Abstract
Background:
The primary objective of this study was to investigate the etiological causes and the underlying mechanism of post-earthquake dizziness in affected persons.
Methods:
The present study utilized an observational case–control design to recruit 69 participants (33 with self-reported dizziness complaints and 36 healthy persons) who were exposed to the 2023 earthquakes in Türkiye. The participants underwent assessments including the Dizziness Handicap Inventory for measuring dizziness-related disability, stress, and anxiety assessment using various scales, and equilibrium evaluation through the use of videonystagmography, video head impulse test, and vestibular evoked myogenic potential. The 2 groups were compared based on these assessments.
Results:
The results indicate that the Dizziness Handicap Inventory score was significantly higher in the patient group compared to the control group (P < .001). The mean score of the Peritraumatic Distress Inventory, as well as the mean scores of the Hospital Anxiety and Depression Scale anxiety score and depression score, were found to be significantly higher in the patient group compared to the control group (P = .012, P < .001, and P < .001, respectively). Furthermore, it was observed that the mean vestibulo-ocular reflex gain of the left posterior semicircular canal exhibited a statistically significant decrease in the patient group (P = .02).
Conclusion:
The observed equilibrium dysfunction experienced by individuals following a significant earthquake is likely attributable to heightened stress and anxiety stemming from multiple sources, including the impact of recurrent vibrations on the inner ear. Therefore, it is essential to establish a holistic healthcare approach that addresses the psychological needs of individuals affected by earthquakes.
Keywords: Earthquake, dizziness, dtress, anxiety, equilibrium
Main Points
Dizziness is a significant contributor to post-earthquake morbidity.
Anxiety and stress are identified as primary factors contributing to post-earthquake dizziness.
Social media misinformation and mainstream media drama can increase imbalance earthquake sufferers.
Recurring seismic vibrations has the potential to result in dysfunction of the inner ear.
Introduction
On February 6, 2023, an earthquake measuring 7.7 on the Richter scale, followed 9 hours later by another with a magnitude of 7.6, occurred in Kahramanmaras in southeastern Türkiye. These 2 earthquakes were followed by more than 1000 aftershocks, some with a magnitude greater than 6. These earthquakes were among the most destructive ever experienced. The earthquakes affected 11 cities and approximately 15 million people in southeastern Türkiye and have created horrific outcomes, such as over 50 000 deaths and 107 000 injuries, as well as various disorders affecting a significant part of society.
The post-earthquake period, particularly in the aftermath of a severe one, has been linked to a range of physiological and psychological disorders. Various types of psychiatric diseases,1 sleep disorders,2 myocardial infarctions, strokes,3 and dizziness4-6 have been observed to have a high prevalence in the population throughout the post-earthquake period. Following the Nepal earthquake in April–May 2015, researchers noted a discernible rise in the prevalence of individuals presenting with vestibular symptoms characterized by instability and dizziness, which could not be definitively classified under any specific category of vestibular disorder.4 Several months after the Kumamoto earthquakes, a considerable number of cases involving the manifestation of dizziness were documented within a wide geographical region encompassing the epicenter of seismic activity.7 In post-earthquake assessments, scholars characterized this state as a non-specific sensation of dizziness and disequilibrium. The clinical appearance described by Nomura et al was designated post-earthquake dizziness syndrome (PEDS).8 Despite the existence of multiple hypotheses on the etiology of PEDS, consensus on the underlying mechanism and etiology has yet to be reached.9 The existing body of research pertaining to this subject matter has been inadequate in terms of studies that possess a substantial level of proof.
The primary objective of this study was to provide a comprehensive understanding of the causes and potential physiological processes involved in post-earthquake dizziness. The disclosure of evidence pertaining to the causation of post-earthquake dizziness will make a valuable contribution to the advancement of an improved treatment strategy.
MATERIAL AND METHODS
Ethical Considerations
The Çukurova Medical Faculty of Medicine Non-Invasive Clinical Research Ethics Committee approved the study, which was conducted in line with the Declaration of Helsinki (Approval Number: 132-26; Date: April 7, 2023). All participants enrolled in the study provided written informed consent.
Participants
Patients who applied to our outpatient clinic between April 1 and May 1, 2023, were chosen as participants. The patients in the study were chosen from among individuals who had been affected by earthquakes and aftershocks, developed dizziness, and sought treatment at our facility. Patients who did not fit the standard presentation of vestibular disorders were evaluated in the context of exposure to earthquake jolts. The control group comprised healthy people who had experienced earthquakes and aftershocks but had no symptoms of dizziness. All individuals' medical histories were thoroughly questioned, and clinical examinations and testing were carried out. Those with known otological illnesses, such as benign paroxysmal positional vertigo, Meniere's disease, and otitis media, as well as those with any psychiatric disorder, those under the age of 18, and those who had suffered head trauma, were excluded from the study. All participants were also questioned about their psychiatric, neurotological, vestibular suppressant drug, and alcohol use. Those who were actively using or had recently used these drugs were excluded from the study. All participants were from Adana, which is near the epicenter of the earthquake, and experienced intense vibrations.
Questionnaires and Vestibular System Assessment
The participants completed a questionnaire that evaluated issues such as environmental conditions, psychosocial characteristics, exposure to disasters, family support, and media influence (Table 1). The Dizziness Handicap Inventory (DHI), Peritraumatic Distress Inventory (PDI), and Hospital Anxiety and Depression Scale (HADS) were implemented (Supplementary Tables 1-3).
Table 1.
Research Survey on Dizziness After the Earthquake
| Q1 | Have you ever experienced an earthquake before? | Yes | No |
| Q2 | Is the house you live in higher than the third floor of the building? | Yes | No |
| Q3 | Do you think you feel the vibration of the earthquake more than other people? | Yes | No |
| Q4 | Were you living alone during the earthquake? | Yes | No |
| Q5 | Do you think you can safely escape from your home in an emergency during an earthquake? | Yes | No |
| Q6 | Have you ever received any treatment for anxiety or depression? | Yes | No |
| Q7 | Is there any people around you to share your troubles with? | Yes | No |
| Q8 | Did you often watch the earthquake footage from news channels and social media? | Yes | No |
| Q9 | Do you believe the rumors circulating after the earthquake? | Yes | No |
Supplementary Table 1.
The Dizziness Handicap Inventory (DHI)
| P1. Does looking up increase your problem? | Yes Sometimes No |
| E2. Because of your problem, do you feel frustrated? | Yes Sometimes No |
| F3. Because of your problem, do you restrict your travel for business or recreation? | Yes Sometimes No |
| P4. Does walking down the aisle of a supermarket increase your problems? | Yes Sometimes No |
| F5. Because of your problem, do you have difficulty getting into or out of bed? | Yes Sometimes No |
| F6. Does your problem significantly restrict your participation in social activities, such as going out to dinner, going to the movies, dancing, or going to parties? | Yes Sometimes No |
| F7. Because of your problem, do you have difficulty reading? | Yes Sometimes No |
| P8. Does performing more ambitious activities such as sports, dancing, household chores (sweeping or putting dishes away) increase your problems? | Yes Sometimes No |
| E9. Because of your problem, are you afraid to leave your home without having someone accompany you? | Yes Sometimes No |
| E10. Because of your problem have you been embarrassed in front of others? | Yes Sometimes No |
| P11. Do quick movements of your head increase your problem? | Yes Sometimes No |
| F12. Because of your problem, do you avoid heights? | Yes Sometimes No |
| P13. Does turning over in bed increase your problem? | Yes Sometimes No |
| F14. Because of your problem, is it difficult for you to do strenuous homework or yard work? | Yes Sometimes No |
| E15. Because of your problem, are you afraid people may think you are intoxicated? | Yes Sometimes No |
| F16. Because of your problem, is it difficult for you to go for a walk by yourself? | Yes Sometimes No |
| P17. Does walking down a sidewalk increase your problem? | Yes Sometimes No |
| E18.Because of your problem, is it difficult for you to concentrate | Yes Sometimes No |
| F19. Because of your problem, is it difficult for you to walk around your house in the dark? | Yes Sometimes No |
| E20. Because of your problem, are you afraid to stay home alone? | Yes Sometimes No |
| E21. Because of your problem, do you feel handicapped? | Yes Sometimes No |
| E22. Has the problem placed stress on your relationships with members of your family or friends? | Yes Sometimes No |
| E23. Because of your problem, are you depressed? | Yes Sometimes No |
| F24. Does your problem interfere with your job or household responsibilities? | Yes Sometimes No |
| P25. Does bending over increase your problem? | Yes Sometimes No |
Used with permission from GP Jacobson. Jacobson GP, Newman CW: The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg 1990;116: 424-427
DHI Scoring Instructions
The patient is asked to answer each question as it pertains to dizziness or unsteadiness problems, specifically considering their condition during the last month. Questions are designed to incorporate functional (F), physical (P), and emotional (E) impacts on disability.
To each item, the following scores can be assigned:
No=0 Sometimes=2 Yes=4
Scores:
Scores greater than 10 points should be referred to balance specialists for further evaluation.
16-34 Points (mild handicap)
36-52 Points (moderate handicap) 54+Points (severe handicap)
Table 3.
Median Values of cVEMP and oVEMP Parameters for Patient Group with Dizziness Compared with Those of Healthy Control Group
|
|
Control (n = 36) | Patient (n = 33) | P a | P b | ||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | |||
| cVEMP | ||||||
| Latency p1 (ms) | 17.0 (14.7-21.0) | 17.2 (14.3-22.0) | 17.4 (13.3-20.3) | 17.2 (14.0-22.0) | .800 | .547 |
| Latency n1 (ms) | 25.6 (21.3-30.7) | 25.9 (22.3-35.7) | 25.6 (22.0-33.0) | 25.9 (22.3-31.0) | .687 | .957 |
| Amplitude asymmetry (%) | 11.0 (3.0-43.0) | 11.0 (3.0-45.0) | .931 | |||
| oVEMP | ||||||
| Latency p1 (ms) | 14.1 (11.3-18) | 13.9 (11.3-18.7) | 14.3 (11.7-19.1) | 14.0 (11.0-19.3) | .757 | .693 |
| Latency n1 (ms) | 19.6 (16.7-25.1) | 19.4 (16.1-26.8) | 19.7 (16.9-25.8) | 19.7 (17.2-25.1) | .859 | .463 |
| Amplitude asymmetry (%) | 10.1 (4.0-36.0) | 10.2 (4.0-35.0) | .871 | |||
Data are expressed as median (minimum–maximum).
a P value for comparison of patient and control groups on the right.
b P value for comparison of patient and control groups on the left.
Dizziness Handicap Inventory
The DHI is a 25-item self-report questionnaire that quantifies the impact of dizziness on daily life by measuring self-perceived handicap. The following scores can be assigned to each item: No = 0, Sometimes = 2, or Yes = 4. Item scores are summed. There is a maximum score of 100 and a minimum score of 0.10
Hospital Anxiety and Depression Scale
The HADS is a 14-item, 4-point Likert-type self-report scale that measures symptoms of anxiety and depression. It consists of anxiety and depression subscales, each comprising 7 questions, and is scored from 0 to 3. The cutoff score for the HADS subscales is 8. The validity and reliability of the Turkish version have been previously demonstrated.11
Peritraumatic Distress Inventory
The PDI is a 13-item self-report scale that measures the stress level experienced during and after a traumatic event. It has 3 subscales: negative emotions, perceived life threat, and bodily arousal. Each item is scored from 0 to 4. The total score is obtained by determining the mean response across all items. Higher scores indicate higher levels of distress. The validity and reliability of the Turkish version have been previously demonstrated.12
For audiological evaluation, tympanometry and pure tone audiometry were performed on all participants. Following this, the participants underwent a complete neurotological examination and vestibular system assessment, which included the tests described below (Supplementary Table 1).
The OTOsuite Vestibular (Software Version: 3.00 Build 1007, Otometrics, Denmark) computer program and special glasses (Type-1085 ICS impulse) with a video camera mounted were employed to perform a video head impulse test (VHIT) and a videonystagmography (VNG) (gaze test, ocular pursuit, saccade test, Dix–Hallpike, and supine roll) test. In this test, the patient wore narrow glasses with a high-speed camera and a mirror to reflect the right eye image, and the fixation target was a 1 m wall point.
Videonystagmography
Spontaneous nystagmus (sitting position with gaze straightforward), positional nystagmus (during the Dix–Hallpike and supine roll maneuvers), gaze-evoked nystagmus, saccadic test, and ocular pursuit test were performed.
Video Head Impulse Test
The study's right-handed operator performed all VHIT exams. Keeping their heads fixed and controlled, the participants calibrated by viewing a laser light alternately on either side of a 1 m-ahead target. To stay concentrated, the subjects were instructed to relax their neck muscles, avoid blinking, and keep their eyes open as long as possible. After calibration, lateral, left anterior right posterior (LARP), and right anterior left posterior (RALP) canal tests were performed. With the head neutral and angled 20° downward, 20 passive lateral head impulses at 10°-20° and 200°/s stimulated the lateral canal. Vertical canal stimulation was performed with the subject's head moved to the left for RALP stimulation and to the right for LARP stimulation at 30°-40° from the LARP and RALP fixation targets. Next, unpredictable passive head movements with 10°-15° amplitude and 150°/s pace were conducted at least 20 times per channel. VHIT analysis relies on vestibulo-ocular reflex (VOR) increase and refixation saccades. Saccades during head thrust were "covert," while those afterward were "overt." The manufacturer's (ICS otometric VHIT) normal VOR gain was 0.8-1.2 for the lateral canal and 0.7-1.2 for LARP/RALP.
Cervical Vestibular Evoked Myogenic Potential Assessment
Cervical vestibular evoked myogenic potential (cVEMP) was measured with a GSI Audera device (Grason-Stadler Inc., MN, USA). The patient sat in a quiet room for the airway tone-burst stimulation test. Regular tonic stimulation of the sternocleidomastoid (SCM) muscle was performed with the patient's head 45° contralateral to the stimulated ear. Electromyography (EMG) responses of the SCM muscle were recorded ipsilaterally via the surface electrode. The active electrode was placed in the middle of the SCM, the reference electrode on the sternoclavicular joint, and the ground electrode on the forehead. The resulting impedance of the recording electrodes was below 5 kΩ. Acoustic stimuli (100 dB nHL and 500 Hz; rate = 5.1/s; rise and fall time = 2 ms; and plateau = 1 ms, duration = 5 ms) were delivered through a headset. Analysis time was 120 ms, and the EMG signal was bandpass filtered from 10 Hz to 750 Hz. Each set of 200 stimuli was averaged and repeated twice to verify response repeatability. The initial waveform created after stimulation had P1 and N1 peaks. Then, the wave delay and amplitude were measured. Positive cVEMP responses had recognizable or reproducible waveforms, while negative responses did not.
Ocular VEMP Assessment
Ocular VEMP (oVEMP) was measured with GSI Audera equipment (Grason-Stadler Inc., MN, USA). The patient sat in a quiet room for the airway tone-burst stimulation test. Surface electrode EMG was collected from the contralateral eye. The patient was requested to look up 30° during the test. The active electrode was positioned 1 cm below the center of the lower eyelid, while the reference electrode was 1 cm below the skin. The ground electrode was placed in the middle of the forehead. The resulting impedance of the recording electrodes was below 5 kΩ. The acoustic stimulus (100 dB nHL and 500 Hz; rate = 5.1/s; rise and fall time = 2 ms; and plateau = 1 ms, duration = 5 ms) was delivered through a headset. Analysis time was 100 ms, and the EMG signal was bandpass filtered from 10 to 750 Hz. Each set of 200 stimuli was averaged and repeated twice to verify response repeatability. The stimulus-induced first waveform peaks were N1 and P1. Then, the wave delay and amplitude were measured. The oVEMP response was considered positive if an identifiable or repeatable waveform was observed and negative otherwise.
The amplitude asymmetry ratio (AR) between a subject's ears was calculated according to the following formula: AR% = |(Right ear EMG normalized amplitude − Left ear EMG normalized amplitude)/(Right ear EMG normalized amplitude + Left ear EMG normalized amplitude)| × 100. Asymmetry ratios of 35% and above were considered asymmetric.
Statistical Analysis
Categorical variables were expressed as numbers and percentages, whereas continuous variables were summarized as mean and standard deviation, and as median and minimum–maximum, where appropriate. A chi-square test was used to compare the categorical variables between the groups. The normality of distribution for continuous variables was confirmed with the Shapiro–Wilk test. For comparison of continuous variables between 2 groups, the student's t-test or Mann–Whitney U-test was used depending on whether the statistical hypotheses were fulfilled. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM SPSS Corp.; Armonk, NY, USA) statistical software package. The statistical level of significance for all tests was considered to be .05.
Results
Our study was a controlled observational study evaluating dizziness in the post-earthquake period. Two groups of participants were included in this study: a patient group with post-earthquake dizziness and a control group of healthy subjects. There was no significant difference between the groups in terms of age and gender distribution. A total of 69 patients, 33 in the patient group and 36 in the control group, with a mean age of 40.1 ± 13.4 (minimum: 21.0, maximum: 68.0), were included in the study. The demographic characteristics and the mean or median values of the survey scores of the study groups are shown in Supplementary Table 4.
Supplementary Table 4.
Demographic characteristics and questionnaire scores of study groups
|
|
Group | P | |
|---|---|---|---|
| Control (n = 36) | Patient (n = 33) | ||
| Age(year) | 36.0 (21.0-68.0) | 42.0 (22.0-64.0) | .471 |
| Gender, n(%) | .267 | ||
| Male | 13 (36.1) | 9 (27.3) | |
| Female | 23 (63.9) | 24 (72.7) | |
| DHI score | 0.0 (0.0-10.0) | 28.0 (12.0-62.0) | <.001 |
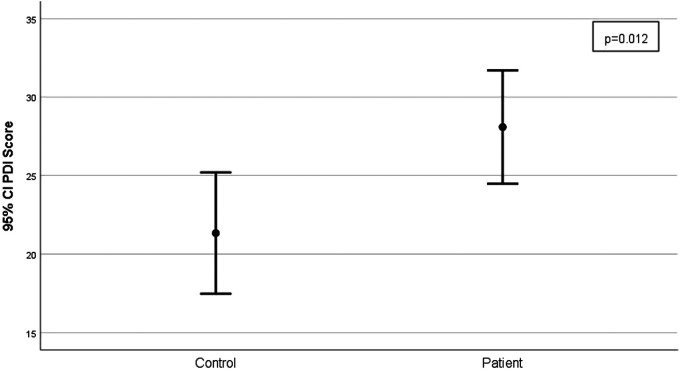
| PDI score | 21.3 ± 11.4 | 28.1 ± 10.2 | .012 |
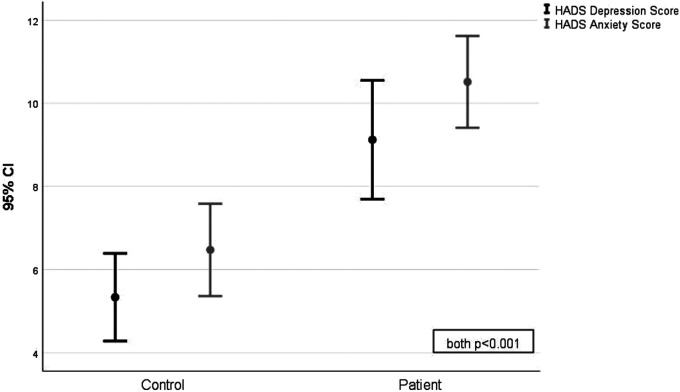
| HADS Depression score | 5.3 ± 3.1 | 9.1 ± 4.0 | <.001 |
| HADS Anxiety score | 6.5 ± 3.3 | 10.5 ± 3.1 | <.001 |
Unless otherwise specified data was expressed as mean±stanard deviation or median (min-max).
DHI: Dizziness handicap inventory, PDI: Peritraumatic distress inventory, HADS: Hospital anxiety and depression scale
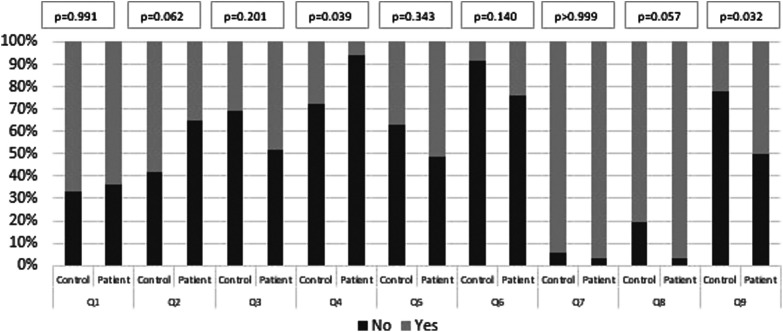
The DHI score was higher in the patient group than in the control group (P < .001). The PDI mean score and the HADS depression and anxiety mean scores were higher in the patient group than in the control group (P = .012, P < .001, and P < .001, respectively; Figures 1 and 2). In addition to completing surveys to assess their psychological state, the subjects underwent an additional survey questioning environmental conditions, social factors, and earthquake experiences (Figure 3). According to this analysis, most patients had experienced an earthquake at least once in their lifetime. Those who experienced it for the first time were in the minority (36.4%). This situation was similar in the control group. No significant difference was observed between the 2 groups (Q1 in Figure 3). Another aspect was that 93.9% of the participants in the patient group were not alone at the time of the earthquake. The status of the participants experiencing the earthquake alone at home was statistically significantly lower than in the control group (P = .039; Q4 in Figure 3).
Figure 1.
Comparison of the mean and standard deviations of the PDI scores of the post-earthquake dizziness patient group and the healthy control group (P = .012). Abbreviation: PDI, peritraumatic distress inventory.
Figure 2.
Comparison of the mean and standard deviations of the HADS depression and anxiety scores of the post-earthquake dizziness patient group and the healthy control group (both P < .001). Abbreviation: HADS, hospital anxiety and depression scale
Figure 3.
The display of the answers given by the participants to the questions (Q1-Q9) in the questionnaire stated in Table 1. The questionnaire administered to the participants assessed their responses to questions pertaining to environmental circumstances, social factors, and earthquake experiences. The chart presented herein displays the percentages of affirmative and negative responses for each question, together with the associated statistical significance levels.
After the earthquake, it was evaluated how the mainstream media, social media content, and rumors in society affected the subjects. The eerie images and portrayals of the earthquake tragedy in the media aroused fear in most (97%) of the patient group. Although this rate was higher than in the control group, it was not statistically significant (P = .057; Q8 in Figure 3). In addition, we observed that rumors spread through social networks or word of mouth negatively affected half of the patient group. This effect was statistically significantly higher than in the control group (P = .032; Q9 in Figure 3).
Spontaneous nystagmus (sitting position with gaze straightforward), positional nystagmus (during the Dix–Hallpike and supine roll maneuvers), and gaze-evoked nystagmus were not detected in either group. The saccadic test and the ocular pursuit test showed no abnormalities in the 2 groups. In the patient group, 3 of the participants had an abnormal sharpened Romberg test, and 1 had an abnormal Fukuda test. No abnormality was observed in the tests of any participants in the control group. Tympanometry was normal in all subjects in the study. Mild sensorineural hearing loss was detected in 9% of the patient group and 11% of the control group.
Table 3 indicates the baseline parameters of the VEMPs for all evaluated subjects. No lack of response for cVEMPs and oVEMPs was observed in either group. The cVEMP and oVEMP responses of all participants were recorded bilaterally. There were no significant differences in VEMPs of both ears for all subjects studied for mean p1-latency, mean n1-latency, and mean percent of amplitude asymmetry between patients and healthy controls (P > .05).
The VOR gain values were measured for the 3 semicircular canals of all subjects investigated with the VHIT. All participants in both groups had a VOR gain in the normal range (0.8-1.2), except for 3 patients in the patient group. Left posterior semicircular canal VOR gains were low (≤0.80) in these 3 patients. There was no statistical difference between the mean VOR gains between the 2 groups, except for the left posterior semicircular canal (P > .05). The right posterior semicircular canal median VOR gain of the patient group was lower than that of the control group, but this was not statistically significant (P = .073). The mean VOR gain of the left posterior semicircular canal was found to be statistically significantly lower in the patient group (P = .02). The distribution of patients and healthy controls according to their VOR gains is presented in Table 2.
Table 2.
Mean or Median Values of VOR Gains of Semicircular Canals in VHIT for Patient Group with Dizziness Compared with Those of Healthy Control Group
|
|
Control (n = 36) | Patient (n = 33) | Pa | Pb | ||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | |||
| Anterior SCC | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | .897 | .793 |
| Lateral SCC | 1.1 (0.9-1.3) | 0.9 (0.9-1.4) | 1.0 (0.9-1.4) | 0.9 (0.8-1.4) | .727 | .665 |
| Posterior SCC | 1.0 (0.9-1.4) | 1.0 ± 0.1 | 0.9 (0.7-1.3) | 0.9 ± 0.1 | .073 | .020 |
Data are expressed as mean ± standard deviation or median (minimum–maximum).
SCC, semicircular canal.
aP value for comparison of patient and control groups on the right.
b P value for comparison of patient and control groups on the left.
Discussion
Major earthquakes are highly destructive natural phenomena that have profound impacts on human populations across various dimensions, including physical, social, and psychological aspects. The inhabitants of our locality were firsthand witnesses to the profound ramifications of the seismic event that occurred in our vicinity, prompting us to engage in empirical observations. Following the earthquakes, there was a notable rise in the volume of individuals seeking medical attention due to post-earthquake-induced dizziness. Studies have documented notable increases in the occurrence of dizziness following severe seismic events in Japan and Nepal.4,5 This observation implies that the observed increase in patients bears a resemblance to the aforementioned scenario. In this controlled observational study, we investigated the potential etiological factors contributing to the manifestation of post-earthquake dizziness by employing a multidimensional approach to comprehensively analyze the phenomenon.
The primary pathology observed in balance disorders is characterized by disturbances in the interplay of the vestibular, neurological, visual, and proprioceptive systems. Nevertheless, it is widely recognized that psychopathological disorders can impact the vestibular system to varying degrees. The observed correlation between the spectra of psychological and vestibular diseases provides additional support for this viewpoint. The literature has highlighted a reciprocal link between psychological and vestibular problems.13 The neuroanatomical linkages between the autonomic and vestibular centers at various levels of the nervous system have been shown to provide a physiological foundation for this interplay.14
It has been observed that psychopathological conditions, such as anxiety disorders,1 mood disorders,15 and post-traumatic stress disorder,16 increase in communities in the post-earthquake period. Physiological and psychological alterations, such as anxiety disorders, can be induced in individuals, particularly in circumstances marked by apprehension and persistent worry. A study examining individuals who survived the Nepal–India earthquake of 2015 revealed that a majority of patients exhibiting post-earthquake equilibrium disorders experienced symptoms associated with anxiety, dread, panic, agoraphobia, or various forms of psychosomatic illness. The researchers postulated that the unexplained symptoms they observed were not associated with vestibular dysfunction. Instead, they proposed that the earthquake either exacerbated an underlying psychosomatic condition or initiated a series of events, leading to the manifestation of dizziness.4 Nevertheless, this study did not comprehensively examine the anxiety and stress levels of the patients, nor did it conduct an objective assessment of their vestibular function.
The concept of PEDS, encompassing symptoms of post-earthquake disequilibrium, was introduced by Nomura et al.8 Miwa et al focused on the potential factors involved in the establishment of PEDS after the 2016 Kumamoto earthquake. They speculated that the disequilibrium caused by the earthquake was more affected by physical stress factors, including sensory disturbances caused by earthquake vibrations, changes in living conditions, and autonomic stress.5 Nonetheless, the absence of a psychological stress assessment and the inability to compare with earthquake-exposed healthy individuals were significant limitations. In the present investigation, individuals who presented with symptoms resembling PEDS underwent a thorough inquiry into their social and environmental circumstances. Additionally, full evaluations of anxiety levels, peritraumatic stress, and mood were conducted. These findings were compared with those of healthy individuals who experienced an earthquake. Based on the findings of the analysis, it was observed that the patient group had significantly higher scores in PDI, HADS depression, and anxiety compared to the control group.
The present study's findings, along with the outcomes of prior research, indicate a reciprocal relationship between the vestibular system and the corticolimbic system, which governs mood and stress-related diseases. The primary factors contributing to post-earthquake equilibrium disorder include heightened levels of stress resulting from a significant seismic event, anticipatory anxiety stemming from subsequent aftershocks, and a prevailing sense of depression due to extensive destruction and deteriorating living conditions. Furthermore, throughout the assessment of participants' individual experiences, as well as their interactions within the environmental and social contexts, certain findings have surfaced that necessitate additional investigation. Our investigation has revealed indications that the dissemination of rumors in society and through social media platforms regarding an impending earthquake can have a detrimental impact on the psychological well-being of individuals, leading to an increase in equilibrium disorders. Furthermore, the inclination of the mainstream media to depict present circumstances through sensationalized storytelling has the potential to intensify this predicament. However, due to the nature of the data collected in this survey, it is not possible to provide a definitive interpretation of the impact of environmental and social interactions on equilibrium disorders. The examination of these causes and the implementation of preventive actions in the future are of significant importance.
The utilization of VEMPs, which assess the utricular and saccular systems, in conjunction with VHIT, which measures the gains of semicircular canals, yields comprehensive insights for the assessment of peripheral vestibular function. No statistically significant differences were seen between the 2 groups in terms of potential latencies or asymmetry between amplitudes in the VEMP test. Furthermore, the patient group did not exhibit peripheral vestibular hypofunction, as defined by a gain of ≤0.80 and/or the presence of saccades, with the exception of 3 patients identified during the VHIT test. Nevertheless, the mean VOR gains in the posterior semicircular channels of the patient group were comparatively lower than those of the control group. However, it is worth noting that these gains still fell within the anticipated range. Nonetheless, the DHI scores of these 3 patients with lower VOR gain were 22, 46, and 48, with a mean DHI score of 38.6. While the mean DHI score of the overall patient group was 28, once these 3 individuals were eliminated, the mean DHI score was 26.9. As a result, patients with low gain in the left posterior semicircular canal (VOR gain ≤ 0.80) had a higher DHI score than those with VOR gain in the usual range (0.8-1.2). However, due to the small number of patients with low VOR gain, it will not be possible to make a definitive conclusion.
A study examined the equilibrium functions of individuals diagnosed with panic disorder using the VHIT and VEMP. The results indicated a lack of supporting evidence for semicircular canal hypofunction and otolith system impairment.17 While there are variations within the patient cohorts, based on the aforementioned results, it is plausible that the diminished VOR gain in the posterior semicircular canals, as reported in the patient group under investigation, can be attributed to the vibrations caused by earthquakes. In a separate investigation concerning individuals who experienced aftershocks following the Japan earthquake, the researchers assessed the participants' balance functions using a stabilometer. The findings of this study substantiate the claim that recurrent exposure to earthquakes leads to dysfunction in the inner ear.9 Due to the utilization of diverse methodologies for evaluating equilibrium functions, it would be inappropriate to engage in a direct comparison. However, similar to the preceding investigation, we posited that the physiological mechanisms of the inner ear were only marginally affected among the individuals who reported experiencing dizziness in our study. Nevertheless, there is uncertainty regarding whether the existing clinical manifestation is a result of this particular influence.
The current study has some limitations. It lacked a third study group with anxiety and stress disorders who had not experienced earthquake vibrations. By establishing a study group, it would become feasible to gain insights into the potential causes of inner ear involvement following an earthquake. Due to the extensive impact of the earthquake on the entire region, it would not be possible to identify suitable subjects who had not been exposed to seismic waves. One additional limitation pertains to the non-uniform distribution of earthquake vibrations across the area, resulting in varying levels of intensity experienced by individuals. In the course of our investigation, we were compelled to exclude this particular circumstance as a result of disparities in geographical locations. The study is an observational case–control study wherein patient treatment and follow-up data have not been incorporated.
The study yielded evidence indicating that post-earthquake dizziness arises from stress and anxiety associated with multiple variables, while the occurrence of inner ear dysfunction resulting from frequent earthquake vibrations contributes to this phenomenon. The results of our study have yielded valuable insights into the phenomenon of post-earthquake dizziness, contributing to a deeper comprehension of this condition and potentially aiding in its effective management. The phenomenon of post-earthquake equilibrium disorder is a significant contributor to morbidity within societies following catastrophic earthquakes, and hence requires inclusion in disaster recovery programs. Given the significant role that stress and anxiety play in the development of diseases, it is crucial to establish a comprehensive healthcare approach that addresses the psychological well-being of those who have experienced earthquakes.
Supplementary Table 2.
Peritraumatic distress inventory (PDI)
| Not at all true | Slightly true | Somewhat true | Very true | Entirely true | ||
|---|---|---|---|---|---|---|
| 1 | I felt helpless to do more | |||||
| 2 | I felt sadness and grief | |||||
| 3 | I felt frustrated or angry | |||||
| 4 | I felt afraid for my own safety | |||||
| 5 | I felt guilty for not doing more | |||||
| 6 | I felt ashamed of my emotional reactions | |||||
| 7 | I felt worried about the safety of others | |||||
| 8 | I had the feeling I was about to lose control of my emotions | |||||
| 9 | I had difficulty controlling my bowel and bladder | |||||
| 10 | I was horrified by what I saw | |||||
| 11 | I had physical reactions like sweating, shaking, and my heart pounding | |||||
| 12 | I felt I might pass out | |||||
| 13 | I thought I might die |
Supplementary Table 3.
Hospital anxiety and depression scale
|
Choose the answers that is closest to how you have been feeling in the past week. Don't take too long over you replies: your immediate is best.
1) I feel tense or 'wound up': 3) most of the time 2) a lot of the time 1) from time to time, occasionally 0) not at all 2) I still enjoy the things I used to enjoy: 0) definitely as much 1) not quite so much 2) only a little 3) hardly at all 3) I get a sort of frightened feeling as if something awful is about to happen: 3) very definitely and quite badly 2) yes, but not too badly 1) a little, but it doesn't worry me 0) not at all 4) I can laugh and see the funny side of things: 0) as much as I always could 1) not quite so much now 2) definitely not so much now 3) not at all 5) Worrying thoughts go through my mind: 3) a great deal of the time 2) a lot of the time 1) from time to time, but not too often 0) only occasionally 6) I feel cheerful: 3) not at all 2) not often 1) sometimes 0) most of the time 7) I can sit at ease and feel relaxed: 0) definitely 1) usually 2) not often 3) not at all |
|
8) I feel as if I am slowed down: 3) nearly all the time 2) very often 1) sometimes 0) not at all 9) I get a sort of frightened feeling like 'butterflies' in the stomach: 0) not at all 1) occasionally 2)quite often 3) very often 10) I have lost interest in my appearance: 3) definitely 2) I don't take as much care as I should 1) I may not take quite as much care 0) I take just as much care as ever 11) I feel restless as I have to be on the move: 3) very much indeed 2) quite a lot 1) not very much 0) not at all 12) I look forward with enjoyment to things: 0) as much as I ever did 1) rather less than I used to 2) definitely less than I used to 3) hardly at all 13) I get sudden feelings of panic: 3) very often indeed 2) quite often 1) not very often 0) not at all 14) I can enjoy a good book or radio or TV program: 0) often 1) sometimes 2) not often 3) very seldom |
Please check you have answered all the questions.
Blue boxes are for anxiety scores, rest are for depression scores.
Total score: Anxiety (__) Depression (__)
0-7 = Normal
8-10 = Borderline abnormal (borderline case)
11-21 = Abnormal (case)
Funding Statement
The authors declare that this study received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of Çukurova University (Approval Number: 132-26; Date: April 7, 2023).
Informed Consent: Informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – C.E., M.D.; Design – C.E., M.D., E.C., Z.N., E.O.; Supervision – M.D.,O.S., Z.N.; Resources – C.E., M.D., O.S., A.A.; Materials – C.E., M.D., O.S., A.A.; Data Collection and/or Processing – C.E., M.D., O.S., E.C., A.A., Z.N., S.P.Y.K., E.O.; Analysis and/or Interpretation – C.E., O.S., E.C., A.A., S.P.Y.K., E.O.; Literature Search – C.E., M.D., A.A., Z.N.; Writing Manuscript – C.E., M.D., O.S., E.C., A.A., Z.N., S.P.Y.K., E.O.; Critical Review – C.E., M.D., O.S, E.C., Z.N.
Declaration of Interests: The authors have no conflicts of interest to declare.
References
- 1. Ehring T, Razik S, Emmelkamp PM. Prevalence and predictors of posttraumatic stress disorder, anxiety, depression, and burnout in Pakistani earthquake recovery workers. Psychiatry Res. 2011;185(1-2):161 166. ( 10.1016/j.psychres.2009.10.018) [DOI] [PubMed] [Google Scholar]
- 2. Varela E, Koustouki V, Davos CH, Eleni K. Psychological consequences among adults following the 1999 earthquake in Athens, Greece. Disasters. 2008;32(2):280 291. ( 10.1111/j.1467-7717.2008.01039.x) [DOI] [PubMed] [Google Scholar]
- 3. Kario K, McEwen BS, Pickering TG. Disasters and the heart: a review of the effects of earthquake-induced stress on cardiovascular disease. Hypertens Res. 2003;26(5):355 367. ( 10.1291/hypres.26.355) [DOI] [PubMed] [Google Scholar]
- 4. Kumar V, Bhavana K. Post earthquake equilibrium disturbance: A study after Nepal-India earthquake 2015. Indian J Otolaryngol Head Neck Surg. 2019;71(suppl 2):1258 1265. ( 10.1007/s12070-018-1296-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miwa T, Matsuyoshi H, Nomura Y, Minoda R. Post-earthquake dizziness syndrome following the 2016 Kumamoto earthquakes, Japan. PLoS One. 2021. 5;16(8):e0255816. ( 10.1371/journal.pone.0255816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tevzadze N, Shakarishvili R. Vertigo syndromes associated with earthquake in Georgia. Georgian Med News. 2007;(148-149):36 39 [PubMed] [Google Scholar]
- 7. Miwa T. Vestibular function after the 2016 Kumamoto earthquakes: A retrospective chart review. Front Neurol. 2021;11:626613. ( 10.3389/fneur.2020.626613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomura Y, Toi T. Post earthquake dizziness syndrome. Equilib Res. 2014;73:167 173. ( 10.1371/journal.pone.0255816) [DOI] [Google Scholar]
- 9. Honma M, Endo N, Osada Y, Kim Y, Kuriyama K. Disturbances in equilibrium function after major earthquake. Sci Rep. 2012;2:749. ( 10.1038/srep00749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham MK, Staab JP, Lohse CM, McCaslin DL. A comparison of dizziness handicap inventory scores by categories of vestibular diagnoses. Otol Neurotol. 2021;42(1):129 136. ( 10.1097/MAO.0000000000002890) [DOI] [PubMed] [Google Scholar]
- 11. Aydemir Ö, Güvenir T, Küey L. Validity and realibility of Turkish version of Hospital Anxiety and Depression Scale. Turk J Psychiatry. 1997;8:280 287. [Google Scholar]
- 12. Ermağan Çağlar E, Sanal Özcan Y, Türk Kurtça T, Hamzaoğlu N. Validity and reliability of the Turkish version of Peritraumatic Distress Inventory (PDI). Trakya Univ J Soc Sci. 2022;24(1):23 42. [Google Scholar]
- 13. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15(1-2):9 26. ( 10.1016/S0887-6185(00)00040-2) [DOI] [PubMed] [Google Scholar]
- 14. Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77(4-5):469 475. ( 10.1016/s0031-9384(02)00935-6) [DOI] [PubMed] [Google Scholar]
- 15. Anwar J, Mpofu E, Matthews LR, Shadoul AF, Brock KE. Reproductive health and access to healthcare facilities: risk factors for depression and anxiety in women with an earthquake experience. BMC Public Health. 2011;11:523. ( 10.1186/1471-2458-11-523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato H, Asukai N, Miyake Y, Minakawa K, Nishiyama A. Post-traumatic symptoms among younger and elderly evacuees in the early stages following the 1995 Hanshin-Awaji earthquake in Japan. Acta Psychiatr Scand. 1996;93(6):477 481. ( 10.1111/j.1600-0447.1996.tb10680.x) [DOI] [PubMed] [Google Scholar]
- 17. Angov G, Mihaylova-Angelova E, Petrova D, Stambolieva K. Vestibular function in panic disorder patients: a vestibular-evoked myogenic potentials and video head impulse test study. Eur Arch Otorhinolaryngol. 2019;276(6):1607 1616. ( 10.1007/s00405-019-05398-5) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a