Abstract
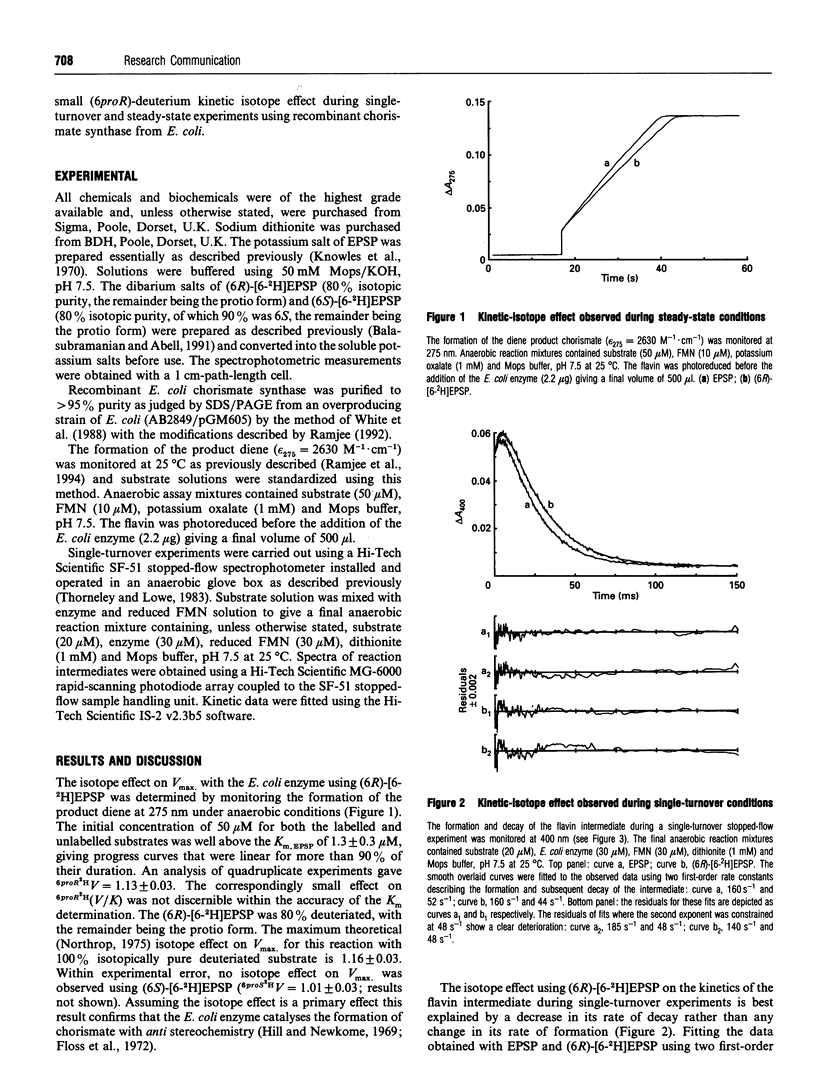
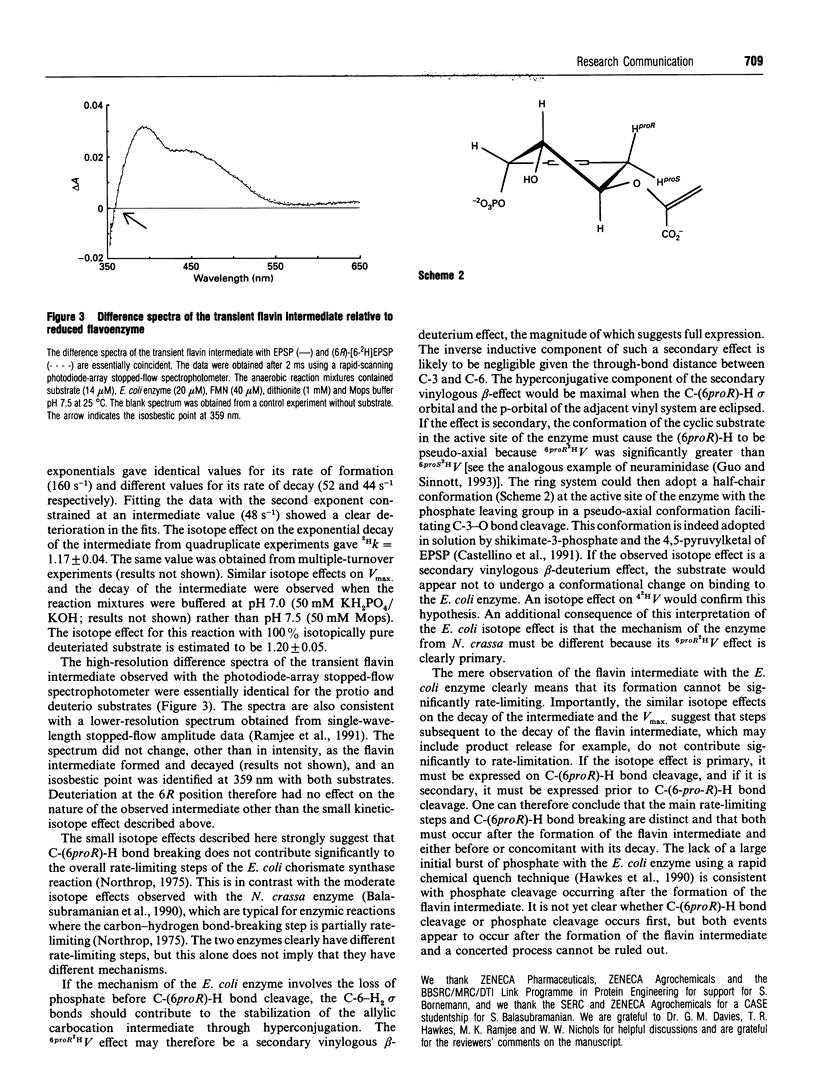
We report the observation of a deuterium kinetic isotope effect for the conversion of 5-enolpyruvylshikimate-3-phosphate into chorismate (6proR2HV = 1.13 +/- 0.03) using recombinant chorismate synthase from Escherichia coli. Similar isotope effects were observed for the decay of a spectroscopically characterized flavin intermediate (6proR2Hk = 1.17 +/- 0.04) during single-turnover experiments. The main rate-limiting steps and C-(6proR)-H bond breaking are therefore distinct and both must occur after the formation of the flavin intermediate and either before or concomitant with its decay.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley R. The shikimate pathway--a metabolic tree with many branches. Crit Rev Biochem Mol Biol. 1990;25(5):307–384. doi: 10.3109/10409239009090615. [DOI] [PubMed] [Google Scholar]
- Charles I. G., Lamb H. K., Pickard D., Dougan G., Hawkins A. R. Isolation, characterization and nucleotide sequences of the aroC genes encoding chorismate synthase from Salmonella typhi and Escherichia coli. J Gen Microbiol. 1990 Feb;136(2):353–358. doi: 10.1099/00221287-136-2-353. [DOI] [PubMed] [Google Scholar]
- Davies G. M., Barrett-Bee K. J., Jude D. A., Lehan M., Nichols W. W., Pinder P. E., Thain J. L., Watkins W. J., Wilson R. G. (6S)-6-fluoroshikimic acid, an antibacterial agent acting on the aromatic biosynthetic pathway. Antimicrob Agents Chemother. 1994 Feb;38(2):403–406. doi: 10.1128/aac.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss H. G., Onderka D. K., Carroll M. Stereochemistry of the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J Biol Chem. 1972 Feb 10;247(3):736–744. [PubMed] [Google Scholar]
- Guo X., Sinnott M. L. A kinetic-isotope-effect study of catalysis by Vibrio cholerae neuraminidase. Biochem J. 1993 Sep 15;294(Pt 3):653–656. doi: 10.1042/bj2940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., Lewis T., Coggins J. R., Mousdale D. M., Lowe D. J., Thorneley R. N. Chorismate synthase. Pre-steady-state kinetics of phosphate release from 5-enolpyruvylshikimate 3-phosphate. Biochem J. 1990 Feb 1;265(3):899–902. doi: 10.1042/bj2650899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. K., Newkome G. R. Stereochemistry of chorismic acid biosynthesis. J Am Chem Soc. 1969 Oct 8;91(21):5893–5894. doi: 10.1021/ja01049a045. [DOI] [PubMed] [Google Scholar]
- Morell H., Clark M. J., Knowles P. F., Sprinson D. B. The enzymic synthesis of chorismic and prephenic acids from 3-enolpyruvylshikimic acid 5-phosphate. J Biol Chem. 1967 Jan 10;242(1):82–90. [PubMed] [Google Scholar]
- Northrop D. B. Steady-state analysis of kinetic isotope effects in enzymic reactions. Biochemistry. 1975 Jun 17;14(12):2644–2651. doi: 10.1021/bi00683a013. [DOI] [PubMed] [Google Scholar]
- Onderka D. K., Floss H. G. Steric course of the chorismate synthetase reaction and the 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) synthetase reaction. J Am Chem Soc. 1969 Oct 8;91(21):5894–5896. doi: 10.1021/ja01049a046. [DOI] [PubMed] [Google Scholar]
- Ramjee M. K., Coggins J. R., Thorneley R. N. A continuous, anaerobic spectrophotometric assay for chorismate synthase activity that utilizes photoreduced flavin mononucleotide. Anal Biochem. 1994 Jul;220(1):137–141. doi: 10.1006/abio.1994.1309. [DOI] [PubMed] [Google Scholar]
- Schaller A., van Afferden M., Windhofer V., Bülow S., Abel G., Schmid J., Amrhein N. Purification and Characterization of Chorismate Synthase from Euglena gracilis: Comparison with Chorismate Synthases of Plant and Microbial Origin. Plant Physiol. 1991 Dec;97(4):1271–1279. doi: 10.1104/pp.97.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. Nitrogenase of Klebsiella pneumoniae. Kinetics of the dissociation of oxidized iron protein from molybdenum-iron protein: identification of the rate-limiting step for substrate reduction. Biochem J. 1983 Nov 1;215(2):393–403. doi: 10.1042/bj2150393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch G. R., Cole K. W., Gaertner F. H. Chorismate synthase of Neurospora crassa: a flavoprotein. Arch Biochem Biophys. 1974 Dec;165(2):505–518. doi: 10.1016/0003-9861(74)90276-8. [DOI] [PubMed] [Google Scholar]
- White P. J., Millar G., Coggins J. R. The overexpression, purification and complete amino acid sequence of chorismate synthase from Escherichia coli K12 and its comparison with the enzyme from Neurospora crassa. Biochem J. 1988 Apr 15;251(2):313–322. doi: 10.1042/bj2510313. [DOI] [PMC free article] [PubMed] [Google Scholar]


