Key Points
Question
Is solid organ transplantation associated with an increased risk of adverse pregnancy outcomes?
Findings
In this systematic review and meta-analysis of 22 studies with 93 565 343 pregnancies, there was a statistically significant 4- to 6-fold increased risk of preeclampsia, preterm birth at less than 37 weeks, and low birth weight of less than 2500 g in pregnancies with a solid organ transplant compared with pregnancies without a solid organ transplant.
Meaning
These findings suggest that pregnancies in solid organ transplant recipients have increased risk of adverse outcomes.
This systematic review and meta-analysis evaluates the association of solid organ transplantation with adverse pregnancy outcomes compared with pregnancy in individuals without solid organ transplantation and assesses the incidence of allograft rejection or loss during pregnancy.
Abstract
Importance
Transplant recipients experience high rates of adverse pregnancy outcomes; however, contemporary estimates of the association between solid organ transplantation and adverse pregnancy outcomes are lacking.
Objective
To evaluate the association between solid organ transplantation and adverse pregnancy outcomes and to quantify the incidence of allograft rejection and allograft loss during pregnancy.
Data Sources
PubMed/MEDLINE, EMBASE and Scopus databases were searched from January 1, 2000, to June 20, 2024, and reference lists were manually reviewed.
Study Selection
Cohort and case-control studies that reported at least 1 adverse pregnancy outcome in pregnant women with solid organ transplantation vs without solid organ transplant or studies that reported allograft outcomes in pregnant women with solid organ transplantation were included following independent dual review of abstracts and full-text articles.
Data Extraction and Synthesis
Two investigators abstracted data and independently appraised risk of bias using the Newcastle Ottawa Scale. A random-effects model was used to calculate overall pooled estimates using the DerSimonian-Laird estimator. Reporting followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.
Main Outcomes and Measures
Primary pregnancy outcomes were preeclampsia, preterm birth (<37 weeks), and low birth weight (<2500 g). Secondary pregnancy outcomes were live birth rate, gestation, very preterm birth (<32 weeks), very low birth weight (<1500 g), and cesarean delivery. Allograft outcomes were allograft loss and rejection during pregnancy.
Results
Data from 22 studies and 93 565 343 pregnancies (4786 pregnancies in solid organ transplant recipients) were included; 14 studies reported adverse pregnancy outcomes, and 13 studies provided data for allograft outcomes. Pregnancies in organ transplant recipients were associated with significantly increased risk of preeclampsia (adjusted odds ratio [aOR], 5.83 [95% CI, 3.45-9.87]; I2 = 77.4%), preterm birth (aOR, 6.65 [95% CI, 4.09-12.83]; I2 = 81.8%), and low birth weight (aOR, 6.51 [95% CI, 2.85-14.88]; I2 = 90.6%). The incidence of acute allograft rejection was 2.39% (95% CI, 1.20%-3.96%; I2 = 68.5%), and the incidence of allograft loss during pregnancy was 1.55% (95% CI, 0.05%-4.44%; I2 = 69.2%).
Conclusions and Relevance
In this systematic review and meta-analysis, pregnancies in recipients of a solid organ transplant were associated with a 4 to 6 times increased risk of preeclampsia, preterm birth, and low birth weight during pregnancy. There was a low overall risk of graft rejection or loss during pregnancy.
Introduction
During the 21st century, the rate of solid organ transplantation has increased dramatically, resulting in an increasing number of women of childbearing age with kidney, liver, heart, or lung transplants.1,2,3 In the US, there were 65 775 organ transplants in 2023, of which 9077 were kidney transplants and 3116 were liver transplants in women aged 18 to 49 years.1 Among transplant recipients, pregnancy is associated with a disproportionately increased risk of adverse pregnancy outcomes, including preeclampsia, low birth weight, and premature birth.4,5,6
Much of our understanding of the risks during pregnancy are derived from registry and single-center reports. Previous meta analyses have reported pooled incidences of various pregnancy outcomes in both kidney and liver transplant recipients.4,5,6 However, to our knowledge, none have measured the strength of association between adverse pregnancy outcomes and solid-organ transplantation compared with pregnancies without transplantation. The estimation of the risk of developing adverse pregnancy outcomes provides valuable data with which to inform prepregnancy counselling and enhance interdisciplinary antenatal care.
As such, the primary goal of this study was to synthesize the available evidence on the associations between solid organ transplantation and adverse pregnancy outcomes. Our secondary goal was to examine the impact of pregnancy on the allograft by summarizing the incidence of acute allograft rejection and loss during pregnancy.
Methods
For this systematic review and meta-analysis, a prespecified protocol was registered with PROPSERO (CRD42021269591). Institutional review board approval was not required for this meta-analysis because it included only previously published research. Reporting for this study adheres to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.7
Data Sources and Search Strategy
A systematic search of PubMed/Medline, EMBASE and Scopus was undertaken from January 1, 2000, to June 20, 2024, by a research librarian (A.A.). The search was limited to articles from 2000 onward to represent more current populations. This was supplemented by hand-searching reference lists of key citations. For the primary goal, we sought studies with the following: a population of pregnant individuals with solid organ transplantation (kidney, liver, heart, or lung), a comparison group of pregnant individuals with no solid organ transplantation, and at least 1 primary or secondary pregnancy outcome of interest. For the secondary goal, we also included studies that reported allograft loss or allograft rejection during pregnancy in pregnant individuals with a solid organ transplant. A detailed search strategy is available in eAppendix 1 in Supplement 1. While the search and eligibility criteria used an inclusive definition of women (ie, pregnant populations that included all gender identities), all studies that we found referred to their populations as women.
Outcomes
The primary pregnancy outcomes were preeclampsia, preterm birth (ie, <37 weeks’ gestation), and low birth weight (ie, <2500 g). Secondary pregnancy outcomes were gestational age at birth in weeks, live birth, very preterm birth (ie, <32 weeks’ gestation), very low birth weight (ie, <1500 g), and cesarean delivery. For the secondary goal, the allograft outcomes were acute allograft rejection during pregnancy and acute allograft loss during pregnancy.
Study Selection
Peer-reviewed, English-language, cohort and case-control studies were included if they published original effect estimates of the association between solid organ transplantation and at least 1 primary or secondary pregnancy outcome or reported the risk of allograft outcomes during pregnancy in solid organ transplant recipients. Independent dual review of abstracts and full text articles was performed by 3 authors (J.H.Y., N.F., or S.A.M.). Discrepancies in study selection were resolved by 2 mediators (K.R.P. and P.G.K.). If the same registry or patient population was used in more than 1 study, the larger of the studies was included.
Data Extraction and Risk of Bias Assessment
Two reviewers (J.H.Y., N.F., or S.A.M.) independently extracted data from eligible studies. Where information was not available from the studies, authors were contacted to request this information. Risk of bias was independently dual rated using the Newcastle-Ottawa Scale (NOS) for observational studies.8 Based on the NOS, studies were classified as high (8-9 stars), moderate (6-7 stars), and low (<6 stars) risk of bias. For studies that reported allograft outcomes only, a modified NOS using selection and outcome was used as the category for comparability was deemed irrelevant for this outcome. For the modified NOS, studies were judged to be low risk of bias (≥4 stars) and high risk of bias (<4 stars). Discrepancies in data extraction and quality appraisal were resolved through consensus with a third reviewer.
Statistical Analysis
Random effects meta-analysis using the DerSimonian-Laird method was used to calculate overall pooled odds ratios (ORs) of the association between solid organ transplantation and primary or secondary adverse pregnancy outcomes.9 Adjusted ORs (aORs) from studies that adjusted for at least 1 confounding factor were included in the meta-analysis. If aORs were unavailable, crude estimates were pooled separately. Incidence rate ratio and risk ratio were used as approximations of each other in the setting of low incidence rate10 and the aOR was computed.11 For continuous outcomes, the mean difference (MD) was calculated. Pooled incidences were expressed as proportions after transforming data using Freeman-Tukey Double Arcsine transformation.12 Heterogeneity was assessed using τ2 statistic and I2 values.
Subgroup analysis by solid organ transplant type and maternal factors were planned but not conducted owing to the small number of included studies. Tests of small study effects were evaluated using the Egger test13 and funnel plots for outcomes with at least 10 studies.14
Sensitivity analyses with restriction of the meta-analyses to studies reporting effect estimates adjusted for important confounding factors specified a priori, namely maternal age and preexisting hypertension, was performed. All significance testing was 2-sided, and results were considered statistically significant at P < .05. Data were analyzed using Stata version 18.0 (StataCorp). Data were analyzed from January 1 to June 25, 2024
Results
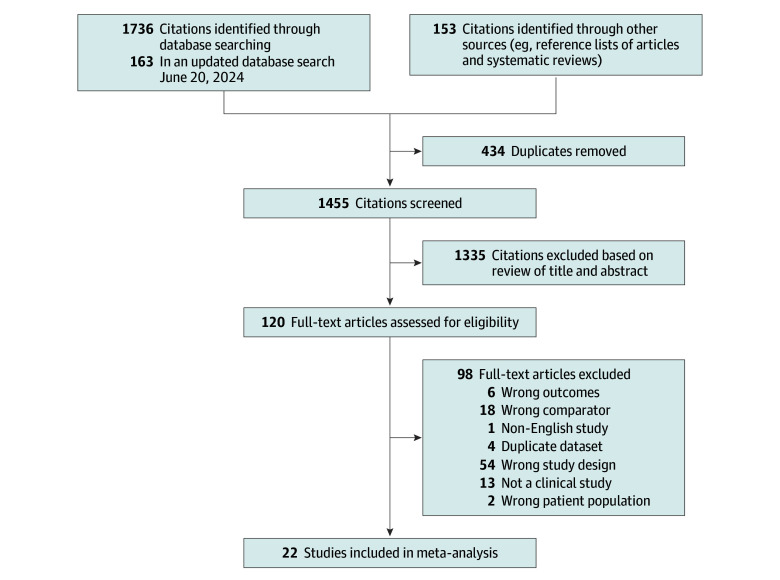
The search yielded 1736 unique results (Figure 1), and 22 articles15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 were selected as relevant studies for our analysis, including a total study population of 92 289 079 women (93 565 343 pregnancies). There were a total of 4431 women who had 4786 pregnancies following a solid organ transplant. Of these, 2758 pregnancies (57.6%) were among women with kidney transplants, 1811 pregnancies (37.8%) were among women with liver transplants, 211 pregnancies (4.4%) were among women with heart transplants, and 6 pregnancies (0.1%) were among women with lung transplants.
Figure 1. Flow Diagram of Study Selection Process.
Of 22 studies, 14 studies16,17,18,19,20,21,22,23,24,25,26,27,28,36 (3187 pregnancies in 3090 women with a solid organ transplant) reported at least 1 primary or secondary adverse pregnancy outcome. Thirteen studies15,16,19,26,27,28,29,30,31,32,33,34,35 (2000 pregnancies in 1728 women with a solid organ transplant) reported the rate of allograft outcomes during pregnancy.
There were 6 studies from North America,15,17,20,24,27,36 2 studies from Australia,18,25 11 studies from Europe,16,19,21,22,23,26,29,30,32,33,35 1 study from Asia34 and 2 studies from the Middle East.28,31 The sample size of included studies ranged widely, from 120 women in an Iranian study28 to a registry-based study of 38 449 030 US women.20
The summary results of all meta-analyses are presented in Table 1. Study characteristics of the 14 articles16,17,18,19,20,21,22,23,24,25,26,27,28,36 that provided effect estimates for at least 1 adverse pregnancy outcome are presented in Table 2 (baseline characteristics in eTable 1 in Supplement 1). Study characteristics of the 13 articles15,16,19,26,27,28,29,30,31,32,33,34,35 that reported allograft outcomes are presented in eTable 2 in Supplement 1.
Table 1. Summary Results of Meta-Analyses.
| Outcome | Studies, No. | Source | Pregnancies, No. | Outcomes, No. | Pooled OR (95% CI) | I2, % | τ2 |
|---|---|---|---|---|---|---|---|
| Preeclampsia | |||||||
| Crude | 11 | Barros et al,16 2022; DeFilippis et al,17 2022; Hewawasam et al,18 2022; Mazanowska et al,19 2022; Sobotka et al,20 2021; Madej et al,22 2016; Majak et al,23 2016; Arab et al,24 2015; Bramham et al,26 2013; Coffin et al,27 2010; Pezeshki et al,28 2004 | 70 069 293 | 2 861 580 | 6.34 (4.94 to 8.14) | 67.6 | 0.09 |
| Adjusted (any) | 5 | DeFilippis et al,17 2022; Hewawasam et al,18 2022; Majak et al,23 2016; Arab et al,24 2015; Bramham et al,26 2013 | 31 615 223 | 1 284 691 | 5.83 (3.45 to 9.87) | 77.4 | 0.25 |
| Adjusted for prespecified covariatesa | 3 | DeFilippis et al,17 2022; Hewawasam et al,18 2022; Majak et al,23 2016 | 24 520 251 | 1 011 433 | 4.14 (2.87 to 5.97) | 1.7 | 0.00 |
| Preterm birth <37 wk | |||||||
| Crude | 12 | Craig et al,36 2023; Barros et al,16 2022; Hewawasam et al,18 2022; Sobotka et al,20 2021; Piccoli et al,21 2017; Madej et al,22 2016; Majak et al,23 2016; Arab et al,24 2015; Wyld et al,25 2013; Bramham et al,26 2013; Coffin et al,27 2010; Pezeshki et al,28 2004 | 91 105 532 | 4 919 542 | 7.48 (4.49 to 12.47) | 95.1 | 0.74 |
| Adjusted (any) | 4 | Craig et al,36 2023; Majak et al,23 2016; Arab et al,24 2015; Bramham et al,26 2013 | 46 995 396 | 3 793 167 | 6.65 (4.09 to 12.83) | 81.8 | 0.19 |
| Adjusted for prespecified covariatesa | 3 | Craig et al,36 2023; Majak et al,23 2016; Arab et al,24 2015 | 46 933 949 | 3 793 002 | 5.31 (3.93 to 7.17) | 41.1 | 0.03 |
| Low birth weight <2500 g | |||||||
| Crude | 11 | Barros et al,16 2022; DeFilippis et al,17 2022; Hewawasam et al,18 2022; Mazanowska et al,19 2022; Sobotka et al,20 2021; Piccoli et al,21 2017; Majak et al,23 2016; Arab et al,24 2015; Wyld et al,25 2013; Bramham et al,26 2013; Coffin et al,27 2010 |
73 168 692 | 1 969 495 | 5.50 (3.42 to 8.86) | 91.7 | 0.54 |
| Adjusted (any) | 4 | DeFilippis et al,17 2022; Hewawasam et al,18 2022; Arab et al,24 2015; Bramham et al,26 2013 | 31 944 922 | 972 050 | 6.51 (2.85 to 14.88) | 90.6 | 0.58 |
| Adjusted for prespecified covariatesa | 3 | DeFilippis et al,17 2022; Hewawasam et al,18 2022; Arab et al,24 2015 | 31 943 476 | 971 894 | 4.96 (1.60 to 15.37) | 92.5 | 0.82 |
| Live birth | |||||||
| Crude | 4 | Barros et al,16 2022; Hewawasam et al,18 2022; Wyld et al,25 2013; Bramham et al,26 2013 | 8 263 840 | 8 199 030 | 0.28 (0.03 to 2.61) | 97.8 | 5.02 |
| Adjusted (any) | NA | NA | NA | NA | NA | NA | NA |
| Adjusted for prespecified covariatesa | NA | NA | NA | NA | NA | NA | NA |
| Preterm birth <32 wk | |||||||
| Crude | 4 | Hewawasam et al,18 2022; Piccoli et al,21 2017; Wyld et al,25 2013; Bramham et al,26 2013 | 5 717 636 | 99 507 | 14.47 (8.29 to 25.28) | 80.5 | 0.25 |
| Adjusted (any) | 2 | Hewawasam et al,18 2022; Bramham et al,26 2013 | 2 947 860 | 55 139 | 10.35 (6.89 to 15.53) | 2.7 | 0.00 |
| Adjusted for prespecified covariates | 1 | Hewawasam et al,18 2022 | 2 946 413 | 55 103 | 11.4 (7.41 to 17.55) | NA | NA |
| Birth weight <1500 g | |||||||
| Crude | 5 | Hewawasam et al,18 2022; Piccoli et al,21 2017; Madej et al,22 2016; Wyld et al,25 2013; Bramham et al,26 2013 | 5 717 634 | 78 258 | 4.97 (2.28 to 10.82) | 87.0 | 0.67 |
| Adjusted (any) | 1 | Bramham et al,26 2013 | 1446 | 33 | 7.76 (3.29 to 18.3) | NA | NA |
| Adjusted for prespecified covariatesa | 0 | NA | NA | NA | NA | NA | NA |
| Gestational age | |||||||
| Crude | 5 | Barros et al,16 2022; Mazanowska et al,19 2022; Majak et al,23 2016; Wyld et al,25 2013; Pezeshki et al,28 2004 | 2 769 094 | NA | −3.37 (−3.76 to −2.99)b | 0.0 | 0.00 |
| Adjusted (any) | 0 | NA | NA | NA | NA | NA | NA |
| Adjusted for prespecified covariatesa | 0 | NA | NA | NA | NA | NA | NA |
| Cesarean delivery | |||||||
| Crude | 11 | Craig et al,36 2023; Barros et al,16 2022; Hewawasam et al,18 2022; Mazanowska et al,19 2022; Sobotka et al,20 2021; Piccoli et al,21 2017; Madej et al,22 2016; Majak et al,23 2016; Arab et al,24 2015; Bramham et al,26 2013; Coffin et al,27 2010 | 88 293 100 | 27 376 648 | 3.67 (2.33 to 5.78) | 96.1 | 0.54 |
| Adjusted (any) | 5 | Craig et al,36 2023; Hewawasam et al,18 2022; Majak et al,23 2016; Arab et al,24 2015; Bramham et al,26 2013 | 49 837 531 | 15 991 969 | 3.30 (2.13 to 5.11) | 85.5 | 0.21 |
| Adjusted for prespecified covariatesa | 4 | Craig et al,36 2023; Hewawasam et al,18 2022; Majak et al,23 2016; Arab et al,24 2015 | 49 836 076 | 10 200 179 | 3.06 (1.89 to 4.94) | 86.1 | 0.20 |
Abbreviations: NA, not applicable; OR, odds ratio.
Prespecified comorbidities: maternal age and preexisting hypertension.
Expressed as mean difference (95% CI) in weeks.
Table 2. Characteristics of Studies That Reported at Least 1 Adverse Pregnancy Outcome.
| Source | Country | Study design, data source | Pregnancies, No. | Exposure | Definition of control group | Outcomes | Confounders adjusted |
|---|---|---|---|---|---|---|---|
| Craig et al,36 2023 | US | Retrospective cohort, National Readmissions Database, HCUP | 39 839 192 | Heart transplant | Women without a history of heart transplant | Preterm birth, cesarean delivery | Age, comorbid conditions (including hypertension), calendar year, demographics, and facility characteristics |
| Barros et al,16 2022 | Portugal | Retrospective cohort, clinical records at single institution | 243 | Kidney transplant | Healthy women without transplantation who had antenatal follow up and delivery at the same institution between 2009 and 2019 | Preeclampsia, preterm birth <37 wk, LBW <2500 g, gestational age, live birth, cesarean delivery | NA |
| DeFilippis et al,17 2022 | US | Retrospective cohort, National Inpatient Sample, HCUP | 21 922 631 | Heart transplant | Women without history of heart transplant or systolic heart failure and had hospitalization for delivery | Preeclampsia, preterm birth <37 wk, LBW <2500 g, cesarean delivery | Maternal age, race, preexisting hypertension, kidney failure, diabetes, Elixhauser Comorbidity Index |
| Hewawasam et al,18 2022 | Australia | Retrospective cohort, Australian & New Zealand Dialysis and Transplant Registry and state-level perinatal datasets | 2 903 135 | Kidney transplant | Babies born to mothers who had either never received KRT or had given birth prior to commencement of KRT | Preeclampsia, LBW <2500 g, preterm birth <32 wk, LBW <1500 g, live birth, cesarean delivery | Maternal age, preexisting hypertension, preexisting diabetes, socioeconomic status, parity, multiple births |
| Mazanowska et al,19 2022 | Poland | Retrospective cohort, clinical records at single institution | 137 | Kidney transplant | Healthy patients with reference range kidney function matched according to age, BMI, and gestational age | Pregnancy-induced hypertension, gestational age at delivery, LBW, cesarean delivery, intrauterine growth restriction (<10%) | NA |
| Sobotka et al,20 2021 | US | Retrospective cohort, NIS (2005-2013) | 38 449 030 | LT | Women age >18 y who had hospitalization for delivery and no LT | Preeclampsia, preterm birth <37 wk, LBW <2500 g, cesarean delivery | Maternal age, race, income, type of insurance, Elixhauser Comorbidity Index, and hospital size, type, and region |
| Piccoli et al,21 2017 | Italy | Retrospective cohort, clinical records across multiple sites | 731 | Kidney transplant | Pregnancies occurring in the absence of hypertension, obesity, diabetes, CKD, cardiovascular disease | Preeclampsia, preterm birth <37 wk, LBW, preterm birth <34 wk, cesarean delivery | NA |
| Madej et al,22 2016 | Poland | Retrospective cohort, clinical records at single institution | 288 | Kidney transplant or LT | Pregnancies in nontransplanted women | Preeclampsia, preterm birth <37 wk, LBW <2500 g, mean gestational age, cesarean delivery | NA |
| Majak et al,23 2016 | Norway | Retrospective cohort, national registry | 357 | Kidney transplant | Random sample nontransplanted women who delivered first baby | Preeclampsia, preterm birth, LBW <2500 g, gestational age, cesarean delivery | Hypertension and twin pregnancy |
| Arab et al,24 2015 | US | Retrospective cohort, NIS | 7 094 400 | Kidney transplant | Deliveries in women without kidney transplant | Preeclampsia, preterm birth <37 wk, cesarean delivery | Maternal age, race, smoking, obesity, preexisting hypertension, diabetes |
| Wyld et al,25 2013 | Australia | Retrospective cohort, Australian & New Zealand Dialysis and Transplant Registry and national perinatal registry | 5 270 337 | Kidney transplant | All deliveries in Australia 1991-2010 | Preeclampsia, preterm birth <37 wk, LBW <2500 g, live birth, mean gestational age | NA |
| Bramham et al,26 2013 | UK | Retrospective cohort, national registry and hospital records at multiple institutions | 1465 | Kidney transplant | Women without kidney transplant | Preeclampsia, preterm birth <37 wk, LBW <2500 g, live birth, preterm birth <32 wk, LBW <1500 g, cesarean delivery | Maternal age, parity, smoking status |
| Coffin et al,27 2010 | US | Retrospective cohort, national registry (1993-2005) | 4266 | LT | Randomly selected controls (no LT) matched on age, hospital, and calendar year | Preeclampsia, preterm birth <37 wk, LBW <2500 g, live birth, cesarean delivery | Maternal age, sex, race, insurance, comorbidities, admission characteristics, year, region, LT hospital center |
| Pezeshki et al,28 2004 | Iran | Retrospective cohort, single institution | 120 | Kidney transplant | Pregnancies in nontransplant patients, matched for age and parity | Preeclampsia, preterm <37 wk, LBW <2500 g, gestational age | NA |
Abbreviations: CKD, chronic kidney disease; BMI, body mass index; HCUP, Healthcare Costs and Utilization Project; KRT, kidney replacement therapy; LBW, low birth weight; LT, liver transplant; NA, not applicable; NIS, National Inpatient Sample.
Preeclampsia
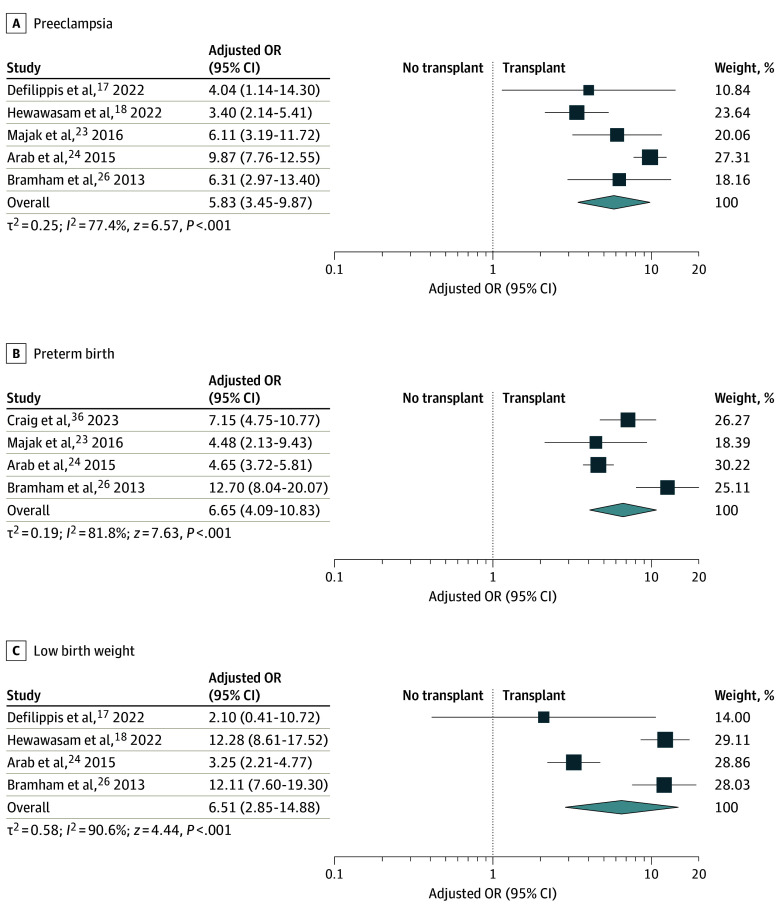
Eleven studies16,17,18,19,20,22,23,24,26,27,28 provided effect estimates for the association between solid organ transplantation and preeclampsia, with 5 studies17,18,23,24,26 also providing adjusted effect estimates. In a meta-analysis, solid organ transplantation was significantly associated with preeclampsia (OR, 6.34 [95% CI, 4.94 to 8.14]; I2 = 67.6%; aOR, 5.83 [95% CI, 3.45 to 9.87]; I2 = 77.4%) (Figure 2). In a sensitivity analysis, results were similar when restricting the meta-analysis to the 3 studies17,18,23 that adjusted for maternal age and preexisting hypertension (aOR, 4.14 [95% CI, 2.87 to 5.97]; I2 = 1.7%).
Figure 2. Summary of Pooled Adjusted Odds Ratios (ORs) for the Association Between Solid Organ Transplantation and Adverse Pregnancy Outcomes.
Preterm birth was defined as birth at less than 37 weeks’ gestation; low birth weight, birth weight less than 2500 g. Size of squares indicates study weight; diamond, overall findings.
Preterm Birth
Twelve studies16,18,19,20,22,23,24,25,26,27,28,36 reported estimates of the association between solid organ transplantation and preterm birth and 4 studies23,24,26,36 reported adjusted effect estimates. Solid organ transplantation was found to be significantly associated with preterm birth in meta-analysis (OR, 7.48 [95% CI, 4.49 to 12.47]; I2 = 95.1%; aOR, 6.65 [95% CI, 4.09 to 12.83]; I2 = 81.8%) (Figure 2). When we limited the meta-analysis to 3 studies23,24,36 in a sensitivity analysis, the association between solid organ transplantation and preterm birth was similar (aOR, 5.31 [95% CI, 3.93 to 7.17]; I2 = 41.1%).
Low Birth Weight
Eleven studies16,17,18,19,20,21,23,24,25,26,27 reported effect estimates of the association between solid organ transplantation and low birth weight, with 4 studies17,18,24,26 also reporting adjusted effect estimates. In a meta-analysis, solid organ transplantation was significantly associated with low birth weight (OR, 5.50 [95% CI, 3.42 to 8.86]; I2 = 91.7%; aOR, 6.51 [95% CI, 2.85 to 14.88]; I2 = 90.6%) (Figure 2). Sensitivity analysis was performed with three studies17,18,24 that reported effect estimates adjusting for preexisting hypertension and maternal age with similar effects (aOR, 4.96 [95% CI, 1.60 to 15.37]; I2 = 92.5%).
Secondary Adverse Pregnancy Outcomes
Four studies16,18,25,26 reported effect estimates of the association between solid organ transplantation and live birth. When the effect estimates were pooled, there was no significant association between solid organ transplantation and live birth (OR, 0.28 [95% CI, 0.03 to 2.61]; I2 = 97.8%) (eFigure 1 in Supplement 1).
Four studies18,21,25,26 provided effect estimates of the associations between solid organ transplantation and very preterm birth. Two studies18,26 also reported adjusted effect estimates. In meta-analysis, solid organ transplantation was significantly associated with very preterm birth (OR, 14.47 [95% CI, 8.29 to 25.28]; I2 = 80.5%; aOR, 10.35 [95% CI, 6.89 to 15.53]; I2 = 2.7%) (eFigure 2 in Supplement 1). Five studies18,21,22,25,26 reported effect estimates of the association between solid organ transplantation and very low birth weight (OR, 4.97 [95% CI, 2.28 to 10.82]; I2 = 87.0%) (eFigure 3 in Supplement 1).
Eleven studies16,18,19,20,21,22,23,24,26,27,36 reported effect estimates of the association between solid organ transplantation and cesarean delivery; 5 studies18,23,24,26,36 provided adjusted effect estimates. In meta-analysis, there was a significant association between solid organ transplantation and cesarean delivery (OR, 3.67 [95% CI, 2.33 to 5.78]; I2 = 96.1%; aOR 3.30 [95% CI, 2.13 to 5.11]; I2 = 85.5%) (eFigure 4 in Supplement 1).
Five studies16,19,23,25,28 reported gestation at birth, measured in weeks. In meta-analysis, there was a statistically significant MD in gestational age at birth in pregnancies comparing solid organ transplantation vs no transplantation (MD, −3.37 [95% CI, −3.37 to −2.99] weeks; I2 = 0.0%) (eFigure 5 in Supplement 1).
Sensitivity analysis for the secondary adverse pregnancy outcomes was only able to be performed for the association between solid organ transplantation and cesarean birth. Four studies18,23,24,36 adjusted specifically for preexisting hypertension and maternal age, resulting in a similar effect estimate (aOR, 3.06 [95% CI, 1.89 to 4.94]; I2 = 86.1%).
Allograft Outcomes
In 13 studies15,16,19,26,27,28,29,30,31,32,33,34,35 (2000 pregnancies in 1728 women with a solid organ transplant) that reported allograft outcomes during pregnancy, most were from kidney transplants (1696 pregnancies [84.8%]), followed by liver transplants (286 pregnancies [14.3%]), lung transplants (6 pregnancies [0.3%]), and heart transplants (12 pregnancies [0.6%]). The pooled incidence of acute allograft rejection during pregnancy was 2.39% (95% CI, 1.20% to 3.96%; I2 = 68.5%) and of allograft loss during pregnancy was 1.55% (95% CI, 0.05% to 4.44%; I2 = 69.2%) (eTable 3, eFigure 6, and eFigure 7 in Supplement 1). Of the 51 episodes of acute rejection, 33 (64.7%) occurred in women with kidney transplants and 18 (35.3%) occurred in women with liver transplants. Nine episodes of allograft loss during pregnancy occurred in women with kidney transplants, with none reported in other organ transplant types.
Publication Bias and Risk of Bias Assessment
An assessment of publication bias for acute allograft rejection during pregnancy indicated there is likely small study effects (eFigure 8 in Supplement 1). There were too few studies to evaluate small study effects for other outcomes. One study was assessed as low quality22 with the remaining studies of high or moderate methodological quality (eTable 4 and eTable 5 in Supplement 1).
Discussion
In this systematic review and meta-analysis of 22 studies and 93 565 343 pregnancies, solid organ transplantation was significantly associated with adverse pregnancy outcomes. The risks of preeclampsia, preterm birth (<37 weeks), and low birth weight (<2500 g) in pregnant women with a solid organ transplant were 4 to 6 times those of pregnant women without an organ transplant.
To date, this is one of the largest contemporary systematic reviews and meta-analyses to quantify the association between solid organ transplantation and adverse pregnancy outcomes. Results of the meta-analysis support and extend the findings of prior reviews.4,5,6,37,38 Shah et al4 described high rates of maternal and fetal outcomes in kidney transplant recipients, with almost one-quarter developing preeclampsia and 41% delivering preterm. Previous meta-analyses of pregnancy outcomes in liver and thoracic transplant recipients also reported high pooled incidences of adverse pregnancy outcomes.6,37,38
Results of the meta-analysis also show that while there was a high risk of preterm birth, most women delivered in the early term period. The MD in gestational age in a pregnancy from a transplant recipient vs nontransplant control was approximately 3 weeks. This is important new knowledge that will inform clinical practice and also provide reassurance to expectant mothers and health care practitioners.
We did not observe a significant difference in live births; however, there is likely a high level of imprecision in our estimate. Previous meta-analyses have reported higher live birth rates in kidney and liver transplant recipients compared with the live birth rate in the US general population.4,5,6 There are a number of potential reasons for this discrepancy. First, the definition of live birth rate is variable and range from a baby born with any signs of life through to any live birth greater than 20 weeks.16,18 Second, there is likely underreporting of early pregnancy loss in transplant registries. Third, the rates of termination in this high risk cohort are unknown, particularly where fetal morbidity may potentially have been increased due to exposure to teratogenic medications or maternal comorbidities.
Graft rejection during pregnancy is a major concern due to the limited treatment options available as a result of potential toxic effects for the fetus. The incidence of rejection during pregnancy reported in previous studies ranged between 4.2% to 9.4%, higher than in the our study.4,5 These differences may be attributed to the overrepresentation of pregnancies from different transplant eras and the resultant changes in obstetric and transplant practices. Advances in immunosuppressive therapy have improved rejection rates over the past 3 decades.39 In addition, the pharmacokinetics of calcineurin inhibitors, a mainstay of immunosuppression regimens, are altered during pregnancy due to changes in drug distribution and metabolism.40,41 Greater recognition of the need to uptitrate and maintain therapeutic levels of calcineurin inhibitors during pregnancy may have also contributed to the lower incidence of rejection found in our study.
Several potential mechanisms underlie the association between adverse pregnancy outcomes and solid organ transplantation. First, risk factors, such as obesity, preexisting hypertension, and diabetes, are more common in transplant recipients, which contribute to the increased risk of preeclampsia and fetal growth restriction.26 With regards to preterm birth, we do not have pregnancy-specific data to differentiate whether this was spontaneous or medically indicated; however, prior studies have shown that it is primarily due to worsening kidney function, preeclampsia, or severe hypertension.26,35 Second, while it is clear that preeclampsia is an important determinant of perinatal outcomes, other unmeasured factors, such as fetal assessment and antenatal kidney function, may further contribute to the high perinatal morbidity seen in this population.18 Finally, immunosuppressive medications may play a role in driving the risk of adverse pregnancy outcomes. Calcineurin inhibitors are a known cause of hypertension and endothelial dysfunction.42,43 A Dutch study found higher rates of preeclampsia in kidney transplant recipients with calcineurin inhibitors compared with recipients not using calcineurin (40% vs 30%), although this study was limited by small sample size.44
Strengths of this systematic review and meta-analysis include the comprehensive literature search, inclusion of studies from contemporary cohorts, and use of adjusted effect estimates and assessment of individual study quality. In addition, sensitivity analysis was performed using a core set of confounders determined a priori to ensure the robustness of our findings.
Limitations
This study has several limitations. First, only English-language articles were included. Second, high levels of heterogeneity were observed, warranting a cautious interpretation of the results. We were unable to explore reasons for heterogeneity in planned subgroup analysis owing to the small number of studies. Potential reasons for heterogeneity may include maternal factors (eg, interval between transplantation and pregnancy) as well as solid organ transplant type. Third, publication bias remains a possibility, as we were unable to assess for this with respect to the adverse pregnancy outcomes due to the small number of studies. Fourth, the inherent biases of observational studies that constitute the evidence base for this meta-analysis limit interpretations. Although adjustment for confounders was sought, studies varied in how they performed this. Furthermore, there is far more experience with pregnancy in kidney and liver transplants compared with thoracic transplants. Heart and lung transplant recipients have unique maternal risks during pregnancy, including response to the physiologic adaptions associated with pregnancy, higher risk of rejection requiring increased levels of immunosuppression, and comorbidity profiles.45,46 These limitations underscore the need for an individualized approach to preconception counselling and risk assessment by an expert multidisciplinary team.
Further research is needed to examine data on the immunosuppression used at time of conception and during pregnancy, as well as effective risk reduction interventions, such as aspirin supplementation for preeclampsia prevention. The minimal data found by our study on early pregnancy losses and reasons for preterm birth during pregnancy support the need for capturing such data in transplant registries to assist with future studies.
Conclusions
In this systematic review and meta-analysis, pregnancies in solid organ transplant recipients, compared with pregnancies in individuals without an organ transplant, were significantly associated with adverse pregnancy outcomes. The overall rate of allograft rejection and loss during pregnancy was low.
eAppendix 1. Search Strategy
eTable 1. Summary of Baseline Characteristics for Studies Reporting at Least 1 Adverse Pregnancy Outcome
eTable 2. Study Characteristics of Studies Reporting Allograft Outcomes During Pregnancy
eFigure 1. Forest Plot for Live Birth
eFigure 2. Forest Plot for Preterm Birth <32 Weeks (Adjusted Estimate)
eFigure 3. Forest Plot for Low Birth Weight <1500 g (Crude Estimate)
eFigure 4. Forest Plot for Caesarean Section (Adjusted Estimate)
eFigure 5. Forest Plot for Mean Difference in Gestational Age (Weeks)
eTable 3. Meta-Analysis of Allograft Outcomes During Pregnancy
eFigure 6. Forest Plot for Allograft Rejection During Pregnancy
eFigure 7. Forest Plot for Allograft Loss During Pregnancy
eFigure 8. Funnel Plot for Studies Reporting Allograft Rejection During Pregnancy
eTable 4. Risk of Bias Assessment for Studies Reporting at Least 1 Adverse Pregnancy Outcome
eTable 5. Risk of Bias Assessment for Allograft Outcomes
Data Sharing Statement
References
- 1.US Department of Health & Human Services . Organ procurement and transplantation network. Accessed April 16, 2024. https://optn.transplant.hrsa.gov/
- 2.Australia & New Zealand Dialysis & Transplant Registry . ANZDATA 46th Annual Report 2023. Accessed March 1, 2024. https://www.anzdata.org.au/anzdata/publications/reports/
- 3.The Renal Association . UK Renal Registry 25th annual report. Accessed March 1, 2024. https://ukkidney.org/sites/renal.org/files/25th%20Annual%20Report%20Final%202.6.23.pdf
- 4.Shah S, Venkatesan RL, Gupta A, et al. Pregnancy outcomes in women with kidney transplant: metaanalysis and systematic review. BMC Nephrol. 2019;20(1):24. doi: 10.1186/s12882-019-1213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11(11):2388-2404. doi: 10.1111/j.1600-6143.2011.03656.x [DOI] [PubMed] [Google Scholar]
- 6.Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes of liver transplant recipients: a systematic review and meta-analysis. Liver Transpl. 2012;18(6):621-629. doi: 10.1002/lt.23416 [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting—Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 8.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed April 16, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 9.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55-79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 11.Zhang J, Yu KF. What’s the relative risk: a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 12.Freeman M, Tukey J. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607-611. doi: 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20(4):641-654. doi: 10.1002/sim.698 [DOI] [PubMed] [Google Scholar]
- 15.Yin O, Coscia L, Constantinescu S, Moritz MJ, Afshar Y, Irani RA. Pregnancy after deceased donor vs living donor kidney transplant: associated obstetric and graft outcomes. Am J Obstet Gynecol. 2024;230(2):256.e1-256.e12. doi: 10.1016/j.ajog.2023.08.009 [DOI] [PubMed] [Google Scholar]
- 16.Barros T, Braga J, Correia A, Correia S, Martins S, Braga A. Pregnancy in kidney transplantation women: perinatal outcomes and impact on kidney function. J Matern Fetal Neonatal Med. 2022;35(26):10355-10361. doi: 10.1080/14767058.2022.2128650 [DOI] [PubMed] [Google Scholar]
- 17.DeFilippis EM, Blumer V, Mentz RJ, Agarwal R, Haythe JH, Kittleson M. In-hospital outcomes in pregnancy after heart transplantation. Am J Cardiol. 2022;172:68-72. doi: 10.1016/j.amjcard.2022.02.026 [DOI] [PubMed] [Google Scholar]
- 18.Hewawasam E, Davies CE, Li Z, et al. Determinants of perinatal outcomes in dialyzed and transplanted women in Australia. Kidney Int Rep. 2022;7(6):1318-1331. doi: 10.1016/j.ekir.2022.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazanowska N, Jarmużek-Orska P, Pietrzak B, Pazik J, Jabiry-Zieniewicz Z, Kosiński P. First-trimester biochemical serum markers in female kidney transplant recipients—the impact of graft function. Int J Environ Res Public Health. 2022;19(23):16352. doi: 10.3390/ijerph192316352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobotka LA, Mumtaz K, Hinton A, Conteh LF. Pregnancy in liver transplantation recipients is associated with increased complications and healthcare utilization. Am J Gastroenterol. 2021;116(3):560-567. doi: 10.14309/ajg.0000000000001135 [DOI] [PubMed] [Google Scholar]
- 21.Piccoli GB, Cabiddu G, Attini R, et al. ; Italian Study group on Kidney and Pregnancy of the Italian Society of Nephrology; Working Group on Pregnancy in Renal Transplantation . Outcomes of pregnancies after kidney transplantation: lessons learned from CKD—a comparison of transplanted, nontransplanted chronic kidney disease patients and low-risk pregnancies: a multicenter nationwide analysis. Transplantation. 2017;101(10):2536-2544. doi: 10.1097/TP.0000000000001645 [DOI] [PubMed] [Google Scholar]
- 22.Madej A, Pietrzak B, Mazanowska N, et al. Hypertension in pregnant renal and liver transplant recipients. Transplant Proc. 2016;48(5):1730-1735. doi: 10.1016/j.transproceed.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 23.Majak GB, Sandven I, Lorentzen B, et al. Pregnancy outcomes following maternal kidney transplantation: a national cohort study. Acta Obstet Gynecol Scand. 2016;95(10):1153-1161. doi: 10.1111/aogs.12937 [DOI] [PubMed] [Google Scholar]
- 24.Arab K, Oddy L, Patenaude V, Abenhaim HA. Obstetrical and neonatal outcomes in renal transplant recipients. J Matern Fetal Neonatal Med. 2015;28(2):162-167. doi: 10.3109/14767058.2014.909804 [DOI] [PubMed] [Google Scholar]
- 25.Wyld ML, Clayton PA, Jesudason S, Chadban SJ, Alexander SI. Pregnancy outcomes for kidney transplant recipients. Am J Transplant. 2013;13(12):3173-3182. doi: 10.1111/ajt.12452 [DOI] [PubMed] [Google Scholar]
- 26.Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol. 2013;8(2):290-298. doi: 10.2215/CJN.06170612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffin CS, Shaheen AAM, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl. 2010;16(1):56-63. doi: 10.1002/lt.21906 [DOI] [PubMed] [Google Scholar]
- 28.Pezeshki M, Taherian AA, Gharavy M, Ledger WL. Menstrual characteristics and pregnancy in women after renal transplantation. Int J Gynaecol Obstet. 2004;85(2):119-125. doi: 10.1016/j.ijgo.2003.09.013 [DOI] [PubMed] [Google Scholar]
- 29.Gosselink ME, van Buren MC, Kooiman J, et al. A nationwide Dutch cohort study shows relatively good pregnancy outcomes after kidney transplantation and finds risk factors for adverse outcomes. Kidney Int. 2022;102(4):866-875. doi: 10.1016/j.kint.2022.06.006 [DOI] [PubMed] [Google Scholar]
- 30.Schwarz A, Schmitt R, Einecke G, et al. Graft function and pregnancy outcomes after kidney transplantation. BMC Nephrol. 2022;23(1):27. doi: 10.1186/s12882-022-02665-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuksel Y, Tekin S, Yuksel D, et al. Pregnancy and delivery in the sequel of kidney transplantation: single-center study of 8 years’ experience. Transplant Proc. 2017;49(3):546-550. doi: 10.1016/j.transproceed.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 32.Stoumpos S, McNeill SH, Gorrie M, et al. Obstetric and long-term kidney outcomes in renal transplant recipients: a 40-yr single-center study. Clin Transplant. 2016;30(6):673-681. doi: 10.1111/ctr.12732 [DOI] [PubMed] [Google Scholar]
- 33.Blume C, Sensoy A, Gross MM, et al. A comparison of the outcome of pregnancies after liver and kidney transplantation. Transplantation. 2013;95(1):222-227. doi: 10.1097/TP.0b013e318277e318 [DOI] [PubMed] [Google Scholar]
- 34.Kim HW, Seok HJ, Kim TH, Han DJ, Yang WS, Park SK. The experience of pregnancy after renal transplantation: pregnancies even within postoperative 1 year may be tolerable. Transplantation. 2008;85(10):1412-1419. doi: 10.1097/TP.0b013e318170f8ed [DOI] [PubMed] [Google Scholar]
- 35.Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK Transplant pregnancy registry. Transplantation. 2007;83(10):1301-1307. doi: 10.1097/01.tp.0000263357.44975.d0 [DOI] [PubMed] [Google Scholar]
- 36.Craig AM, Campbell A, Snow SC, et al. Maternal and pregnancy outcomes following heart transplantation in the United States. JACC Heart Fail. 2023;11(12):1666-1674. doi: 10.1016/j.jchf.2023.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jha N, Jha AK, Mishra SK, Parida S. Thoracic organ transplantation and pregnancy outcomes: systematic review and meta-analysis. Arch Gynecol Obstet. 2024;309(2):385-396. doi: 10.1007/s00404-023-07065-x [DOI] [PubMed] [Google Scholar]
- 38.Valentin N, Guerrido I, Rozenshteyn F, et al. Pregnancy outcomes after liver transplantation: a systematic review and meta-analysis. Am J Gastroenterol. 2021;116(3):491-504. doi: 10.14309/ajg.0000000000001105 [DOI] [PubMed] [Google Scholar]
- 39.Cooper JE. Evaluation and treatment of acute rejection in kidney allografts. Clin J Am Soc Nephrol. 2020;15(3):430-438. doi: 10.2215/CJN.11991019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng S, Easterling TR, Umans JG, et al. Pharmacokinetics of tacrolimus during pregnancy. Ther Drug Monit. 2012;34(6):660-670. doi: 10.1097/FTD.0b013e3182708edf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95(7):908-915. doi: 10.1097/TP.0b013e318278d367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues-Diez R, González-Guerrero C, Ocaña-Salceda C, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep. 2016;6:27915. doi: 10.1038/srep27915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481-508. doi: 10.2215/CJN.04800908 [DOI] [PubMed] [Google Scholar]
- 44.Koenjer LM, Meinderts JR, van der Heijden OWH, et al. ; Members of the PARTOUT network* . Comparison of pregnancy outcomes in Dutch kidney recipients with and without calcineurin inhibitor exposure: a retrospective study. Transpl Int. 2021;34(12):2669-2679. doi: 10.1111/tri.14156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kittleson MM, DeFilippis EM, Bhagra CJ, et al. Reproductive health after thoracic transplantation: an ISHLT expert consensus statement. J Heart Lung Transplant. 2023;42(3):e1-e42. doi: 10.1016/j.healun.2022.10.009 [DOI] [PubMed] [Google Scholar]
- 46.Khush KK, Hsich E, Potena L, et al. ; International Society for Heart and Lung Transplantation . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult heart transplantation report—2021; focus on recipient characteristics. J Heart Lung Transplant. 2021;40(10):1035-1049. doi: 10.1016/j.healun.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy
eTable 1. Summary of Baseline Characteristics for Studies Reporting at Least 1 Adverse Pregnancy Outcome
eTable 2. Study Characteristics of Studies Reporting Allograft Outcomes During Pregnancy
eFigure 1. Forest Plot for Live Birth
eFigure 2. Forest Plot for Preterm Birth <32 Weeks (Adjusted Estimate)
eFigure 3. Forest Plot for Low Birth Weight <1500 g (Crude Estimate)
eFigure 4. Forest Plot for Caesarean Section (Adjusted Estimate)
eFigure 5. Forest Plot for Mean Difference in Gestational Age (Weeks)
eTable 3. Meta-Analysis of Allograft Outcomes During Pregnancy
eFigure 6. Forest Plot for Allograft Rejection During Pregnancy
eFigure 7. Forest Plot for Allograft Loss During Pregnancy
eFigure 8. Funnel Plot for Studies Reporting Allograft Rejection During Pregnancy
eTable 4. Risk of Bias Assessment for Studies Reporting at Least 1 Adverse Pregnancy Outcome
eTable 5. Risk of Bias Assessment for Allograft Outcomes
Data Sharing Statement