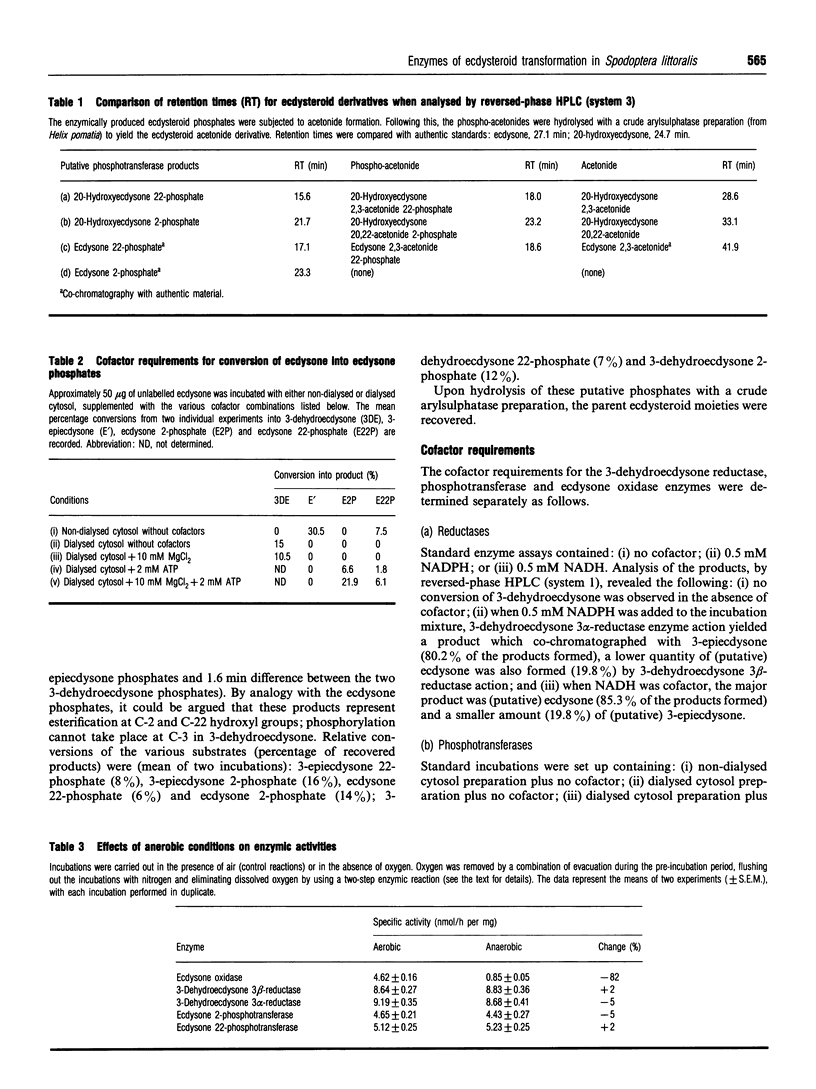
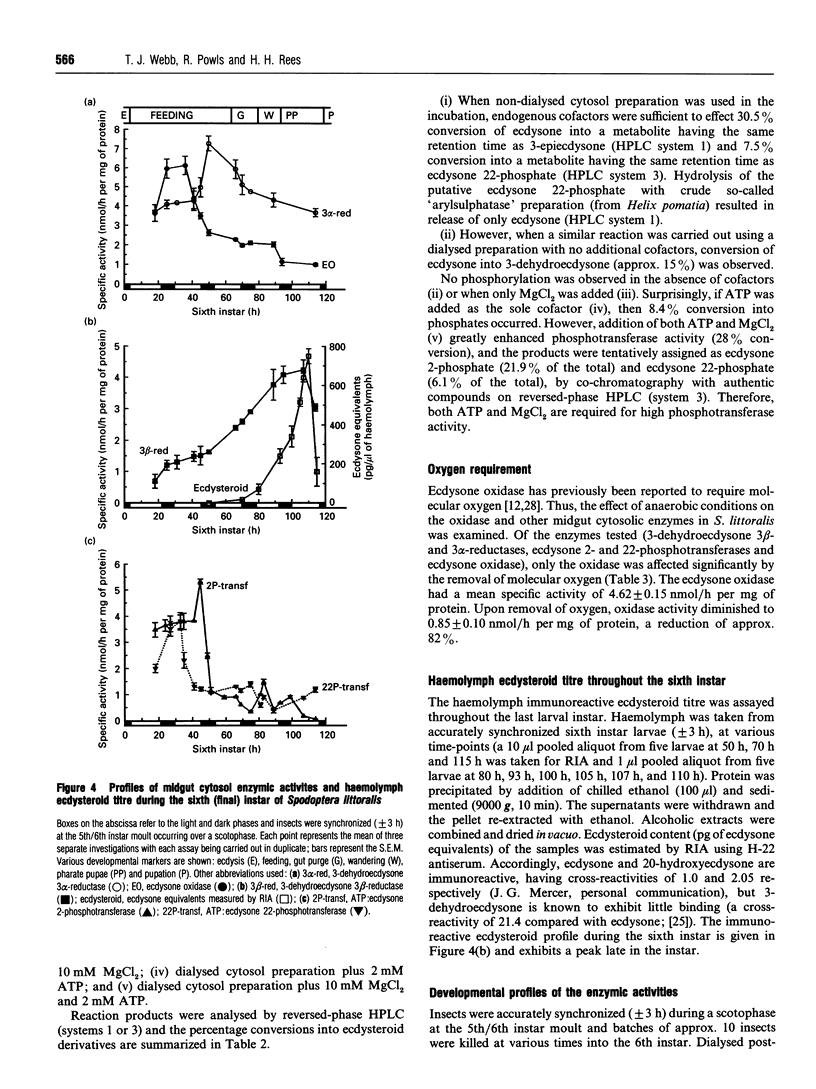
Abstract
In the midgut cytosol of Lepidoptera, ecdysteroids undergo inactivation by transformation via the 3-dehydro derivative to the corresponding 3-epiecdysteroid (3 alpha-hydroxy) and by phosphate conjugation. The oxygen-dependent oxidase catalyses formation of 3-dehydroecdysteroid, which can be reduced either irreversibly by 3-dehydroecdysone 3 alpha-reductase to 3-epiecdysteroid, or by 3-dehydroecdysone 3 beta-reductase back to the initial ecdysteroid. Furthermore, these ecdysteroids undergo further inactivation by phosphorylation. These ecdysteroid transformations have been investigated in last instar larvae of the cotton leafworm, Spodoptera littoralis. The products of the phosphorylation have been characterized as predominantly ecdysteroid 2-phosphate accompanied by smaller amounts of the corresponding 22-phosphate. The phosphotransferases require Mg2+ and ATP. Whereas the 3-dehydroecdysone 3 alpha-reductase has a clear preference for NADPH rather than NADH, the corresponding 3 beta-reductase markedly favours NADH. The physiological significance of the latter enzyme is unclear. The profiles of the various enzymic activities in dialysed midgut cytosol supplemented with appropriate cofactors were determined throughout the last larval instar. All activities were detectable throughout the instar, but the respective enzymes exhibited maxima at different times. Ecdysone oxidase showed a peak early in the instar, with 3-dehydroecdysone 3 alpha-reductase increasing to a peak as the former activity declined. The 3-dehydroecdysone 3 beta-reductase exhibited peak activity late in the instar, a profile similar to that observed for the corresponding haemolymph enzyme involved in reduction of the 3-dehydroecdysone product of the prothoracic glands to ecdysone. Thus, the significance of the midgut 3 beta-reductase may be related to production of active hormone. Both ecydsteroid 22- and 2-phosphotransferases showed high activities early in the instar and then declined. The physiological significance of the profiles for the ecdysone oxidase, the 3-dehydroecdysone 3 alpha-reductase and phosphotransferases is unclear.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blais C., Lafont R. Ecdysteroid metabolism by soluble enzymes from an insect. Metabolic relationships between 3 beta-hydroxy-, 3 alpha-hydroxy- and 3-oxoecdysteroids. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):809–817. doi: 10.1515/bchm2.1984.365.2.809. [DOI] [PubMed] [Google Scholar]
- Dinan L., Rees H. H. Preparation of 3-epi-ecdysone and 3-epi-20-hydroxyecdysone. Steroids. 1978 Dec;32(5):629–638. doi: 10.1016/0039-128x(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Gumaa K. A., McLean P., Greenbaum A. L. Compartmentation in relation to metabolic control in liver. Essays Biochem. 1971;7:39–86. [PubMed] [Google Scholar]
- Isaac R. E., Rees H. H. Isolation and identification of ecdysteroid phosphates and acetylecdysteroid phosphates from developing eggs of the locust, Schistocerca gregaria. Biochem J. 1984 Jul 15;221(2):459–464. doi: 10.1042/bj2210459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R. E., Rose M. E., Rees H. H., Goodwin T. W. Identification of the 22-phosphate esters of ecdysone, 2-deoxyecdysone, 20-hydroxyecdysone and 2-deoxy-20-hydroxyecdysone from newly laid eggs of the desert locust, Schistocerca gregaria. Biochem J. 1983 Aug 1;213(2):533–541. doi: 10.1042/bj2130533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanis J. N., Thompson M. J., Dutky S. R., Robbins W. E. The ecdysteroids from the tobacco hornworm during pupal-adult development five days after peak titer of molting hormone activity. Steroids. 1979 Sep;34(3):333–345. doi: 10.1016/0039-128x(79)90084-9. [DOI] [PubMed] [Google Scholar]
- Koolman J. Ecdysone oxidase in insects. Hoppe Seylers Z Physiol Chem. 1978 Oct;359(10):1315–1321. doi: 10.1515/bchm2.1978.359.2.1315. [DOI] [PubMed] [Google Scholar]
- Koolman J., Karlson P. Ecdysone oxidase: reaction and specificity. Eur J Biochem. 1978 Sep 1;89(2):453–460. doi: 10.1111/j.1432-1033.1978.tb12548.x. [DOI] [PubMed] [Google Scholar]
- Koolman J., Spindler K. D. Enzymatic and chemical synthesis of 3-dehydroecdysterone, a metabolite of the moulting hormone of insects. Hoppe Seylers Z Physiol Chem. 1977 Oct;358(10):1339–1344. doi: 10.1515/bchm2.1977.358.2.1339. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lafont R., Beydon P., Sommé-Martin G., Blais C. High-performance liquid chromatography of ecdysone metabolites applied to the cabbage butterfly, Pieris brassicae L. Steroids. 1980 Aug;36(2):185–207. doi: 10.1016/0039-128x(80)90018-5. [DOI] [PubMed] [Google Scholar]
- Mayer R. T., Durrant J. L., Holman G. M., Weirich G. F., Svoboda J. A. Ecdysone 3-epimerase from the midgut of Manduca sexta (L.). Steroids. 1979 Nov;34(5):555–562. doi: 10.1016/s0039-128x(79)80016-1. [DOI] [PubMed] [Google Scholar]
- Mercer J. G., Munn A. E., Rees H. H. Echinococcus granulosus: occurrence of ecdysteroids in protoscoleces and hydatid cyst fluid. Mol Biochem Parasitol. 1987 Jun;24(2):203–214. doi: 10.1016/0166-6851(87)90107-1. [DOI] [PubMed] [Google Scholar]
- Milner N. P., Rees H. H. Involvement of 3-dehydroecdysone in the 3-epimerization of ecdysone. Biochem J. 1985 Oct 15;231(2):369–374. doi: 10.1042/bj2310369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg H. N., Svoboda J. A., Thompson M. J., Kaplanis J. N., Dutky S. R. Ecdysone metabolism: ecdysone dehydrogenase-isomerase. Lipids. 1974 Dec;9(12):971–974. doi: 10.1007/BF02533820. [DOI] [PubMed] [Google Scholar]
- Rees H. H., Isaac R. E. Biosynthesis and metabolism of ecdysteroids and methods of isolation and identification of the free and conjugated compounds. Methods Enzymol. 1985;111:377–410. doi: 10.1016/s0076-6879(85)11024-4. [DOI] [PubMed] [Google Scholar]
- Shimizu K. Requirement of NADPH and molecular oxygen for the side-chain cleavage of 20-alpha, 22-xi-dihydroxycholesterol. Arch Biochem Biophys. 1968 Jun;125(3):1016–1017. doi: 10.1016/0003-9861(68)90539-0. [DOI] [PubMed] [Google Scholar]
- Soumoff C., Horn D. H., O'Connor J. D. Production of a new antiserum to arthropod molting hormone and comparison with two other antisera. J Steroid Biochem. 1981 May;14(5):429–435. doi: 10.1016/0022-4731(81)90353-8. [DOI] [PubMed] [Google Scholar]
- Warren J. T., Sakurai S., Rountree D. B., Gilbert L. I., Lee S. S., Nakanishi K. Regulation of the ecdysteroid titer of Manduca sexta: reappraisal of the role of the prothoracic glands. Proc Natl Acad Sci U S A. 1988 Feb;85(3):958–962. doi: 10.1073/pnas.85.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. S., Wilkinson C. F. Sulphotransferases and phosphotransferases in insects. Comp Biochem Physiol B. 1973 Dec;46(4):717–726. doi: 10.1016/0305-0491(73)90116-8. [DOI] [PubMed] [Google Scholar]


