Abstract
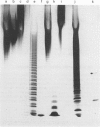
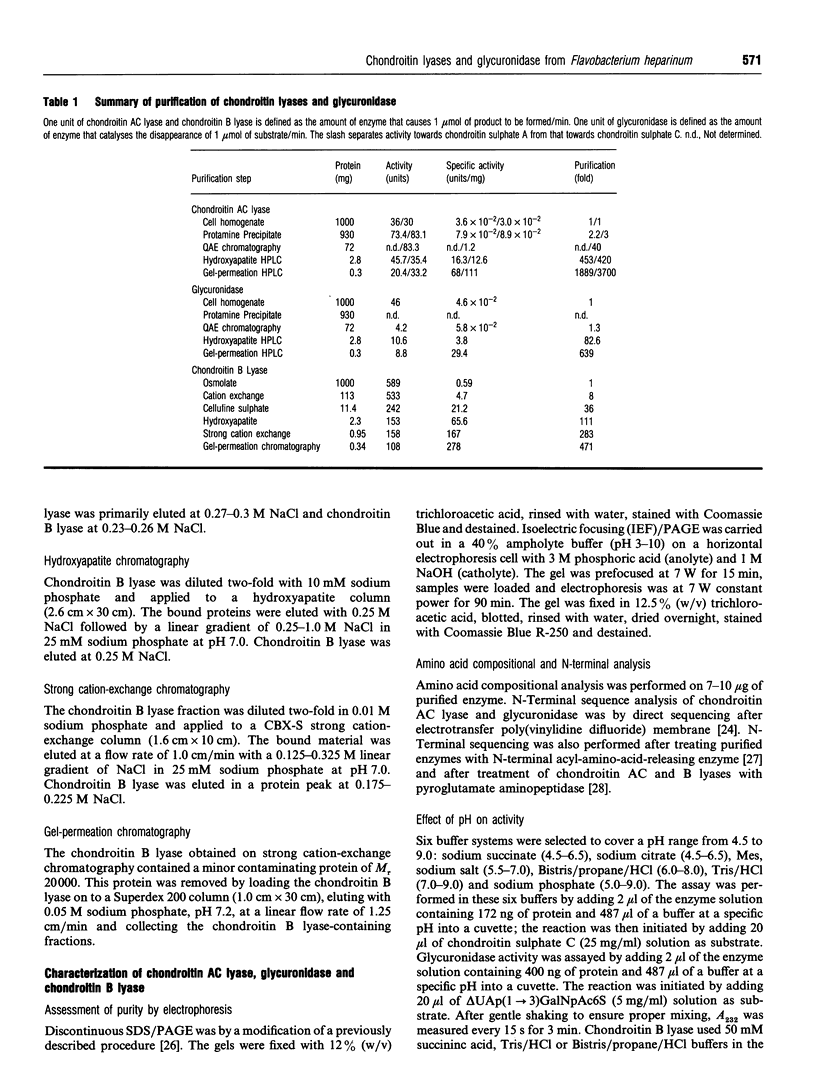
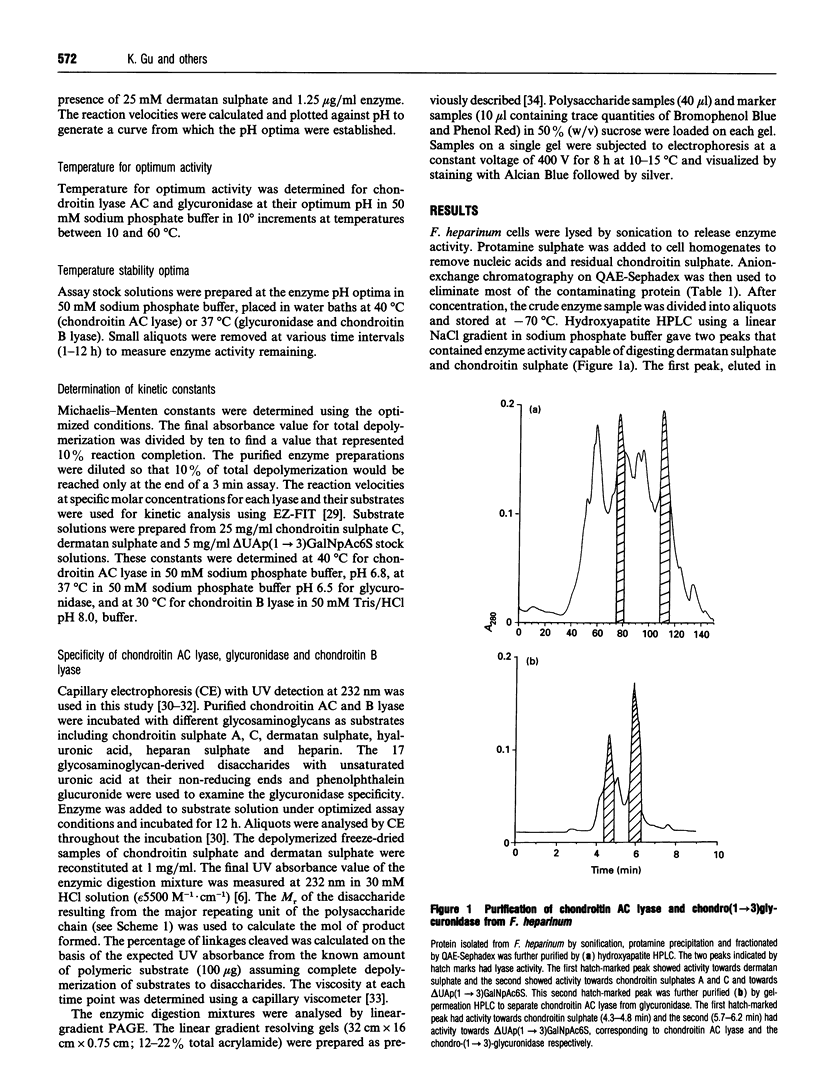
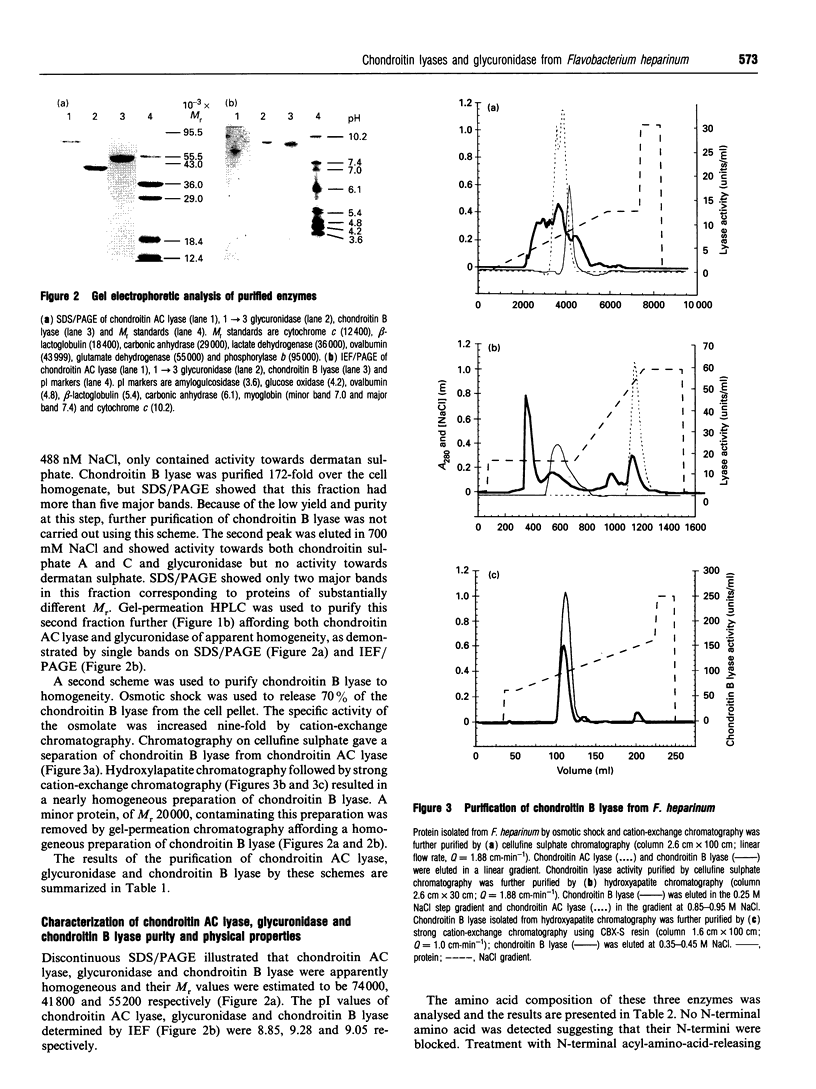
The chondroitin lyases from Flavobacterium heparinum (Cytophaga heparinia) have been widely used in depolymerization of glycosaminoglycan and proteoglycan chondroitin sulphates. Oligosaccharide products derived from chondroitin sulphate can be further degraded by glycuronidases and sulphatases obtained from the same organism. There has been no reported purification of these enzymes to homogeneity nor is there any information on their physical and kinetic characteristics. The absence of pure enzymes has resulted in a lack of understanding of the optimal conditions for their catalytic activity and their substrate specificity. This has limited the use of these enzymes as reagents for preparation of oligosaccharides for structure and activity studies. Reproducible schemes to purify a chondroitin AC lyase, a glycuronidase and chondroitin B lyase from Flavobacterium heparinum to apparent homogeneity are described. Chondroitin AC lyase (chondroitinase AC, EC 4.2.2.5), glycuronidase [chondro-(1-->3)-glycuronidase, no EC number] and chondroitin B lyase (chondroitinase B, no EC number) have M(r) values (assessed by SDS/PAGE) of 74,000, 41,800 and 55,200 respectively, and isoelectric points (determined by isoelectric focusing) of 8.85, 9.28 and 9.05 respectively. Chondroitin lyase AC and B contain pyroglutamic acid at their N-termini precluding their analysis by Edman degradation. Deblocking with pyroglutamate aminopeptidase facilitated the determination of their N-terminal sequences. The kinetic properties of these enzymes have been determined as well as the optimum conditions for their catalytic activity. The specificity of the glycouronidase, determined using 17 different disaccharide substrates, shows that it only acts on unsulphated or 6-O-sulphated 1-->3 linkages. The chondroitin lyases are both endolytic enzymes, and oligosaccharide mapping shows their expected specificity towards the chondroitin and dermatan sulphate polymers.
Full text
PDF

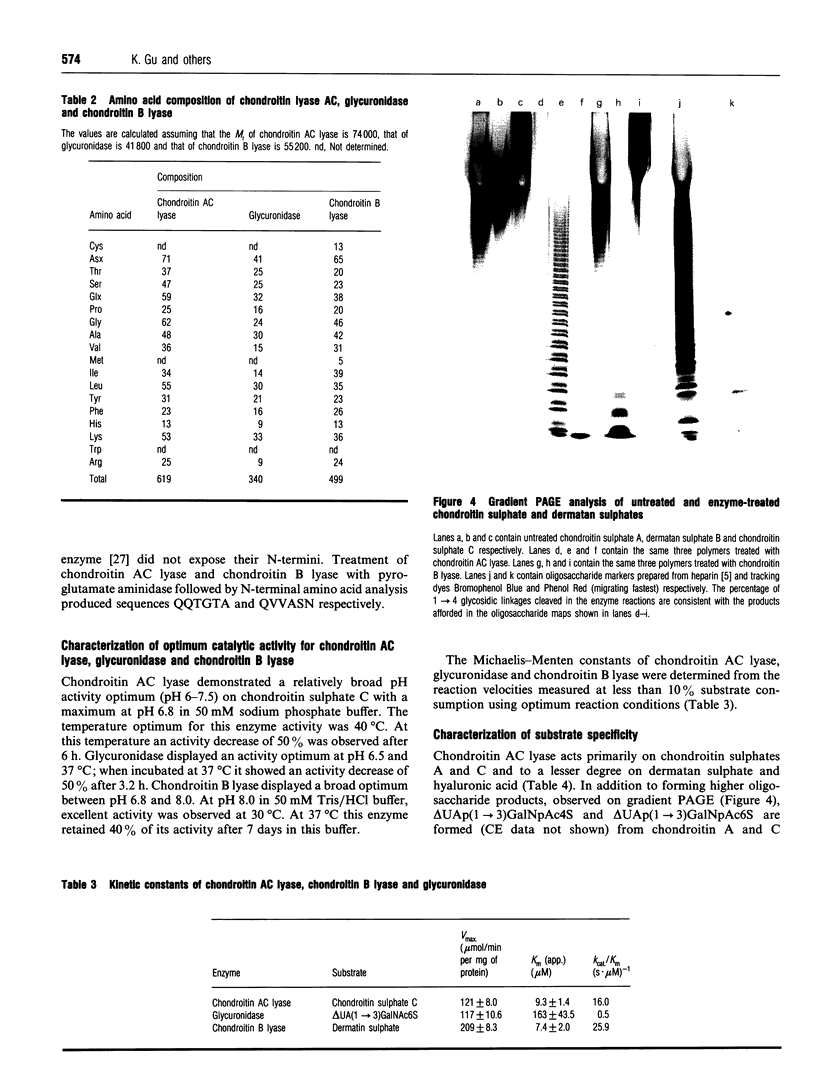

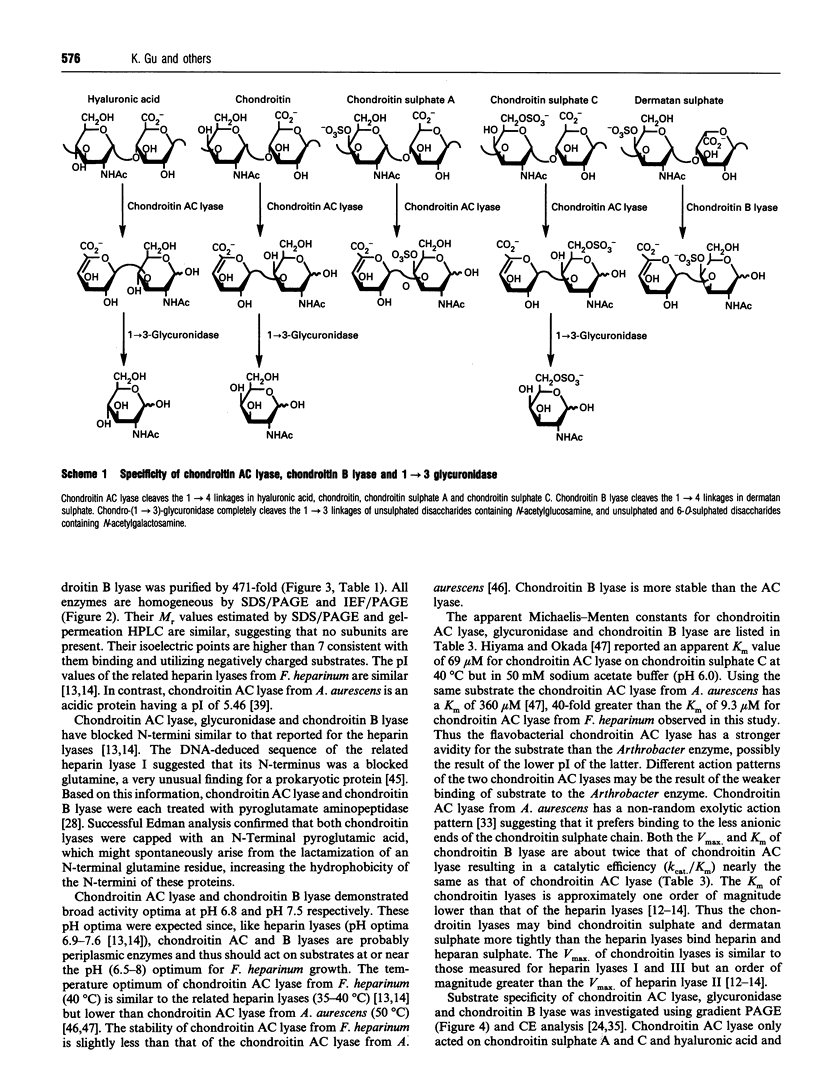

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampofo S. A., Wang H. M., Linhardt R. J. Disaccharide compositional analysis of heparin and heparan sulfate using capillary zone electrophoresis. Anal Biochem. 1991 Dec;199(2):249–255. doi: 10.1016/0003-2697(91)90098-e. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruce J. S., McLean M. W., Long W. F., Williamson F. B. Flavobacterium heparinum 3-O-sulphatase for N-substituted glucosamine 3-O-sulphate. Eur J Biochem. 1985 Apr 15;148(2):359–365. doi: 10.1111/j.1432-1033.1985.tb08847.x. [DOI] [PubMed] [Google Scholar]
- Bruce J. S., McLean M. W., Long W. F., Williamson F. B. Flavobacterium heparinum sulphamidase for D-glucosamine sulphamate. Purification and characterisation. Eur J Biochem. 1987 Jun 15;165(3):633–638. doi: 10.1111/j.1432-1033.1987.tb11487.x. [DOI] [PubMed] [Google Scholar]
- Bruce J. S., McLean M. W., Williamson F. B., Long W. F. Flavobacterium heparinum 6-O-sulphatase for N-substituted glucosamine 6-O-sulphate. Eur J Biochem. 1985 Oct 1;152(1):75–82. doi: 10.1111/j.1432-1033.1985.tb09165.x. [DOI] [PubMed] [Google Scholar]
- Desai U. R., Wang H., Ampofo S. A., Linhardt R. J. Oligosaccharide composition of heparin and low-molecular-weight heparins by capillary electrophoresis. Anal Biochem. 1993 Aug 15;213(1):120–127. doi: 10.1006/abio.1993.1394. [DOI] [PubMed] [Google Scholar]
- Galliher P. M., Cooney C. L., Langer R., Linhardt R. J. Heparinase production by Flavobacterium heparinum. Appl Environ Microbiol. 1981 Feb;41(2):360–365. doi: 10.1128/aem.41.2.360-365.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu K., Liu J., Pervin A., Linhardt R. J. Comparison of the activity of two chondroitin AC lyases on dermatan sulfate. Carbohydr Res. 1993 Jun 21;244(2):369–377. doi: 10.1016/0008-6215(83)85014-9. [DOI] [PubMed] [Google Scholar]
- HOFFMAN P., LINKER A., LIPPMAN V., MEYER K. The structure of chondroitin sulfate B from studies with Flavobacterium enzymes. J Biol Chem. 1960 Nov;235:3066–3069. [PubMed] [Google Scholar]
- Hirano H., Komatsu S., Takakura H., Sakiyama F., Tsunasawa S. Deblocking and subsequent microsequence analysis of N alpha-blocked proteins electroblotted onto PVDF membrane. J Biochem. 1992 Jun;111(6):754–757. doi: 10.1093/oxfordjournals.jbchem.a123831. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Okada S. Action of chondroitinases. III. Ionic strength effects and kinetics in the action of chondroitinase AC. J Biochem. 1977 Aug;82(2):429–436. [PubMed] [Google Scholar]
- Hiyama K., Okada S. Crystallization and some properties of chondroitinase from Arthrobacter aurescens. J Biol Chem. 1975 Mar 10;250(5):1824–1828. [PubMed] [Google Scholar]
- Hovingh P., Linker A. Specificity of flavobacterial glycuronidases acting on disaccharides derived from glycosaminoglycans. Biochem J. 1977 Aug 1;165(2):287–293. doi: 10.1042/bj1650287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovingh P., Linker A. The enzymatic degradation of heparin and heparitin sulfate. 3. Purification of a heparitinase and a heparinase from flavobacteria. J Biol Chem. 1970 Nov 25;245(22):6170–6175. [PubMed] [Google Scholar]
- Jandik K. A., Gu K., Linhardt R. J. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology. 1994 Jun;4(3):289–296. doi: 10.1093/glycob/4.3.289. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- LINKER A., HOFFMAN P., MEYER K., SAMPSON P., KORN E. D. The formation of unsaturated disacharides from mucopoly-saccharides and their cleavage to alpha-keto acid by bacterial enzymes. J Biol Chem. 1960 Nov;235:3061–3065. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Desai U. R., Liu J., Pervin A., Hoppensteadt D., Fareed J. Low molecular weight dermatan sulfate as an antithrombotic agent. Structure-activity relationship studies. Biochem Pharmacol. 1994 Mar 29;47(7):1241–1252. doi: 10.1016/0006-2952(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Galliher P. M., Cooney C. L. Polysaccharide lyases. Appl Biochem Biotechnol. 1986 Apr;12(2):135–176. doi: 10.1007/BF02798420. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The enzymatic degradation of heparin and heparitin sulfate. I. The fractionation of a crude heparinase from flavobacteria. J Biol Chem. 1965 Oct;240(10):3724–3728. [PubMed] [Google Scholar]
- Linker A., Hovingh P. The uses of degradative enzymes as tools for identification and structural analysis of glycosaminoglycans. Fed Proc. 1977 Jan;36(1):43–46. [PubMed] [Google Scholar]
- Lohse D. L., Linhardt R. J. Purification and characterization of heparin lyases from Flavobacterium heparinum. J Biol Chem. 1992 Dec 5;267(34):24347–24355. [PubMed] [Google Scholar]
- McLean M. W., Bruce J. S., Long W. F., Williamson F. B. Flavobacterium heparinum 2-O-sulphatase for 2-O-sulphato-delta 4,5-glycuronate-terminated oligosaccharides from heparin. Eur J Biochem. 1984 Dec 17;145(3):607–615. doi: 10.1111/j.1432-1033.1984.tb08600.x. [DOI] [PubMed] [Google Scholar]
- Michelacci Y. M., Dietrich C. P. A comparative study between a chondroitinase B and a chondroitinase AC from Flavobacterium heparinum: Isolation of a chondroitinase AC-susceptible dodecasaccharide from chondroitin sulphate B. Biochem J. 1975 Oct;151(1):121–129. doi: 10.1042/bj1510121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelacci Y. M., Dietrich C. P. Isolation and partial characterization of an induced chondroitinase B from Flavobacterium heparinum. Biochem Biophys Res Commun. 1974 Feb 27;56(4):973–980. doi: 10.1016/s0006-291x(74)80284-6. [DOI] [PubMed] [Google Scholar]
- Ototani N., Yosizawa Z. Interaction of mucopolysaccharides with glycosaminoglycans on glycosaminoglycan-bound AH-Sepharose 4B. J Biochem. 1978 Oct;84(4):1005–1008. doi: 10.1093/oxfordjournals.jbchem.a132181. [DOI] [PubMed] [Google Scholar]
- PAYZA A. N., KORN E. D. Bacterial degradation of heparin. Nature. 1956 Jan 14;177(4498):88–89. doi: 10.1038/177088a0. [DOI] [PubMed] [Google Scholar]
- Perrella F. W. EZ-FIT: a practical curve-fitting microcomputer program for the analysis of enzyme kinetic data on IBM-PC compatible computers. Anal Biochem. 1988 Nov 1;174(2):437–447. doi: 10.1016/0003-2697(88)90042-5. [DOI] [PubMed] [Google Scholar]
- Pervin A., al-Hakim A., Linhardt R. J. Separation of glycosaminoglycan-derived oligosaccharides by capillary electrophoresis using reverse polarity. Anal Biochem. 1994 Aug 15;221(1):182–188. doi: 10.1006/abio.1994.1395. [DOI] [PubMed] [Google Scholar]
- Podell D. N., Abraham G. N. A technique for the removal of pyroglutamic acid from the amino terminus of proteins using calf liver pyroglutamate amino peptidase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):176–185. doi: 10.1016/0006-291x(78)91646-7. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Warnick C. T., Linker A. Purification of an unusual -glycuronidase from flavobacteria. Biochemistry. 1972 Feb 15;11(4):568–572. doi: 10.1021/bi00754a014. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yang V. C., Linhardt R. J., Bernstein H., Cooney C. L., Langer R. Purification and characterization of heparinase from Flavobacterium heparinum. J Biol Chem. 1985 Feb 10;260(3):1849–1857. [PubMed] [Google Scholar]
- Zimmermann J. J., Langer R., Cooney C. L. Specific plate assay for bacterial heparinase. Appl Environ Microbiol. 1990 Nov;56(11):3593–3594. doi: 10.1128/aem.56.11.3593-3594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Hakim A., Linhardt R. J. Capillary electrophoresis for the analysis of chondroitin sulfate- and dermatan sulfate-derived disaccharides. Anal Biochem. 1991 May 15;195(1):68–73. doi: 10.1016/0003-2697(91)90296-6. [DOI] [PubMed] [Google Scholar]
- al-Hakim A., Linhardt R. J. Electrophoresis and detection of nanogram quantities of exogenous and endogenous glycosaminoglycans in biological fluids. Appl Theor Electrophor. 1991;1(6):305–312. [PubMed] [Google Scholar]