Abstract
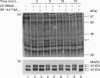
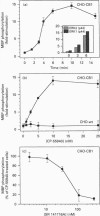
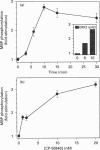
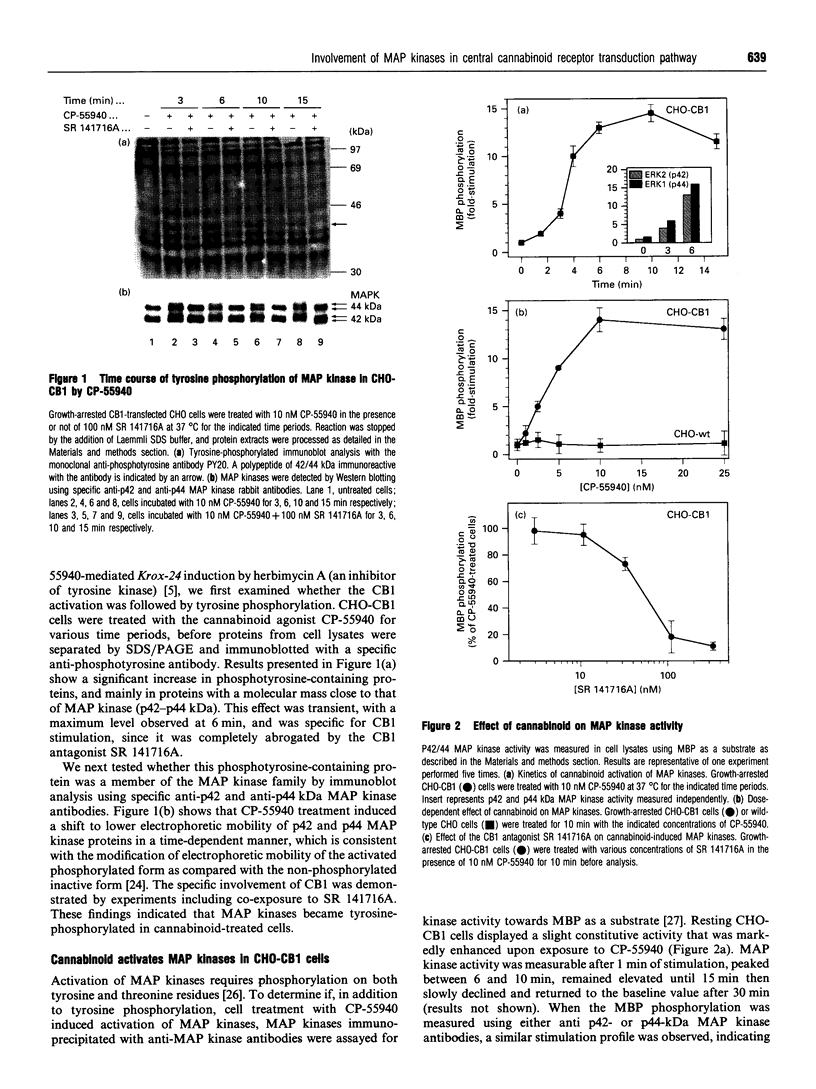
The G-protein-coupled central cannabinoid receptor (CB1) has been shown to be functionally associated with several biological responses including inhibition of adenylate cyclase, modulation of ion channels and induction of the immediate-early gene Krox-24. Using stably transfected Chinese Hamster Ovary cells expressing human CB1 we show here that cannabinoid treatment induces both phosphorylation and activation of mitogen-activated protein (MAP) kinases, and that these effects are inhibited by SR 141716A, a selective CB1 antagonist. The two p42 and p44 kDa MAP kinases are activated in a time- and dose-dependent manner. The rank order of potency for the activation of MAP kinases with various cannabinoid agonists is CP-55940 > delta 9-tetrahydrocannabinol > WIN 55212.2, in agreement with the pharmacological profile of CB1. The activation of MAP kinases is blocked by pertussis toxin but not by treatment with hydrolysis-resistant cyclic AMP analogues. This suggests that the signal transduction pathway between CB1 and MAP kinases involves a pertussis-toxin-sensitive GTP-binding protein and is independent of cyclic AMP metabolism. This coupling of CB1 subtype and mitogenic signal pathway, also observed in the human astrocytoma cell line U373 MG, may explain the mechanism of action underlying cannabinoid-induced Krox-24 induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bading H., Ginty D. D., Greenberg M. E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993 Apr 9;260(5105):181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bouaboula M., Bourrié B., Rinaldi-Carmona M., Shire D., Le Fur G., Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J Biol Chem. 1995 Jun 9;270(23):13973–13980. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- Bouaboula M., Rinaldi M., Carayon P., Carillon C., Delpech B., Shire D., Le Fur G., Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993 May 15;214(1):173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Burstein S., Budrow J., Debatis M., Hunter S. A., Subramanian A. Phospholipase participation in cannabinoid-induced release of free arachidonic acid. Biochem Pharmacol. 1994 Sep 15;48(6):1253–1264. doi: 10.1016/0006-2952(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Veluz J. S., Williams H. L., Briley E. M., Matsuda L. A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992 Nov;42(5):838–845. [PubMed] [Google Scholar]
- Frödin M., Peraldi P., Van Obberghen E. Cyclic AMP activates the mitogen-activated protein kinase cascade in PC12 cells. J Biol Chem. 1994 Feb 25;269(8):6207–6214. [PubMed] [Google Scholar]
- Glass M., Dragunow M. Induction of the Krox 24 transcription factor in striosomes by a cannabinoid agonist. Neuroreport. 1995 Jan 26;6(2):241–244. doi: 10.1097/00001756-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985 Apr;27(4):429–436. [PubMed] [Google Scholar]
- Koch W. J., Hawes B. E., Allen L. F., Lefkowitz R. J. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P., Verslype M., Preud'homme X., Vanderhaeghen J. J. Activation of multiple transcription factor genes by tetrahydrocannabinol in rat forebrain. Neuroreport. 1994 Jun 2;5(10):1265–1268. doi: 10.1097/00001756-199406020-00028. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Monroe J. G. Activation of the p21ras pathway couples antigen receptor stimulation to induction of the primary response gene egr-1 in B lymphocytes. J Exp Med. 1995 Jan 1;181(1):417–422. doi: 10.1084/jem.181.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloux B., Lupker J. H. Rapid isolation of highly productive recombinant Chinese hamster ovary cell lines. Gene. 1994 Nov 18;149(2):341–344. doi: 10.1016/0378-1119(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Seifert R., Metzger J. W., Jung G., Lieberknecht A., Schmidt U., Schultz G. Lipopeptides are effective stimulators of tyrosine phosphorylation in human myeloid cells. Biochem J. 1992 Mar 1;282(Pt 2):551–557. doi: 10.1042/bj2820551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M., Barth F., Héaulme M., Shire D., Calandra B., Congy C., Martinez S., Maruani J., Néliat G., Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994 Aug 22;350(2-3):240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Wartmann M., Campbell D., Subramanian A., Burstein S. H., Davis R. J. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Lett. 1995 Feb 13;359(2-3):133–136. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]
- Winitz S., Russell M., Qian N. X., Gardner A., Dwyer L., Johnson G. L. Involvement of Ras and Raf in the Gi-coupled acetylcholine muscarinic m2 receptor activation of mitogen-activated protein (MAP) kinase kinase and MAP kinase. J Biol Chem. 1993 Sep 15;268(26):19196–19199. [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]