Abstract
Pediatric bone sarcomas, particularly osteosarcomas, present unique challenges in the realm of orthopedic oncology, given their predilection for the metaphyseal regions of long bones and the intricate balance required between achieving oncologic control and preserving limb function. This abstract encapsulates findings from a comprehensive review aimed at advancing pediatric bone sarcoma care, focusing on navigating the complications and innovating solutions for complications of limb salvage and reconstruction focusing on limb length inequalities and accompanying bone defects.
Advancements in imaging, surgical techniques, and adjuvant therapies have shifted the paradigm from amputation to limb-sparing surgeries, albeit with significant challenges, especially in young patients where growth potential complicates reconstructive outcomes. The series highlights the complexity of managing limb length discrepancies (LLD), the cornerstone of limb salvage challenges, and the innovative approaches to address them, including modular endoprosthetic reconstruction with expandable prostheses, magnetic lengthening nails and biological reconstruction strategies like vascularized fibula grafts.
This review underlines the importance of a multidisciplinary approach in managing pediatric bone sarcomas, where the aim extends beyond mere survival to ensuring quality of life through functional limb preservation. It highlights the need for ongoing innovation in surgical and reconstructive techniques tailored to the pediatric population’s unique needs, emphasizing the potential of emerging technologies and methodologies to improve outcomes. Future research should aim to fill the existing knowledge gaps, particularly in comparing pediatric and adult surgical outcomes, to refine treatment protocols and improve patient care in this challenging domain.
Keywords: Pediatric bone sarcoma, Limb salvage surgery, Limb length discrepancy, Modular endoprosthetic reconstruction, Expandable prostheses, Biological reconstruction, Vascularized fibula graft, Orthopedic oncology
Highlights
Advancements in imaging and surgical techniques have shifted treatment paradigms from amputation to limb-sparing surgeries, enhancing the quality of life in pediatric bone sarcoma cases.
Innovative approaches like modular endoprosthetic reconstruction with expandable prostheses and magnetic lengthening nails effectively address limb length discrepancies.
Vascularized fibula grafts provide successful outcomes for complex bone defect reconstruction in pediatric patients.
Plate-assisted bone segment transport with motorized lengthening nails and locking plates is a complex yet reliable reconstruction technique, particularly for diaphyseal bone defects following tumor resection.
Introduction
Bone sarcomas represent the predominant primary bone malignancies encountered in pediatric patients, predominantly manifesting in the long bones’ metaphyseal regions. The treatment paradigm has evolved significantly from the erstwhile norm of amputation to now favoring limb-sparing surgeries, courtesy of advancements in surgical techniques and oncological treatments.1-3 Osteosarcomas exhibit a peak prevalence during the second decade of life, with Ewing’s sarcoma presenting in approximately 30% of patients before the age of 10.4 Recent data from a single-center cohort indicate a 5-year overall survival rate of 78.5% in high-grade osteosarcoma patients without metastases, dropping to 21.7% in those with distant metastases, in patients younger than 16.5
In young patients, especially given the proximity of tumors to the growth plates, achieving oncological clearance while preserving limb function and length can be challenging. The anatomical extent of the tumor necessitates considerable resections (including total or partial growth plate resections) and/or systemic chemotherapy followed by inhibition of the preserved growth plate particularly around the knee, leading to potential limb length discrepancies. This review aims to provide an updated discourse on the biological and technological advancements in reconstructive options available for young patients afflicted with malignant bone tumors of the extremities, thereby offering a guide to the latest in limb salvage and reconstruction strategies.
Limb length discrepancy
In the realm of limb preservation for the skeletally immature, accurately estimating residual growth is pivotal, given the myriad factors influencing limb-length discrepancy (LLD). These factors range from the impact of systemic chemotherapy and the surgical sacrifice of adjacent growth plates, to the deceleration of growth in preserved plates, and the sequela of muscle atrophy, as well as compensatory overgrowth in the contralateral limb, leading to further deformities.6
To predict future limb length and LLD, 4 principal methodologies are employed: Anderson’s method of remaining growth, Moseley’s straight-line graph, Menelaus’ charts of growth remaining by chronological age, and Paley’s multiplier method. The latter, leveraging only current age and limb length, has shown superior applicability for pre-surgical planning due to its simplicity and directness.7-9
However, the interaction between antineoplastic treatments and bone growth is intricate, with a notable dearth of methodological analysis for such data. Pediatric osteosarcoma patients often exhibit a reduced growth velocity during chemotherapy, followed by a rebound phase post-treatment. Studies, such as those by Gilg et al and Li et al, have highlighted the limitations of predictive methods like Paley’s multiplier, which can significantly overestimate the final height in patients with bone tumors, leading to errors that can exceed 1 cm in over a third of cases.10-12
LLDs exceeding 2 cm can have profound clinical and functional repercussions, including pelvic tilt, altered gait mechanics, and joint contractures. For discrepancies anticipated to be between 2 cm and 4 cm, contralateral temporary epiphysiodesis can offer a regulatory mechanism. When discrepancies are expected to be more than 4 cm, endoprosthetic reconstruction using modular reconstruction endoprosthetics with/without expandable components and biologic reconstruction using guided growth modalities or distraction osteogenesis with external fixators or magnetic implantable nails are among various treatments of choice, if feasible. For intramedullary lengthening devices, recent published data suggests younger age for antegrade femoral intramedullary lengthening, which is 8 years in girls and 10 years in boys.13-16 Additionally, non-affected long bone of the ipsilateral side could also be selected for lengthening if the predicted discrepancy among the level of knee joints is less than 3 cm (Figure 1). For LLDs greater than 10 cm, more radical options such as rotationplasty or a lifetime surgical plan including serial lengthening procedures are commonly considered, taking into account the extensive discrepancy and its impact on the patient’s quality of life and functionality.17
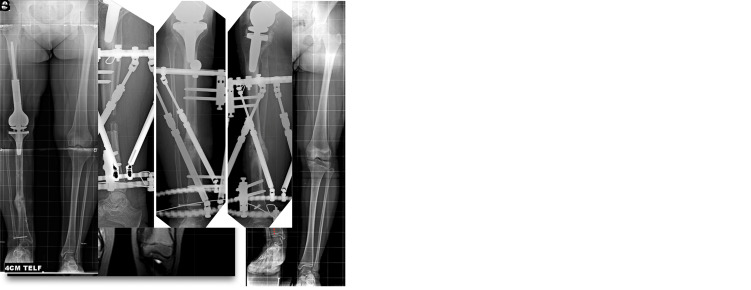
Figure 1.
(a) Coronal section MRI image of a bone sarcoma located in the right distal femur of an 11-year-old girl. Following the wide resection performed in the first stage, the projected adult period limb length discrepancy (LLD) was calculated as 8 cm. (b) Radiographic image at the 5-year follow-up of the tumor prosthesis placed for endoprosthetic reconstruction after the patient’s wide resection. The orthoroentgenogram shows that 4.5 cm of the 8 cm LLD originates from the femur and 3.5 cm from the tibia. Please note the dashed line indicating the alignment of the lower extremity and the osseointegration at the proximal bone-host implant junction. (c and d) Anteroposterior and lateral radiographs of the patient during lengthening from the ipsilateral tibial diaphysis with a computer-assisted external fixator. (e and f) Anteroposterior and lateral radiographs of the patient after 4 cm lengthening during the consolidation period. (g) Orthoroentgenogram with 4 cm compensation taken during the follow-up of the patient’s first lengthening surgery.
Modular endoprosthetic reconstruction with expandable prostheses
Endoprosthetic reconstruction represents a significant advancement for adult sarcoma patients, and this method has been adapted for pediatric patients, particularly those beyond the ages of 13 for males and 12 for females.18 The pioneering MARK I prosthesis, introduced by Scales et al in 1976, marked the inception of extendable prostheses, featuring an expandable spacer to facilitate limb lengthening.15 Despite its initial promise, the early models were plagued by suboptimal functional outcomes, prompting a series of enhancements aimed at improving efficacy and reducing complications. These efforts have culminated in the development of modular, self-actuating, and self-expanding prostheses that offer a new horizon in limb salvage surgery.19-23
These advanced prostheses consist of 4 key components: an articular segment, an intercalary expandable segment, exchangeable graduated spacer segments, and a final intercalary segment. The expansion process primarily occurs within the intercalary segment, with a systematic approach allowing for incremental lengthening. This modular design not only facilitates expansion but also ensures that the prosthesis can be adjusted in tandem with the patient’s growth, offering unprecedented adaptability.
The lengthening procedure for these prostheses, typically performed in 1- to 2-cm increments, necessitates surgical intervention under general anesthesia. The surrounding soft tissues, particularly muscles and neurovascular structures, impose a natural limit on the extent of expansion, with the risk of complications such as nerve palsy if these structures are overly strained.21,24,25
Postoperative care is critical, with patients usually requiring a brief hospital stay followed by the immediate initiation of physical therapy, including continuous passive motion to promote recovery. Successful rehabilitation demands a high level of commitment from both the patient and their support network to prevent complications such as flexion contractures, which can severely impact functional outcomes.
Patient selection for expandable prostheses necessitates meticulous evaluation of a multitude of factors, such as the patient’s age, size, potential for further growth, and the magnitude of the predicted limb length discrepancy (LLD). Candidates ideally suited for this intervention often present with an LLD exceeding 4 cm and possess stable bone conditions amenable to stem integration. The prosthesis may not be suitable for all children, especially those under the age of 6 or those with significant potential for growth due to genetic factors, where achieving symmetrical limb lengths poses a greater challenge. In such cases, where the diaphysis is wider than 8 mm and the child is older than 8 years, yet the LLD is projected to be more than 4 cm, expandable prostheses are considered. However, in scenarios where the potential growth could render the prosthetic solution ineffective or if equalizing leg lengths becomes impractical due to the child’s very young age or the parents’ tall stature, alternative reconstructive strategies may be warranted to ensure optimal functional and cosmetic outcomes.
Expandable prostheses, while transformative in pediatric orthopedic oncology, are not without their complications, which predominantly include infection, mechanical failure of the expansion mechanism, aseptic loosening, and stem migration. A pivotal study by Eckardt et al in 1993 illuminated the complexities associated with the LEAP (Lewis Adjustable Expandable Prosthesis), reporting a complication rate of 67%. The identified issues ranged from prosthesis collapse and mechanical failures to limb rotation and infection, leading to prosthetic revisions in over half of the cases in their cohort.21 A subsequent review by the same group later revealed that only half of the patients with expandable prostheses underwent successful expansion, highlighting a 50% complication rate.24
The frequency of surgical interventions a patient requires is influenced not just by their physical characteristics but also by the incidence of these complications. Kenan and Lewis projected that a patient might undergo 10 to 15 surgeries over the course of treatment to manage or mitigate expansion-related issues.19 This was echoed by Schiller et al, who documented an average of 11 surgeries among their patients aged 9 to 11 years, underscoring the intensive surgical journey these young patients often face.26 The advent of self-actuating, non-invasive prosthetic designs has significantly reduced the need for repeated surgeries, thereby diminishing the associated infection risks. However, Henderson et al reported that reoperations were still necessary in 50% of cases, with aseptic loosening occurring in 20%, indicating that while advancements have been made, the journey toward optimizing these life-changing prostheses continues.27
Modular endoprosthetic reconstruction with limb lengthening
In cases where expandable modular endoprostheses are impractical or unavailable, and where the risk of existing complications is considered unacceptable, limb lengthening techniques can be combined with non-expandable endoprostheses for endoprosthetic reconstruction. In situations with uncertain survival estimates, staged treatments that involve bone lengthening procedures are necessary. It is also possible to adjust modular endoprostheses up to 2 cm longer than the contralateral side as an option.12,28
The first of these combined treatments is temporary resection arthrodesis (TRA). If local recurrence and metastatic conditions are excluded in the first 2-3 years, the treatment initially involves soft tissue lengthening with an external fixator, followed by a transition to a mature-type tumor prosthesis. This constitutes a 3-stage treatment for the patient. Kong and colleagues reported in their series of 56 cases that they were able to provide a mobile joint for 35 patients. The 3-stage treatment is acknowledged to be an exhausting reconstruction in cases with low expected survival rates. Another obstacle is the potential for the staged treatment to be interrupted by infection, negatively affecting the functional outcomes.28
Another option for temporary arthrodesis involves staged treatment with hemiarthroplasty. In the series reported by Chung and colleagues, major complications occurred in 3 out of 25 cases. Two of these cases continued treatment with temporary arthrodesis following component infection. Key points highlighted by the authors include the subchondral collapse caused by the metallic component in contact with the anatomical neighboring surface and the development of cortical atrophy at the host bone prosthesis interface. This situation can result in stress shielding around the stem. The pediatric population is much more sensitive to this entity.29
Alpan and colleagues reported an average extension rate of 45 mm and a bone-healing index of 41 days/cm in 6 sarcoma patients who underwent lengthening with a femoral intramedullary magnetic nail as an extension option.13
In another study, staged surgical treatment was applied again, but in the first stage, lengthening was performed with a unilateral external fixator following the installation of a hinged tumor prosthesis, and finally, the treatment was completed with an adult-type endoprosthesis. The authors state that in cases with a minimum of 2 years of recurrence-free survival, it is necessary to proceed to the distraction osteogenesis part of the staged treatment. For achieving a higher knee level, tibial lengthening is indicated as more advantageous in knee area reconstructions. According to the authors, recurrent dislocation of the knee component can be a significant problem.30
The integration of limb lengthening techniques with modular endoprosthetic reconstruction, while innovative, brings forth a complex array of potential complications that necessitate careful consideration and planning. These complications can range from mechanical failures of the prosthesis, such as wear or breakage of components, to biological challenges including infection, delayed union, or nonunion of the bone. Particularly in pediatric patients, there’s an added layer of complexity due to their ongoing growth and development, which can lead to asymmetric growth or joint misalignment. Moreover, the extensive surgical interventions required for both limb lengthening and modular endoprosthetic insertion increase the risk of soft tissue damage, leading to scarring, reduced mobility, and potential neurovascular compromise. The psychological impact on the patient, especially children and adolescents, also cannot be underestimated, as repeated surgeries and the long recovery periods can be emotionally taxing. Therefore, a multidisciplinary approach involving orthopedic surgeons, pediatricians, physiotherapists, and psychologists is crucial in managing these patients, ensuring that both the physical and emotional aspects of their recovery are adequately addressed (Figure 2).
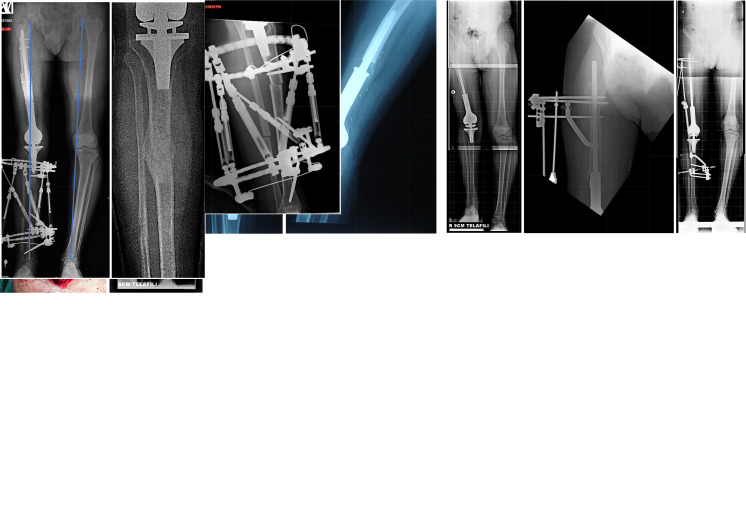
Figure 2.
(A) Coronal section MRI image of a bone sarcoma located in the right distal femur of a 10-year-old boy. After the wide resection performed in the first stage, the projected adult limb length discrepancy (LLD) was 9.5 cm. (B and C) Anteroposterior and lateral roentgenograms of the tumor prosthesis placed during the first stage of reconstruction after the patient’s resection. Note the gap formation at the prosthesis’s proximal attachment area. (D) At the 5-year follow-up orthoroentgenogram with 9 cm compensation, a 10 cm LLD was identified, with 6.3 cm originating from the femur and 3.2 cm from the tibia. Despite all infection parameters being negative, radiolucency suggestive of loosening was observed at the bone–implant interface both distally and proximally. (E) The patient’s bone lengthening surgery was planned from the proximal side. Lengthening over nail (LON) was applied via a nail integrated into the proximal stem, along with a circular external fixator. Early postoperative radiography is shown. (F) The orthoroentgenogram taken at the end of the lengthening period. A lengthening of 4 cm was achieved. (G and H) Grafting with a titanium cage at the proximal distraction area of the implant––bone composite for osteointegration purposes after lengthening. (I) The orthoroentgenogram during follow-up showing full consolidation and osteointegration with 6 cm compensation. (J and K) Early postoperative anteroposterior and lateral radiography of the tibial lengthening procedure using a computer-assisted hexapod external fixator. (L) At the end of the final tibial lengthening procedure, an anteroposterior orthoroentgenogram of the patient revealed proper mechanical axis and lower limb alignment. (M) Successful healing of the distraction gap is shown after fixator removal.
Biological reconstruction
Following tumor resection in the extremities, segmental bone defects present a significant reconstructive challenge. Vascularized fibula grafting has emerged as a leading technique for addressing these defects, utilizing either intramedullary or onlay grafting approaches.31-33 In the intramedullary technique, the defect is bridged with an intercalary allograft, complemented by a vascularized fibula flap inserted into the allograft’s medullary canal. This process involves creating a passage for the fibula’s vascular pedicle through the allograft, ensuring the fibula flap integrates seamlessly with the host bone, secured in place with a locking plate. Conversely, the onlay method positions the vascularized fibula flap at the allograft–host interface, affixing it with cortical screws to facilitate microvascular connections and bone integration.32 Liquid nitrogen recycled bone and free vascular fibula graft combination is another technique of choice named “frozen hotdog” for biological reconstruction, which has been proven as a safe and effective method of reconstruction.33
The efficacy of vascularized fibula flaps is notable, achieving bony union in 86% to 92% of cases, with union times spanning 4.5 to 12 months.34,35 This success rate persists despite the potential delays in healing associated with adjuvant therapies such as chemotherapy and radiation, underscoring the resilience and viability of this reconstructive option.35 Critical factors influencing the outcome include the anatomical site of the reconstruction and the use of allograft materials, with trunk reconstructions generally reaching union more swiftly than those in the extremities.
In the landscape of reconstructive surgery for segmental bone defects, non-vascularized or free fibula grafts are occasionally favored in certain centers for their straightforwardness and reduced surgical time.34 Despite their appeal, these grafts are associated with a notable risk of infection and nonunion, affecting as many as 50% of cases. This highlights a critical consideration: the ease of free fibula grafting comes with potential delays in achieving bony union, particularly when compared to vascularized fibula grafts, which are known for their ability to bear greater weight due to post-operative hypertrophy.
In specific instances, free fibula grafts have demonstrated considerable success. Reports by Krieg and Hefti have shown an 89% rate of primary union within 12 months across 46 non-vascularized grafts, indicating a strong potential for healing under optimal conditions. Zaretski et al further support the utility of free fibula grafts in their study of 30 patients, suggesting their effectiveness, especially in non-weight-bearing areas such as the upper extremities or certain diaphyseal bone defects.36 This body of evidence points toward a nuanced application of free fibula grafts, underscoring their value in selected cases where the advantages of a less complex procedure align with clinical goals, despite the superior weight-bearing capacity and hypertrophic potential of vascularized grafts.
The journey of segmental bone reconstruction, particularly with fibular grafting, is fraught with challenges, among which the risk of nonunion stands prominent. This complication can significantly impact patient morbidity, especially in cases involving complex reconstructions. Notably, Minami et al have documented a high success rate, with 95% bony union achieved across 104 cases utilizing vascularized fibula flaps for a range of traumatic, oncologic, and congenital reconstructions. Despite this success, a small subset experienced nonunion, leading to severe outcomes such as pseudoarthrosis in 3 patients and necessitating amputation in 2.37 Contrastingly, Clemens et al observed no need for amputation among their cohort of 52 patients undergoing vascularized fibula reconstruction, with nonunion cases effectively salvaged through endoprosthesis in 2 instances, ultimately resulting in satisfactory long-term function for all affected patients.35 However, Adam et al reported a higher rate of nonunion, at 30%, highlighting the variability in outcomes across different studies and techniques.31
Fractures of the fibula flap, with reported rates varying from 5% to 48%, represent another critical complication that can lead to nonunion.31,35,38 Despite this, vascularized fibula grafts have shown resilience against angular and torsional stresses, even in the repair of extensive skeletal defects.39 The key to minimizing fracture risk lies in meticulous surgical technique, ensuring proper alignment of the fibula flap, optimizing the interface with the host bone, and adhering to a strict protocol of offloading until radiographic evidence of bony union is confirmed. De Boer and Wood emphasize the importance of gradual loading to foster graft remodeling and hypertrophy, further enhancing the structural integrity of the reconstructed segment (Figure 3).40
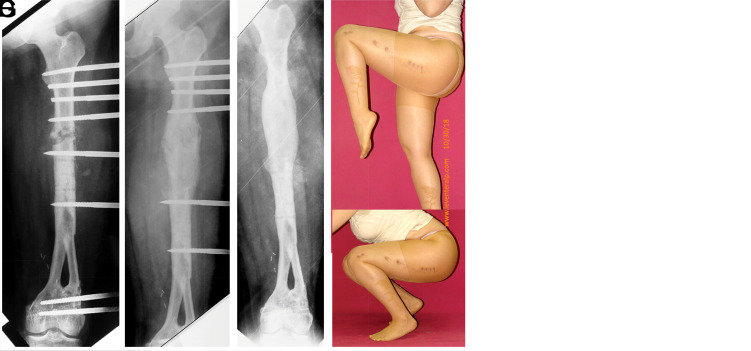
Figure 3.
(A) MRI image of an osteosarcoma located in the distal femur of a 7-year-old girl (initial sarcoma surgery performed by Professor Harzem Ozger). (B) The patient underwent a double barrel free vascularized fibula graft. An orthoroentgenogram taken 11 years later shows an 8 cm shortening in the patient. (C-G) Serial roentgenograms taken after lengthening from the femoral diaphysis with a unilateral monorail fixator and the postoperative functional outcome.
In addition to fibular grafting, distraction osteogenesis and segment transport techniques play crucial roles in the biological reconstruction of bone defects following tumor resection. Distraction osteogenesis involves the gradual mechanical distraction of bone segments to promote new bone formation, while segment transport utilizes single or dual osteotomies to move bone segments into defect areas.41 Segment transport techniques can be applied either during or after elongation with plating, with isolated external fixators, or with combined techniques. In recent years, combined techniques involving magnetic intramedullary nails with elongation and plating have gained prominence. The technique described in the article on plate-assisted bone segment transport with motorized lengthening nails and locking plates (PABST) highlights that increased vascularity and better soft tissue coverage in femur applications make this technique more feasible.42 (Figure 4) In conclusion, while we have numerous techniques and instruments that enhance our options for biological reconstruction, the choice of technique depends on the experience of the treatment team, the suitability of the soft tissue and donor sites, and the richness of the soft tissue coverage and vascularity.
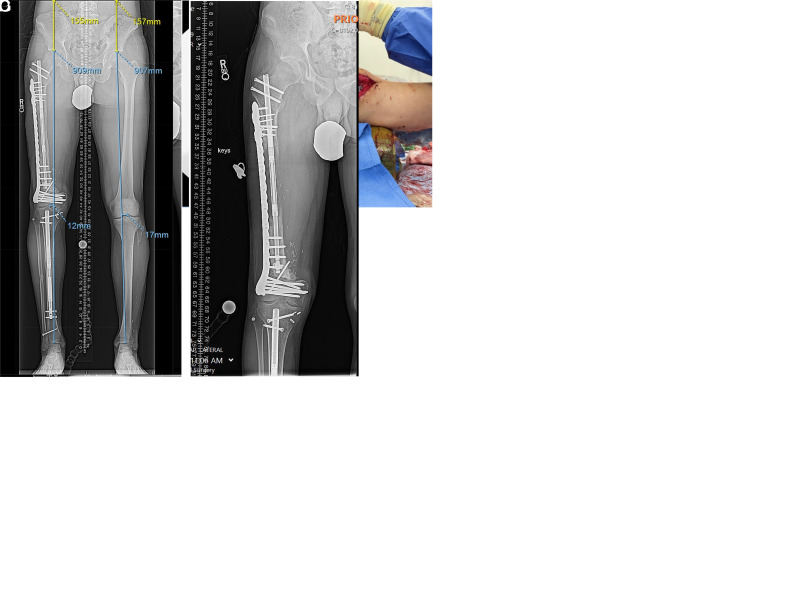
Figure 4.
(A) Radiograph of the right femur of an adolescent boy who underwent Ewing sarcoma resection followed by failed vascularized fibular grafting. (B) Intraoperative clinical image showing the external fixator-assisted application of the plate bridging the entire segmental defect. (C and D) Intraoperative fluoroscopy and radiography displaying the intramedullary magnetic nail at the proximal femur and fixation of the plate at the distal femur. (E and F) Serial radiographs of the right femur during and at the end of the distraction period. The distraction area is 16 cm long, with additional fixation using an oblique screw visible in the radiograph. (G) Orthoroentgenogram of the patient after completion of the treatment, involving plate-assisted segment transport with a magnetic intramedullary nail in his right femur and internal lengthening of the tibia for final equalization of lower limb length.
Amputation and rotationplasty
Despite advances in limb-salvage techniques, amputation remains a significant surgical approach in managing pediatric extremity bone tumors.43 Schrager et al highlighted that, from 1988 to 2007, one-third of such cases resulted in amputation, underscoring its prevalence in situations where limb preservation may not be viable.44
Rotationplasty presents an innovative alternative, particularly in scenarios necessitating an above-knee amputation. This surgical technique ingeniously transforms an above-knee amputation into a below-knee amputation, facilitating more effective local sarcoma control around the knee in children. Notably, rotationplasty has demonstrated promising functional outcomes, offering an improved quality of life with a lower complication rate compared to more intricate limb-salvage procedures. It enables patients to utilize modified below-knee prosthetics, offering a blend of functionality and adaptability.45
The decision to proceed with rotationplasty is critical and requires thorough discussions with the patient’s family, considering both the clinical benefits and the psychological impacts. While the functional advantages of rotationplasty are compelling, many families opt for biological or endoprosthetic reconstructions due to aesthetic and psychological considerations. Renard et al’s comparison of ablative surgeries, including rotationplasty, with limb-salvage techniques revealed superior functional outcomes in limb-salvage procedures for patients under ten, highlighting the nuanced decision-making process in choosing the optimal surgical intervention.46
Conclusion
In conclusion, the evolution of pediatric bone sarcoma treatment has shifted toward limb-salvage and reconstructive strategies, emphasizing the balance between oncological control and the preservation of function and quality of life. The integration of advanced modular endoprosthetics, sophisticated limb-lengthening techniques, and biological reconstruction options highlights the nuanced, multidisciplinary approach essential in contemporary oncological orthopedics. Notably, reconstructions at specific anatomical sites such as the proximal and distal femur, proximal tibia, and upper extremity present unique challenges and exhibit distinct patterns of postoperative complications and failure rates, underscoring the complexity of surgical interventions in these regions. Furthermore, the comparative analysis of surgical outcomes between pediatric patients and adults remains an area ripe for investigation, potentially revealing critical insights into age-related differences in treatment efficacy and complication profiles. Despite these challenges, including the potential for mechanical complications and the psychological burden of multiple surgeries, the advancements in this field offer significant hope for not only improved survival rates but also for enabling survivors to lead active, fulfilling lives. As the field progresses, continued research and interdisciplinary collaboration will be vital in refining these treatments, minimizing complications, and enhancing patient outcomes.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – L.E.; Design – L.E.; Supervision – L.E., S.R.R.; Resources – L.E., S.R.R.; Materials – L.E., S.R.R.; Data Collection and/or Processing – M.C.; Analysis and/or Interpretation – L.E., M.C.; Literature Search – M.C., L.E.; Writing Manuscript – M.C.; Critical Review – L.E., S.R.R.
Declaration of Interests: The authors have no conflict of interest to declare.
References
- 1. Klein MJ, Kenan S, Lewis MM. Osteosarcoma. Clinical and pathological considerations. Orthop Clin North Am. 1989;20(3):327 345. [PubMed] [Google Scholar]
- 2. Rosen G, Marcove RC, Huvos AG, et al. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106(suppl):55 67. ( 10.1007/BF00625054) [DOI] [PubMed] [Google Scholar]
- 3. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331 1337. ( 10.2106/00004623-198668090-00005) [DOI] [PubMed] [Google Scholar]
- 4. Moore DD, Haydon RC. Ewing’s sarcoma of bone. Cancer Treat Res. 2014;162:93 115. ( 10.1007/978-3-319-07323-1_5) [DOI] [PubMed] [Google Scholar]
- 5. Evenhuis RE, Acem I, Rueten-Budde AJ, et al. Survival analysis of 3 different age groups and prognostic factors among 402 patients with skeletal high-grade osteosarcoma. Real world data from a single tertiary sarcoma center. Cancers (Basel). 2021;13(3). ( 10.3390/cancers13030486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis VO. Limb salvage in the skeletally immature patient. Curr Oncol Rep. 2005;7(4):285 292. ( 10.1007/s11912-005-0052-7) [DOI] [PubMed] [Google Scholar]
- 7. Moseley CF. A straight line graph for leg length discrepancies. Clin Orthop Relat Res. 1978;(136):33 40. ( 10.1097/00003086-197810000-00004) [DOI] [PubMed] [Google Scholar]
- 8. Aguilar JA, Paley D, Paley J, et al. Clinical validation of the multiplier method for predicting limb length discrepancy and outcome of epiphysiodesis, part II. J Pediatr Orthop. 2005;25(2):192 196. ( 10.1097/01.bpo.0000150808.90052.7c) [DOI] [PubMed] [Google Scholar]
- 9. Aguilar JA, Paley D, Paley J, et al. Clinical validation of the multiplier method for predicting limb length at maturity, part I. J Pediatr Orthop. 2005;25(2):186 191. ( 10.1097/01.bpo.0000150809.28171.12) [DOI] [PubMed] [Google Scholar]
- 10. Hoshi M, Oebisu N, Iwai T, Ban Y, Nakamura H. Does systemic chemotherapy influence skeletal growth of Young osteosarcoma patients as a treatment-related late adverse effect? Curr Oncol. 2022;29(6):4081 4089. ( 10.3390/curroncol29060325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilg MM, Wibmer C, Andreou D, et al. Paley’s multiplier method does not accurately predict adult height in children with bone sarcoma. Clin Orthop Relat Res. 2014;472(8):2506 2513. ( 10.1007/s11999-014-3636-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Liao F, Xu HR, Niu XH. Is there a reliable method to predict the limb length discrepancy after chemotherapy and limb salvage surgery in children with osteosarcoma? Chin Med J (Engl). 2016;129(16):1912 1916. ( 10.4103/0366-6999.187849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alpan B, Eralp L, Sungur M, Valiyev N, Özger H. Femoral discrepancy after childhood bone sarcoma surgery can be treated with magnetic intramedullary nails. Orthopedics. 2023;46(1):27 34. ( 10.3928/01477447-20221024-03) [DOI] [PubMed] [Google Scholar]
- 14. Vogt B, Laufer A, Gosheger G, et al. Evaluation of simultaneous bilateral femoral distraction osteogenesis with antegrade intramedullary lengthening nails in achondroplasia with rhizomelic short stature: a retrospective study of 15 patients with a minimum follow-up of 2 years. Acta Orthop. 2024;95:47 54. ( 10.2340/17453674.2024.35226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCoy TH, Kim HJ, Cross MB, et al. Bone tumor reconstruction with the Ilizarov method. J Surg Oncol. 2013;107(4):343 352. ( 10.1002/jso.23217) [DOI] [PubMed] [Google Scholar]
- 16. Safi İKA, Samadov F, Kanar M, Tüter İ, Özdemir HM. Deformity correction and limb lengthening with externally controlled motorized extendable intramedullary nails: comparison of 2 different nails. Acta Orthop Traumatol Turc. 2023;57(4):169 175. ( 10.5152/j.aott.2023.23026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevens PM. The role of guided growth as it relates to limb lengthening. J Child Orthop. 2016;10(6):479 486. ( 10.1007/s11832-016-0779-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Topkar OM, Sofulu Ö, Şirin E, Erol B. Limb salvage surgery of primary and metastatic bone tumors of the lower extremity: functional outcomes and survivorship of modular endoprosthetic reconstruction. Acta Orthop Traumatol Turc. 2021;55(2):147 153. ( 10.5152/j.aott.2021.20101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenan S, Lewis MM. Limb salvage in pediatric surgery. The use of the expandable prosthesis. Orthop Clin North Am. 1991;22(1):121 131. ( 10.1016/S0030-5898(20)31635-7) [DOI] [PubMed] [Google Scholar]
- 20. Kenan S, Bloom N, Lewis MM. Limb-sparing surgery in skeletally immature patients with osteosarcoma. The use of an expandable prosthesis. Clin Orthop Relat Res. 1991;(270):223 230. [PubMed] [Google Scholar]
- 21. Eckardt JJ, Safran MR, Eilber FR, Rosen G, Kabo JM. Expandable endoprosthetic reconstruction of the skeletally immature after malignant bone tumor resection. Clin Orthop Relat Res. 1993;(297):188 202. ( 10.1097/00003086-199312000-00032) [DOI] [PubMed] [Google Scholar]
- 22. Wilkins RM, Soubeiran A. The Phenix expandable prosthesis : early American experience. Clin Orthop Relat Res. 2001;(382):51 58. ( 10.1097/00003086-200101000-00009) [DOI] [PubMed] [Google Scholar]
- 23. Gitelis S, Neel MD, Wilkins RM, Rao BN, Kelly CM, Yao TK. The use of a closed expandable prosthesis for pediatric sarcomas. Chir Organi Mov. 2003;88(4):327 333. [PubMed] [Google Scholar]
- 24. Eckardt JJ, Kabo JM, Kelley CM, et al. Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res. 2000;(373):51 61. ( 10.1097/00003086-200004000-00008) [DOI] [PubMed] [Google Scholar]
- 25. Ward WG, Yang RS, Eckardt JJ. Endoprosthetic bone reconstruction following malignant tumor resection in skeletally immature patients. Orthop Clin North Am. 1996;27(3):493 502. ( 10.1016/S0030-5898(20)32095-2) [DOI] [PubMed] [Google Scholar]
- 26. Schiller C, Windhager R, Fellinger EJ, Salzer-Kuntschik M, Kaider A, Kotz R. Extendable tumour endoprostheses for the leg in children. J Bone Joint Surg Br. 1995;77(4):608 614. [PubMed] [Google Scholar]
- 27. Henderson ER, Pepper AM, Marulanda G, Binitie OT, Cheong D, Letson GD. Outcome of lower-limb preservation with an expandable endoprosthesis after bone tumor resection in children. J Bone Joint Surg Am. 2012;94(6):537 547. ( 10.2106/JBJS.I.01575) [DOI] [PubMed] [Google Scholar]
- 28. Kong CB, Lee SY, Jeon DG. Staged lengthening arthroplasty for pediatric osteosarcoma around the knee. Clin Orthop Relat Res. 2010;468(6):1660 1668. ( 10.1007/s11999-009-1117-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung SH, Jeon DG, Cho WH, et al. Temporary hemiarthroplasty with a synthetic device in children with osteosarcoma around the knee as a bridging procedure until skeletal maturity. J Surg Oncol. 2015;112(1):107 114. ( 10.1002/jso.23964) [DOI] [PubMed] [Google Scholar]
- 30. Ji T, Yang Y, Li DS, Tang XD, Guo W. Limb salvage using non-hinged endoprosthesis and staged correction of leg-length discrepancy for children with distal femoral malignant tumors. Orthop Surg. 2019;11(5):819 825. ( 10.1111/os.12525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adam D, Hamel A, Perrot P, Duteille F. Long-term behavior of the vascularized fibular free flap for reconstruction of bony defects in children. Ann Chir Plast Esthet. 2020;65(3):219 227. ( 10.1016/j.anplas.2019.07.004) [DOI] [PubMed] [Google Scholar]
- 32. Capanna R, Campanacci DA, Belot N, et al. A new reconstructive technique for intercalary defects of long bones: the association of massive allograft with vascularized fibular autograft. Long-term results and comparison with alternative techniques. Orthop Clin North Am. 2007;38(1):51 60, vi. ( 10.1016/j.ocl.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 33. Özger H, Alpan B, Eralp L, et al. Is liquid nitrogen recycled bone and vascular fibula combination the biological reconstruction of choice in lower extremity long bone tumor-related defects? J Surg Oncol. 2023;128(5):902 915. ( 10.1002/jso.27385) [DOI] [PubMed] [Google Scholar]
- 34. Hsu RW, Wood MB, Sim FH, Chao EY. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br. 1997;79(1):36 42. ( 10.1302/0301-620x.79b1.6818) [DOI] [PubMed] [Google Scholar]
- 35. Clemens MW, Chang EI, Selber JC, Lewis VO, Oates SD, Chang DW. Composite extremity and trunk reconstruction with vascularized fibula flap in postoncologic bone defects: a 10-year experience. Plast Reconstr Surg. 2012;129(1):170 178. ( 10.1097/PRS.0b013e3182362171) [DOI] [PubMed] [Google Scholar]
- 36. Zaretski A, Amir A, Meller I, et al. Free fibula long bone reconstruction in orthopedic oncology: a surgical algorithm for reconstructive options. Plast Reconstr Surg. 2004;113(7):1989 2000. ( 10.1097/01.prs.0000122213.82011.c5) [DOI] [PubMed] [Google Scholar]
- 37. Minami A, Kasashima T, Iwasaki N, Kato H, Kaneda K. Vascularised fibular grafts. An experience of 102 patients. J Bone Joint Surg Br. 2000;82(7):1022 1025. ( 10.1302/0301-620x.82b7.10332) [DOI] [PubMed] [Google Scholar]
- 38. Minami A, Kimura T, Matsumoto O, Kutsumi K. Fracture through united vascularized bone grafts. J Reconstr Microsurg. 1993;9(3):227 232. ( 10.1055/s-2007-1006649) [DOI] [PubMed] [Google Scholar]
- 39. Pederson WC, Person DW. Long bone reconstruction with vascularized bone grafts. Orthop Clin North Am. 2007;38(1):23 35, v. ( 10.1016/j.ocl.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 40. de Boer HH, Wood MB. Bone changes in the vascularised fibular graft. J Bone Joint Surg Br. 1989;71(3):374 378. ( 10.1302/0301-620X.71B3.2722923) [DOI] [PubMed] [Google Scholar]
- 41. Fidan F, Kılıç F, Lapçin O, Polat A, Kılıç M, Sökücü S. Effect of transported segment size on the new bone formation of the rabbit femur in the Ilizarov bone transport method. Acta Orthop Traumatol Turc. 2023;57(5):215 220. ( 10.5152/j.aott.2023.22087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olesen UK, Nygaard T, Prince DE, et al. Plate-assisted bone segment transport with motorized lengthening nails and locking plates: a technique to treat femoral and tibial bone defects. J Am Acad Orthop Surg Glob Res Rev. 2019;3(8):e064. ( 10.5435/JAAOSGlobal-D-19-00064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baysal Ö, Sağlam F, Sofulu Ö, Yiğit O, Şirin E, Erol B. Indications of amputation after limb-salvage surgery of patients with extremity-located bone and soft-tissue sarcomas: a retrospective clinical study. Acta Orthop Traumatol Turc. 2021;55(2):154 158. ( 10.5152/j.aott.2021.20115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schrager J, Patzer RE, Mink PJ, Ward KC, Goodman M. Survival outcomes of pediatric osteosarcoma and Ewing’s sarcoma: a comparison of surgery type within the SEER database, 1988-2007. J Regist Manag. 2011;38(3):153 161. [PubMed] [Google Scholar]
- 45. Hillmann A, Gosheger G, Hoffmann C, Ozaki T, Winkelmann W. Rotationplasty--surgical treatment modality after failed limb salvage procedure. Arch Orthop Trauma Surg. 2000;120(10):555 558. ( 10.1007/s004020000175) [DOI] [PubMed] [Google Scholar]
- 46. Renard AJ, Veth RP, Schreuder HW, van Loon CJ, Koops HS, van Horn JR. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73(4):198 205. () [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a