244. doi: 10.1111/jpn.13260 [DOI] [PubMed] [Google Scholar]
- Chen, G., Gharib T. G., Huang C. C., Taylor J. M., Misek D. E., Kardia S. L., Giordano T. J., Iannettoni M. D., Orringer M. B., Hanash S. M.,. et al. 2002. Discordant protein and mRNA expression in lung adenocarcinomas. Mol. Cell. Proteomics. 1:304–313. doi: 10.1074/mcp.m200008-mcp200 [DOI] [PubMed] [Google Scholar]
- Chen, X., He C., Zhang K., Wang J., Ding X., Zeng Q., Peng H., Bai J., Lv L., Xuan Y.,. et al. 2022. Comparison of zinc bioavailability in zinc-glycine and zinc-methionine chelates for broilers fed with a corn-soybean meal diet. Front. Physiol. 13:983954. doi: 10.3389/fphys.2022.983954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner-Beattie, J., Huang Z. L., Geiser J., Xu W., and Andrews G. K... 2006. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 44:239–251. doi: 10.1002/dvg.20211 [DOI] [PubMed] [Google Scholar]
- Gao, S., Yin T., Xu B., Ma Y., and Hu M... 2014. Amino acid facilitates absorption of copper in the Caco-2 cell culture model. Life Sci. 109:50–56. doi: 10.1016/j.lfs.2014.05.021 [DOI] [PubMed] [Google Scholar]
- Geiser, J., De Lisle R. C., and Andrews G. K... 2013. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PLoS One. 8:e82149. doi: 10.1371/journal.pone.0082149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B., Bortoluzzi C., King W. D., Graugnard D., Dawson K. A., and Applegate T. J... 2019. Zinc source influences the gene expression of zinc transporters in jejunum and cecal tonsils during broiler challenge with Eimeria maxima and Clostridium perfringens. Poult. Sci. 98:1146–1152. doi: 10.3382/ps/pey484 [DOI] [PubMed] [Google Scholar]
- Hu, Y., Wang C., Wu W., Qu Y., Zhang W., Li D., Zhu L., Gao F., Wu B., Zhang L.,. et al. 2022. Organic zinc with moderate chelation strength enhances zinc absorption in the small intestine and expression of related transporters in the duodenum of broilers. Front. Physiol. 13:952941. doi: 10.3389/fphys.2022.952941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., Huang Y., Wang C., Zhang W., Qu Y., Li D., Wu W., Gao F., Zhu L., Wu B.,. et al. 2023. The organic zinc with moderate chelation strength enhances the expression of related transporters in the jejunum and ileum of broilers. Poult. Sci. 102:102477. doi: 10.1016/j.psj.2023.102477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L., and Gitschier J... 1997. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 17:292–297. doi: 10.1038/ng1197-292 [DOI] [PubMed] [Google Scholar]
- Huang, Y. L., Lu L., Li S. F., Luo X. G., and Liu B... 2009. Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J. Anim. Sci. 87:2038–2046. doi: 10.2527/jas.2008-1212 [DOI] [PubMed] [Google Scholar]
- Huang, L., Cao C., Lin X., Lu L., Lin X., Liu H. C., Odle J., See M. T., Zhang L., Wu W.,. et al. 2024. Zinc alleviates thermal stress-induced damage to the integrity and barrier function of cultured chicken embryonic primary jejunal epithelial cells via the MAPK and PI3K/AKT/mTOR signaling pathways. Poult. Sci. 103:103696. doi: 10.1016/j.psj.2024.103696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe, T. 2013. Overview of and update on the physiological functions of mammalian zinc transporters. Nihon Eiseigaku Zasshi. 68:92–102. doi: 10.1265/jjh.68.92 [DOI] [PubMed] [Google Scholar]
- Khan, M. I., Chand N., Naz S., Alonaizan R., Hu H., Shamsi S., and Khan R. U... 2024. Effects of zinc supplementation from organic and inorganic sources on growth, blood biochemical indices, and intestinal microarchitecture in broilers. Vet. Q. 44:1–7. doi: 10.1080/01652176.2023.2298491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, T., and Kambe T... 2016. The Functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int. J. Mol. Sci. 17:336. doi: 10.3390/ijms17030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Xie J., Lu L., Zhang L., Zhang L., Zou Y., Wang Q., Luo X., and Li S... 2013. Kinetics of manganese transport and gene expressions of manganese transport carriers in Caco-2 cell monolayers. Biometals. 26:941–953. doi: 10.1007/s10534-013-9670-y [DOI] [PubMed] [Google Scholar]
- Li, T., He W., Liao X., Lin X., Zhang L., Lu L., Guo Y., Liu Z., and Luo X... 2021. Zinc alleviates the heat stress of primary cultured hepatocytes of broiler embryos via enhancing the antioxidant ability and attenuating the heat shock responses. Anim. Nutr. 7:621–630. doi: 10.1016/j.aninu.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D... 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Min, Y. N., Liu F. X., Qi X., Ji S., Cui L., Wang Z. P., and Gao Y. P... 2019. Effects of organic zinc on tibia quality, mineral deposit, and metallothionein expression level of aged hens. Poult. Sci. 98:366–372. doi: 10.3382/ps/pey386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhamed, P. K., and Vadstrup S... 2014. Zinc is the most important trace element. Ugeskr. Laeger. 176:V11120654. [PubMed] [Google Scholar]
- Ogbuewu, I. P., and Mbajiorgu C. A... 2023. Potentials of dietary zinc supplementation in improving growth performance, health status, and meat quality of broiler chickens. Biol. Trace Elem. Res. 201:1418–1431. doi: 10.1007/s12011-022-03223-5 [DOI] [PubMed] [Google Scholar]
- Rao, S. B. N., Elangovan A. V., Madiajagan B., Rajendran D., Franklin M. E. E., Gopi M., Pal D., Parthipan S., Nalina M., Dey D. K.,. et al. 2023. Production and evaluation of encapsulated zinc oxide on performance, ileal digestibility and zinc transporter gene expression in broiler chicken. Biol. Trace Elem. Res. 201:5774–5785. doi: 10.1007/s12011-023-03614-2 [DOI] [PubMed] [Google Scholar]
- Saper, R. B., and Rash R... 2009. Zinc: an essential micronutrient. Am. Fam. Physician. 79:768–772. [PMC free article] [PubMed] [Google Scholar]
- Sekler, I., Sensi S. L., Hershfinkel M., and Silverman W. F... 2007. Mechanism and regulation of cellular zinc transport. Mol. Med. 13:337–343. doi: 10.2119/2007-00037.Sekler [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, Y., Wolf P. G., Guo S., Guo Y., Gaskins H. R., and Zhang B... 2017. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 43:18–26. doi: 10.1016/j.jnutbio.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Tang, Z. G., Chen G. Y., Li L. F., Wen C., Wang T., and Zhou Y. M... 2015. Effect of zinc-bearing zeolite clinoptilolite on growth performance, zinc accumulation, and gene expression of zinc transporters in broilers. J. Anim. Sci. 93:620–626. doi: 10.2527/jas.2014-8165 [DOI] [PubMed] [Google Scholar]
- Vallee, B. L., and Auld D. S... 1990. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 29:5647–5659. doi: 10.1021/bi00476a001 [DOI] [PubMed] [Google Scholar]
- Wu, A., Bai S., Ding X., Wang J., Zeng Q., Peng H., Wu B., and Zhang K... 2020. The systemic zinc homeostasis was modulated in broilers challenged by salmonella. Biol. Trace Elem. Res. 196:243–251. doi: 10.1007/s12011-019-01921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, S., Duan M., Fang B., Zhao G., Leng X., and Zhang T... 2023. Zinc homeostasis and regulation: Zinc transmembrane transport through transporters. Crit. Rev. Food Sci. Nutr. 63:7627–7637. doi: 10.1080/10408398.2022.2048292 [DOI] [PubMed] [Google Scholar]
- Yu, Y., Lu L., Luo X. G., and Liu B... 2008. Kinetics of zinc absorption by in situ ligated intestinal loops of broilers involved in zinc transporters. Poult. Sci. 87:1146–1155. doi: 10.3382/ps.2007-00430 [DOI] [PubMed] [Google Scholar]
- Yu, Y., Lu L., Li S. F., Zhang L. Y., and Luo X. G... 2017. Organic zinc absorption by the intestine of broilers in vivo. Br. J. Nutr. 117:1086–1094. doi: 10.1017/S0007114517001040 [DOI] [PubMed] [Google Scholar]
- Zhang, S. M., Liao X. D., Lu L., Zhang L. Y., and Luo X. G... 2018. Isolation and identification of duodenal epithelial cells of broiler embryos in vitro and its establishment and evaluation of primary cultured absorption model. Chin. J. Anim. Nutr. 30:3159–3167. In Chinese. [Google Scholar]