Abstract
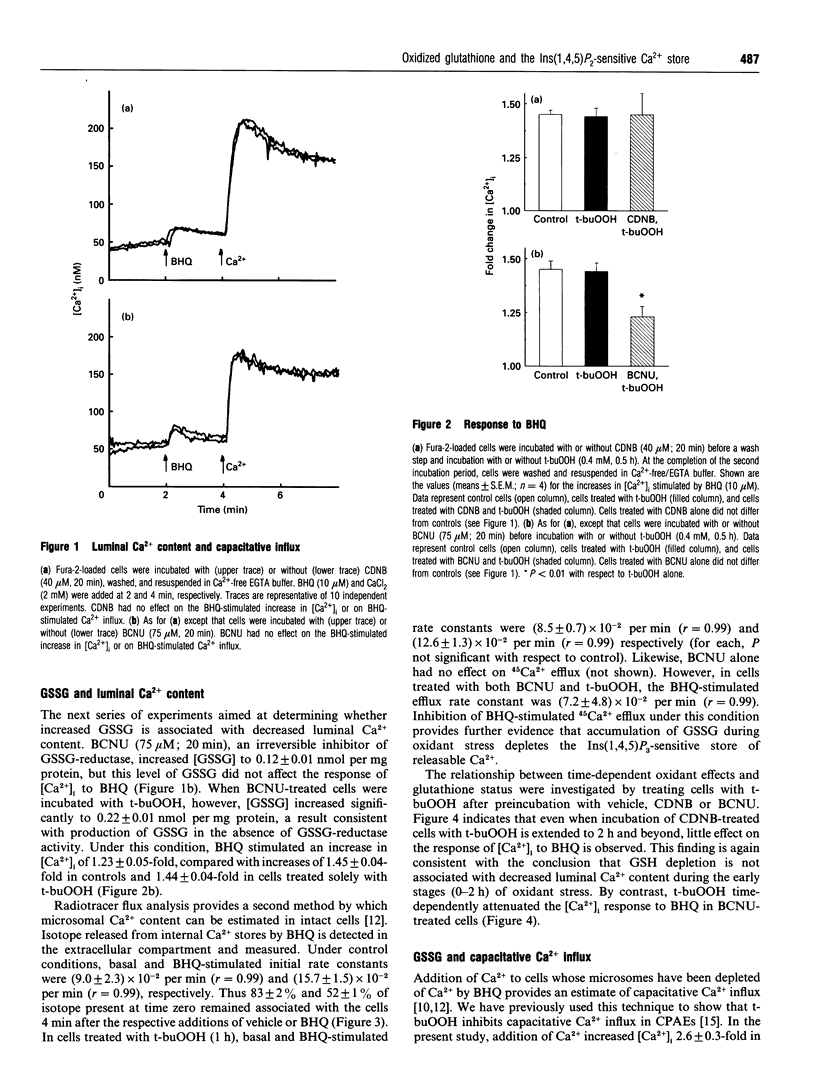
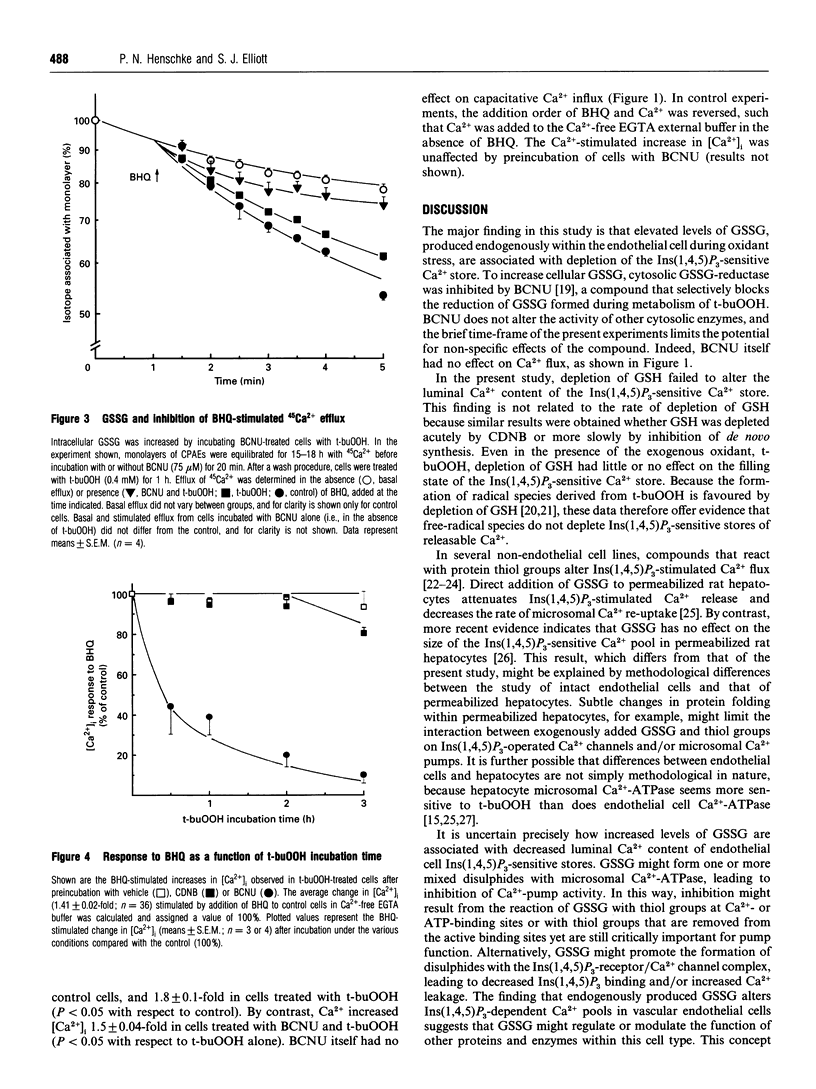
The model oxidant, t-butyl hydroperoxide (t-buOOH), inhibits Ins(1,4,5)P3-dependent Ca2+ signalling in calf pulmonary artery endothelial cells. Metabolism of t-buOOH within the cytosol is coupled to the oxidation of glutathione. In this study, we investigated whether oxidized glutathione (GSSG) is the intracellular moiety responsible for mediating the effects of t-buOOH on Ca2+ signalling. The increase in cytosolic [Ca2+] stimulated by application of 2,5-di-t-butylhydroquinone (BHQ) was used to estimate the luminal Ca2+ content of the Ins(1,4,5)P3-sensitive store in intact cells. Luminal Ca2+ content was unaffected by t-buOOH (0.4 mM, 0-3 h) unless intracellular GSSG content was concomitantly elevated. The effect was specific for increased GSSG and was not replicated by depletion of GSH. These results suggest that cytosolic GSSG, produced endogenously within the endothelial cell, decreases the luminal Ca2+ content of Ins(1,4,5)P3-sensitive Ca2+ stores. Depletion of internal Ca2+ stores by GSSG may represent a key mechanism by which some forms of oxidant stress inhibit signal transduction in vascular tissue. At the plasma membrane, t-buOOH is known to inhibit the capacitative Ca2+ influx pathway. Increased intracellular GSSG potentiated the inhibitory effect of t-buOOH on Ca2+ influx, thereby providing the first evidence that activity of the capacitative Ca2+ influx channel is sensitive to thiol reagents formed endogenously within the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adunyah S. E., Dean W. L. Effects of sulfhydryl reagents and other inhibitors on Ca2+ transport and inositol trisphosphate-induced Ca2+ release from human platelet membranes. J Biol Chem. 1986 Oct 5;261(28):13071–13075. [PubMed] [Google Scholar]
- Alpert A. J., Gilbert H. F. Detection of oxidized and reduced glutathione with a recycling postcolumn reaction. Anal Biochem. 1985 Feb 1;144(2):553–562. doi: 10.1016/0003-2697(85)90153-8. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Taylor C. W., Berridge M. J. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1992 Dec 15;267(35):25113–25119. [PubMed] [Google Scholar]
- Cadenas E., Sies H. Low level chemiluminescence of liver microsomal fractions initiated by tert-butyl hydroperoxide. Relation to microsomal hemoproteins, oxygen dependence, and lipid peroxidation. Eur J Biochem. 1982 May 17;124(2):349–356. doi: 10.1111/j.1432-1033.1982.tb06599.x. [DOI] [PubMed] [Google Scholar]
- Elliott S. J., Doan T. N. Oxidant stress inhibits the store-dependent Ca(2+)-influx pathway of vascular endothelial cells. Biochem J. 1993 Jun 1;292(Pt 2):385–393. doi: 10.1042/bj2920385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. J., Eskin S. G., Schilling W. P. Effect of t-butyl-hydroperoxide on bradykinin-stimulated changes in cytosolic calcium in vascular endothelial cells. J Biol Chem. 1989 Mar 5;264(7):3806–3810. [PubMed] [Google Scholar]
- Frischer H., Ahmad T. Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU" [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med. 1977 May;89(5):1080–1091. [PubMed] [Google Scholar]
- Gilbert H. F. Biological disulfides: the third messenger? Modulation of phosphofructokinase activity by thiol/disulfide exchange. J Biol Chem. 1982 Oct 25;257(20):12086–12091. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jones D. P., Thor H., Smith M. T., Jewell S. A., Orrenius S. Inhibition of ATP-dependent microsomal Ca2+ sequestration during oxidative stress and its prevention by glutathione. J Biol Chem. 1983 May 25;258(10):6390–6393. [PubMed] [Google Scholar]
- Kalyanaraman B., Mottley C., Mason R. P. A direct electron spin resonance and spin-trapping investigation of peroxyl free radical formation by hematin/hydroperoxide systems. J Biol Chem. 1983 Mar 25;258(6):3855–3858. [PubMed] [Google Scholar]
- Kwan C. Y., Takemura H., Obie J. F., Thastrup O., Putney J. W., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990 Jun;258(6 Pt 1):C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Parys J. B., Casteels R. Co-activation of inositol trisphosphate-induced Ca2+ release by cytosolic Ca2+ is loading-dependent. J Biol Chem. 1994 Mar 11;269(10):7238–7242. [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Parys J. B., Missiaen L., De Smedt H., Casteels R. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. Implications for the mechanism of quantal Ca2+ release. J Biol Chem. 1993 Nov 25;268(33):25206–25212. [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Renard D. C., Seitz M. B., Thomas A. P. Oxidized glutathione causes sensitization of calcium release to inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1992 Jun 1;284(Pt 2):507–512. doi: 10.1042/bj2840507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Renard D. C., Sass E. J., Thomas A. P. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991 Jul 5;266(19):12272–12282. [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Schilling W. P., Cabello O. A., Rajan L. Depletion of the inositol 1,4,5-trisphosphate-sensitive intracellular Ca2+ store in vascular endothelial cells activates the agonist-sensitive Ca(2+)-influx pathway. Biochem J. 1992 Jun 1;284(Pt 2):521–530. doi: 10.1042/bj2840521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L., Sinkins W. G., Hu Y., Kunze D. L., Schilling W. P. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994 Nov;267(5 Pt 1):C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]


