Abstract
Aim:
Several studies have found subcutaneous (SC) and intravenous (IV) administration of similar drugs for long-lasting immunological and autoimmune diseases to have similar clinical effectiveness, meaning that what patients report they prefer is, or should be, a major factor in treatment choices. Therefore, it is important to systematically compile evidence regarding patient preferences, treatment satisfaction and health-related quality of life (HRQL) using SC or IV administration of the same drug.
Materials & methods:
PubMed database searches were run on 15 October 2021. Studies involving patients with experience of both home-based SC and hospital-based IV administration of immunoglobulins or biological therapies for the treatment of any autoimmune disease or primary immunodeficiencies (PIDs) were included. The outcomes assessed were patient preferences, treatment satisfaction and HRQL. Preference data were meta-analyzed using a random-effects model.
Results:
In total, 3504 citations were screened, and 46 publications describing 37 studies were included in the review. There was a strong overall preference for SC over IV administration, with similar results seen for PIDs and autoimmune diseases: PID, 80% (95% confidence interval [CI], 64–94%) preferred SC; autoimmune diseases, 83% (95% CI: 73–92%); overall, 82% (95% CI: 75–89%). The meta-analysis also found that 84% (95% CI: 75–92%) of patients preferred administration at home to treatment in hospital. Analysis of treatment satisfaction using the life quality index found consistently better treatment interference and treatment setting scores with SC administration than with IV administration.
Conclusion:
Compared with IV infusions in hospital, patients tend to prefer, to be more satisfied with and to report better HRQL with SC administration of the same drug at home, primarily due to the greater convenience. This study contributes to evidence-based care of patients with autoimmune diseases or PIDs.
Keywords: administration route, intravenous, meta-analysis, patient preference, quality of life, subcutaneous, systematic literature review, treatment satisfaction
Plain language summary
What is this article about?
Where two therapies have similar effectiveness, patient preferences, satisfaction with treatment and health-related quality of life (a patient's general perception of the effect of the illness and treatment on the physical, psychological and social aspects of their life) are important factors in treatment choices. This literature review sought to compile the relevant published evidence concerning the choice of subcutaneous administration (injection/infusion in the fat under the skin) at home or intravenous infusion (administration directly into a vein) of the same drug in hospital. In particular, data for autoimmune disease or primary immunodeficiencies were investigated.
What were the results?
A total of 37 studies were included in the review. The results showed that, compared with intravenous treatment in hospital, patients tend to prefer subcutaneous administration of the same drug at home. Similar results were seen for both autoimmune disease and primary immunodeficiencies. Most patients in the included studies also preferred administration at home to treatment in hospital, independently of preferences for administration route. In addition, patients were consistently more satisfied with subcutaneous treatment at home, compared with intravenous treatment in hospital, primarily due to the greater convenience.
What do the results mean?
The results of this review show that patients prefer subcutaneous administration at home to intravenous administration in hospital. These findings contribute to evidence-based care of patients with autoimmune diseases or primary immunodeficiencies.
A number of parenteral drugs can either be administered as subcutaneous (SC) injections/infusions or as intravenous (IV) infusions. One of the most commonly used SC infusion therapies is SC immunoglobulins (SCIg), used for both antibody deficiencies and autoimmune diseases [1]. SCIg is today considered in most countries to be the first-choice administration route for immunoglobulin therapy for primary immunodeficiency (PID) [1,2]. A number of monoclonal antibodies can also be administered by IV infusion or by SC injection. These include the autoimmune disease treatments infliximab, alemtuzumab and tocilizumab [3–5].
Several studies have found similar clinical effectiveness for the SC and IV versions of therapies – including both immunoglobulins and antibody treatments – for immunological and autoimmune diseases [5–8]. Accordingly, for an individual patient, the choice of administration route may depend on their preferences and family situation, rather than being specific to their disease. In addition to patient preference, treatment satisfaction and health-related quality of life (HRQL) are major considerations when choosing treatments. Therefore, it is important to understand not only patients' overall preferences for SC or IV administration, but also to compile the patient-reported benefits of each administration route that underly these preferences.
SC therapies for autoimmune diseases and PID can most often, after education and training of adult patients or children with their parents, be administered by patients or families themselves, rather than by healthcare providers. In addition, administration can typically take place at home, rather than in hospital. The potential for home self-administration of treatments for chronic disorders may benefit healthcare systems and patients. For example, home SCIg administration has been found to reduce nurse time and healthcare system costs in Europe, North America and Australia [9–12]. For patients, the benefits of home self-administration include improved HRQL, treatment satisfaction, convenience and comfort [13,14]. In addition, home self-administration may allow patients to avoid having to attend hospital and potentially take a day off work or school, leading to reductions in out-of-pocket travel and visiting costs and income losses [15,16].
The aim of this systematic literature review (SLR) and meta-analysis is to compile evidence regarding patient preferences, treatment satisfaction and HRQL for SC administration at home or IV administration of the same drug in hospital. Because patient preferences for treatment administration route may differ according to the duration of their therapy, treatments for oncological diseases were not included (as shown in the search strategy, Supplementary Table 1), with the SLR and meta-analysis focusing on long-term treatments.
Methods
Systematic literature review
An SLR was performed to identify relevant evidence. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) reporting guidelines were followed throughout [17].
Eligibility criteria
Inclusion and exclusion criteria are shown in full in Supplementary Table 2, according to the PICOS (Participants, Intervention, Comparator, Outcomes and Study design) approach. In brief, studies were selected as follows:
Participants, adult patients with any autoimmune disease or PID.
Intervention, long-term use of immunoglobulins, monoclonal antibodies, abatacept or romiplostim, administered by home-based SC or hospital-based IV administration.
Comparator, long-term use of the same compound administered by the alternative delivery route (home-based SC or hospital-based IV administration) to the intervention.
Outcomes, patient preferences and patient-reported outcomes (PROs) assessing treatment satisfaction and HRQL.
Study designs, clinical trials or observational studies including least five adult patients (or reporting pooled results for adults and children); any study design could be included, provided other inclusion criteria were met.
If studies included groups of patients treated with IV therapies at home, these groups were excluded from the analysis to avoid complicating the interpretation of the meta-analysis. Studies published in languages other than English were excluded.
Information sources
Searches of the PubMed database were conducted on 15 October 2021 using the National Library of Medicine Esearch and Efetch application programming interfaces.
Search strategy
Searches were designed to identify clinical trials or observational studies comparing SC and IV routes of administration for PID and autoimmune diseases, identified using terms for individual indications and for relevant therapies. Search terms are described in Supplementary Table 1.
Selection process
Duplicate citations were removed using Microsoft Excel (Microsoft Corporation, WA, USA). Titles and abstracts were screened by a single reviewer to assess the potential relevance of the study, according to strict, predefined inclusion and exclusion criteria (as described above and in Supplementary Table 2). For potentially relevant citations, full-text articles were obtained and reviewed. The reference lists of all references included in the SLR were checked for additional potentially relevant references that may not have been captured by the initial search.
Data collection process & data items
For the included studies, data were extracted into predefined tables. Patient preference data were captured from studies that asked patients which administration route they preferred, or which they would like to continue, after trying both options. For the 36-item short-form health survey (SF-36), only overall scores and Physical and Mental Component Summary scores (PCS and MCS), rather than individual domain scores, were included. All extracted data were checked for accuracy by a second researcher.
Effect measures
Measures assessed were percentages of patients (for preference and treatment satisfaction data) and patient-reported outcome instrument scores (for HRQL data). Results in individual studies were considered to be statistically significant if a p-value < 0.05 was reported, or if the result was described as statistically significant in the original papers.
Synthesis methods
Patient preference data were meta-analysed with MetaXL (EpiGear International Pty Ltd). In the analysis of the proportion of patients preferring SC therapies, preferences were considered dichotomously: patients reporting ‘no preference’ were considered not to prefer SC administration. Consistent with previous meta-analyses of preference data, a random-effects model was used [18]. Heterogeneity was assessed using the Q statistic and the I2 index [19].
Results
Study selection
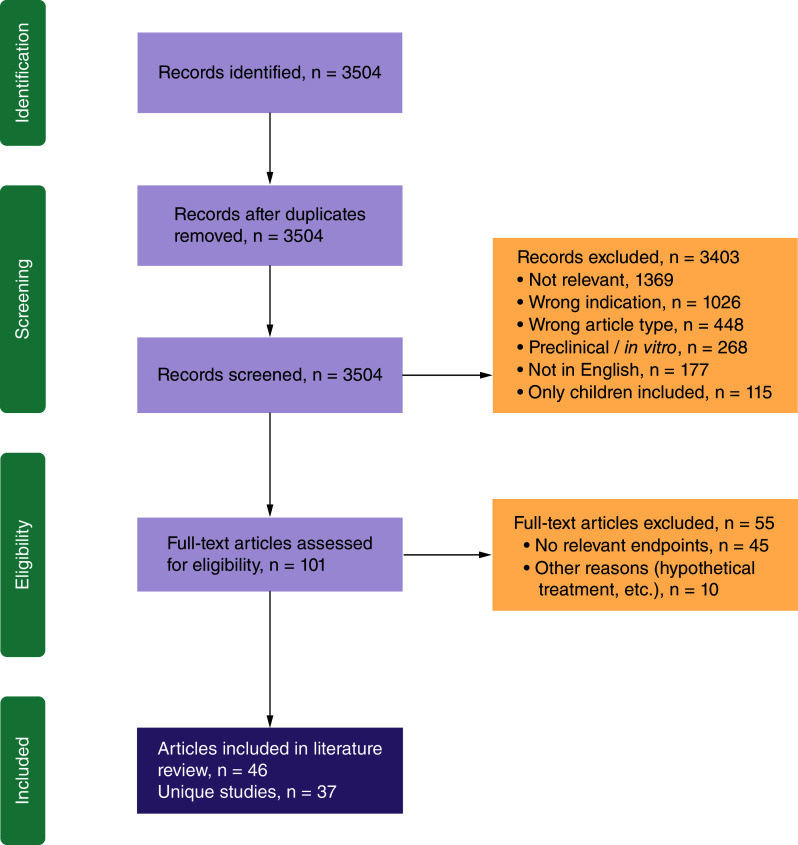
The study selection process is shown in Figure 1. In total, 3504 citations were screened, and full-text versions of 101 were reviewed. After full-text review, 46 publications corresponding to 37 studies were included in the SLR [7,14,20–63].
Figure 1. . PRISMA diagram.
PRISMA: Preferred reporting item for systematic review and meta-analysis.
Study characteristics
Of the 37 studies included in the SLR (Table 1), 33 investigated SC and IV administration of immunoglobulins [7,14,20–29,32–51,54–56,58–63]. The most common indications were PID (14 studies) [14,21,22,24,32,35,43,45,46,48,54,55,58,62], chronic inflammatory demyelinating polyneuropathy (CIDP, six studies) [7,27–29,38,42,60,61] and multifocal motor neuropathy (MMN, six studies) [27,28,33,37,41,47,51,56] (including one study [27,28] that reported data separately for CIDP and MMN groups). Additional studies reported data for populations of patients with a mix of CIDP and MMN (two studies) [26,40], myasthenia gravis (MG, two studies) [20,23], polymyositis and dermatomyositis (one study) [25] or mixed autoimmune indications (three studies) [39,59,63]. A further two studies investigated belimumab for systemic lupus erythematosus (SLE) [31,53,57] while one study each assessed abatacept [52] and tocilizumab [30] for rheumatoid arthritis (RA).
Table 1. . Summary of included studies.
| Study | Study design | Indication and therapy | IV treatment and setting | SC treatment and setting | Patient population | Relevant end points | Ref. |
|---|---|---|---|---|---|---|---|
| Alcantara et al., 2021 Canada |
Retrospective study; Switch design; Patients who had been switched from IV to SC | MG; Ig | IVIg 1 g/kg every 3–4 weeks for a mean of 21.8 (range: 3–64) months; hospital | SCIg mean of 31.4 (range: 15–80) g/week for a mean of 19.5 (range: 5–45) months; home | n = 34; adults only; mean age, 58.9 years | MGII; Patient-reported “Percentage of normal” (0–100%) | [14] |
| Bienvenu et al., 2016 [VISAGES] France |
Observational study; Switch design; Patients received IVIg at study start, some switched to SCIg at any time within observation period | PID; Ig | IVIg, dose and duration NR; hospital | SCIg, dose and duration NR; home | n = 10; adults and adolescents | LQI; SF-36 | [15] |
| Borte et al., 2017 Meckley et al., 2020 [NCT01412385] Europe |
Interventional – Phase II/III; Switch design; IV prior to study, standardized IV schedule at study start (for 13 weeks) followed by SC (for 52 weeks) | PID; Ig | IVIg (Gammagard liquid/Kiovig, 10%) every 3–4 weeks for 13 weeks; dose NR; hospital | SCIg (20%) weekly for 52 weeks; dose NR; home | n = 30; all age groups | LQI; Patient preference | [16,45] |
| Bourque et al., 2016 Canada |
Retrospective Study; Switch design; Patients who had been switched from IV to SC | MG; Ig | IVIg, mean of 18.3 (range: 13.8–25) g/week, duration NR; hospital | SCIg (Hizentra), mean of 24.3 (range: 16–30) g/week 1–2 times a week for a mean of 6.8 (range: 2–11) months; home | n = 6; adults only; mean age, 41.5 years | MG-QOL 15; Overall treatment satisfaction score (0–10) | [17] |
| Chapel et al., 2000 Europe |
Interventional; Cross-over | PID; Ig | IVIg mean of 629 mg/kg/month (Sweden) or 414 mg/kg/month (UK) 2 times a month for 12 months; hospital | SCIg mean of 632 mg/kg/month (Sweden) or 494 mg/kg/month (GB) 3–4 times a month for 12 months; home | n = 30; adults only; mean age, 44 years | Patient preference | [18] |
| Chérin et al., 2020 France |
Survey/Interview; Switch design; Patients having experience with both IV and SC RoA | Dermatomyositis, polymyositis; Ig | IVIg, dose and duration NR; hospital | SCIg (Gammanorm), dose and duration NR; home | n = 6; adults only; mean age, 52.5 years | Patient preference | [19] |
| Christiansen et al., 2018 [NCT02111590] Denmark |
Observational study; Switch design; IV prior to study, standardized IV schedule at study start (for 8 weeks) followed by SC (for 12 weeks) | CIDP, MMN; Ig | IVIg (Privigen, 10%) mean of 24.2 (range: 12.5–50.0) g/week for 8 weeks; hospital | SCIg (Gammanorm, 16.5%) 2–3 times a week for 12 weeks; dose NR; home | n = 17; adults only; mean age, 60 years | EQ-5D-5L | [20] |
| Cocito et al., 2011 Italy |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | CIDP; Ig | IVIg mean of 64 (range: 50–80) g/kg/month for ≥12 months; hospital | SCIg (Vivaglobin) mean of 64 (range: 50–80) g/kg/month for 6 months; home | n = 5; adults only | LQI; Patient preference; SF-36 | [23] |
| Cocito et al., 2014 Cocito et al., 2016 Italy |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | CIDP, MMN; Ig | IVIg 1–2 g/kg/month every 2–5 weeks for ≥6 months; hospital | SCIg (16% or 20%) 1–3 times/week for a mean of 24.2 (range: 6–65) months; dose NR; home | n = 66; adults only; mean age, 56.7 years | LQI | [21,22] |
| Darloy et al., 2019 [RoSwitch] France |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | RA; tocilizumab | Tocilizumab 7.2 mg/kg every 4 weeks for a mean of 35 months; hospital | Tocilizumab 162 mg weekly for 12 months; home (assumed) | n = 94; adults only | Patient preference | [24] |
| Desai et al., 2009 USA |
Interventional – pilot study; Cross-over | PID; Ig | IVIg monthly for 6 months; dose NR; hospital | SCIg (Gamunex, 10%) weekly for 6 months; dose NR; home | n = 11; all age groups; mean age, 29 years | Patient preference | [26] |
| Eftimov et al., 2009 Netherlands |
Interventional – pilot study; Switch design; IV prior to study start, then switch to SC at study start | MMN; Ig | IVIg mean of 0.46 g/kg/month for ≥6 months; hospital | SCIg (GammaQuin) weekly for 6 months; dose NR; home | n = 5; adults only; mean age, 57 years | LQI; Patient preference; SF-36 | [27] |
| Gardulf et al., 1995 Europe |
Interventional (assumed); Switch design; SC treatment prior to study start but experienced with IV and/or IM RoA prior to SC | PID; Ig | IVIg or IMIg, dose and duration NR; hospital | SCIg 220–465 mg/month 1–4 times a week for a mean of 36 (range: 5–116) months; home | n = 112; adults only | Patient preference | [28] |
| Gardulf et al., 2004 Gardulf et al., 2008 Europe, South America |
Interventional (assumed); Switch design; IV or SC prior to study, continue or switch to SC at study start | PID; Ig | IVIg for ≥6 months; dose NR; hospital | SCIg (16%) 50–150 mg/kg weekly for 10 months; home | n = 22; adults and adolescents | LQI; SF-36; Patient preference | [29,30] |
| Gentile et al., 2020 Italy |
Retrospective study; Switch design; Patients who had been switched from IV to SC | CIDP; Ig | IVIg monthly for a mean of 39.6 (range: 6–132) months; dose NR; hospital | SCIg 18.5 (range: 9.6–30) g/week for a mean of 57.6 (range: 24–84) months; home | n = 17; adults only; mean age, 59 years | LQI; Patient preference | [31] |
| Gentile et al., 2021 Italy |
Retrospective study; Switch design; Patients who had been switched from IV to SC | MMN; Ig | IVIg monthly for a mean of 78 (range: 12–228) months; dose NR; hospital | SCIg mean of 21.7 (range: 20–30) g/week for a mean of 77.3 (range: 54–96) months; home | n = 8; adults only; mean age, 55.9 years | LQI | [32] |
| Gingele et al., 2021 Germany |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | CIDP; Ig | IVIg mean of 21.5 g/week monthly for a median of 20 months; hospital | SCIg weekly for 6 months; dose NR; home (assumed) | n = 41; adults only; mean age, 60 years | Patient preference; Treatment satisfaction | [33] |
| Hachulla et al., 2017 France |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | Various autoimmune diseases; Ig | IVIg for a mean of 67.4 months; dose NR; hospital | SCIg 9.6–60 g/week 1–2 times a week for a mean of 5.7 months; home | n = 23; adults only; mean age, 51.1 years | SF-36 | [34] |
| Hadden et al., 2015 UK |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | CIDP, MMN; Ig | IVIg (various brands) mean of 16.8 (range: 8.0–24.0) g/week for a mean of 98.4 (range: 13.2–231.6) months; hospital | SCIg (various brands) mean of 17.2 (range: 7.3–24.0) g/week weekly for a mean of 33 (18–64) months; home | n = 8; adults only; mean age, 57.4 years | Treatment satisfaction; Patient preference | [35] |
| Harbo et al., 2009 [NCT00268788] Denmark |
Interventional – Phase II; Cross-over | MMN; Ig | IVIg (Endobulin) mean of 1.66 (range: 1.0–2.1) g/kg/week every 4–6 weeks for 3 treatment intervals; hospital | SCIg (Subcuvia) mean of 21.0 (range: 12.8–24.8) g/week 2–3 times per week for a mean of 2.8 (range: 1.2–3.7) months; home | n = 9; adults only; mean age, 49.2 years | Patient preference; SF-36 | [36] |
| Hoffmann et al., 2010 Germany |
Observational study; Switch design; IVIg- or Ig-naive prior to study start, then switch to SC at study start | PID; Ig | IVIg, dose and duration NR; hospital | SCIg (Vivaglobin, 16%), dose and duration NR; home | n = 24; adults and adolescents | Patient preference; SF-36 | [38] |
| Jolles et al., 2011 Mallick et al., 2018 [NCT00542997] Europe |
Interventional – Phase III; Switch design; IV or SC prior to study start, then wash-in/wash-out period with switch to SC Hizentra, then efficacy period | PID; Ig | IVIg mean of 131.5 mg/kg/week for ≥6 months; hospital | SCIg (Hizentra, 20%) weekly for 9.3 months; dose NR; home | n = 27; all age groups; mean age, 24.3 years | SF-36; TSQM | [40,44] |
| Kanegane et al., 2014 Igarashi et al., 2014 Mallick et al., 2018 [NCT01199705] Japan |
Interventional – Phase III; Switch design; IV prior to and at study start, then wash-in/wash-out period with switch to SC, followed by efficacy period | PID; Ig | IVIg 77.3 (range: 21.5–144.3) mg/kg every 3–4 weeks for 3 cycles (at study start); hospital | SCIg (Hizentra, 20%) mean of 87.8 (range: 26.7–172.7) mg/kg/week weekly for 5.6 months; home | n = 24; all age groups; mean age, 17.5 years | LQI | [39,41,44] |
| Katzberg et al., 2016 Rasutis et al., 2017 Canada |
Interventional (assumed); Switch design; IV prior to study start, then switch to SC at study start | MMN; Ig | IVIg mean of 1.15 (range: 0.3–2.0) g/kg/month for ≥2 months; hospital | SCIg (Hizentra, 20%) 1–2 times a week for 6 months; dose NR; home | n = 15; adults only; mean age, 52.2 years | HUI-QoL; Treatment satisfaction | [42,51] |
| Latysheva et al., 2020 [NCT03988426] Russia |
Interventional – Phase III; Switch design; IV prior to study start, wash-in/wash-out period with switch to SC at study start, followed by efficacy period | PID; Ig | IVIg 0.2–0.8 g/kg/month for ≥9 weeks; hospital | SCIg (Octanorm) mean of 0.11 g/kg/week weekly for 8 months; home (assumed) | n = 25; adults only; mean age, 35.2 years | SF-36 | [43] |
| Misbah et al., 2011 [NCT00701662] Europe |
Interventional – Phase II; Switch design; IV prior to study start, wash-in/wash-out period with switch to SC at study start, followed by efficacy period | MMN; Ig | IVIg mean of 1.2 (range: 0.4–1.9) g/kg/month for ≥12 weeks; hospital | SCIg (Vivaglobin) mean of 271.8 (range: 100–488) mg/kg/week weekly for 5.6 months; home | n = 8; adults only; mean age, 57.25 years | LQI; Patient preference; HRQL questionnaire | [46] |
| Monti et al., 2015 Italy |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | RA; abatacept | Abatacept for a mean of 14.4 months; dose NR; hospital | Abatacept for 6 months; dose NR; home | n = 21; adults only; mean age, 60.9 years | Patient preference | [47] |
| Mucke et al., 2019 Germany |
Observational study; Switch design; IV prior to study start, then switch to SC at study start | SLE; belimumab | Belimumab 10 mg/kg monthly for a median of 48 months; hospital | Belimumab 200 mg weekly for 6 months; home (assumed) | n = 9; adults only; mean age, 45 years | Patient preference; Treatment satisfaction | [48] |
| Nicolay et al., 2005 Europe, South America |
Observational study; Switch design; IV or SC prior to study start, then switch to interventional SC at study start | PID; Ig | IVIg monthly for ≥6 months; dose NR; hospital | SCIg (16%) 50–150 mg/kg/week weekly for 10 months; home | n = 39; all age groups | LQI | [49] |
| Nicolay et al., 2006 Gardulf et al., 2008 North America |
Observational study; Switch design; IV prior to study start (group A: hospital-based; group B: home-based), then switch to SC at study start | PID; Ig | IVIg for ≥4 months; dose NR; hospital | SCIg (Vivaglobin, 16%) median of 152 mg/kg/week weekly for 12 months; home | n = 28; adults only; mean age, 36.1 years | LQI; Patient preference; Treatment satisfaction; SF-36 | [29,50] |
| Sheikh et al., 2016 Dashiell-Aje et al., 2018 [NCT02124798] USA |
Interventional – Phase II; Switch design; IV prior to study start, then switch to SC (with an autoinjector) at study start | SLE; belimumab | Belimumab (70% of patients for >1 year); dose NR; hospital | Belimumab 200 mg weekly for 8 weeks; home | n = 43; adults only; mean age, 46.2 years | Treatment satisfaction; Patient preference | [25,52] |
| Suez et al., 2016 Meckley et al., 2020 [NCT01218438] North America |
Interventional – Phase II/III; Switch design; SC or IV prior to study, IV at study start (period 1, 13 weeks), followed by SC (period 2–4, total 58 weeks) | PID; Ig | IVIg (10%) every 3–4 weeks for 3 months; dose NR; hospital | SCIg (20%) weekly for 13.5 months; dose NR; home | n = 68; all age groups | LQI; Treatment satisfaction | [45,53] |
| Suleman et al., 2019 Canada |
Retrospective study; Switch design; Patients who had been switched from IV to SC | Various autoimmune diseases; Ig | IVIg mean of 23.3 (range: 12.5–56.7) g/week for a mean of 31.5 (range: 4–98) months; hospital | SCIg (Hizentra, 20%) mean of 26.2 (range: 12–60) g/week 1–3 times a week for 12 months; home | n = 19; adults only; mean age, 54 years | Patient preference | [54] |
| van Schaik et al., 2018 van Schaik et al., 2019 Hartung et al., 2020 [NCT01545076 PATH study] Europe, North America, East Asia, Australia, Israel |
Interventional – Phase III; Switch design; IV prior to study, IV at study start (period 1 & 2, total 25 weeks) followed by SC (period 3, 24 weeks) | CIDP; Ig | IVIg (Privigen, 10%) 1 g/kg every 3 weeks for 13 weeks; hospital | SCIg (20%) 0.2 (LD) or 0.4 (HD) g/kg/week weekly for 24 weeks; home | n = 57; adults only; mean age, 58.9 years | EQ-VAS; Patient preference; EQ-5D; TSQM | [10,37,55] |
| Vu et al., 2021 [NCT02465359] USA |
Interventional; Switch design; IV prior to study start, then switch to SC at study start | CIDP; Ig | IVIg for a mean of 11.4 (range: 5–69) months; hospital | SCIg (Hizentra, 20%) mean of 0.38 g/kg/week weekly for 6 months; home (assumed) | n = 15; adults only; mean age, 54.5 years | CAP-PRI; SF-36; TSQM | [56] |
| Vultaggio et al., 2015 [VISPO] Italy |
Observational study; Switch design; IV or SC prior to study start, then switch to SC (Vivaglobin) at study start | PID; Ig | IVIg, dose and duration NR; hospital | SCIg (Vivaglobin, 16%) weekly for 24 months; dose NR; home | n = 50; all age groups; mean age, 31.7 years | LQI; SF-36 | [57] |
| Yoon et al., 2015 Germany |
Retrospective Study; Switch design; Patients who had been switched from IV to SC | Various autoimmune diseases; Ig | IVIg 0.3–0.75 g/kg/month, duration NR; hospital | SCIg 0.3–0.75 g/kg/month for a mean of 39 months; home | n = 6; adults only; mean age, 56.5 years | Patient preference | [58] |
CAP-PRI: Chronic acquired polyneuropathy patient-reported Index; CIDP: Chronic inflammatory demyelinating polyneuropathy; EQ-5D: 5-level EuroQol questionnaire; EQ-5D-5L: 5-dimension: 5-level EuroQol questionnaire; EQ-VAS: EuroQol visual analogue scale; HD: High dose; HUI-QoL: Health utility index – quality of life; Ig: Immunoglobulin; IM: Intramuscular; IMIg: Intramuscular immunoglobulin; IV: Intravenous; IVIg: Intravenous immunoglobulin; LD: Low dose; LQI: Life quality index; MG: Myasthenia gravis; MGII: Myasthenia gravis impairment index; MG-QOL 15: 15-item Myasthenia Gravis Quality of Life questionnaire; MMN: Multifocal motor neuropathy; NR: Not reported; PID: Primary immunodeficiency; QoL: Quality of life; RA: Rheumatoid arthritis; RoA: Route of administration; SC: Subcutaneous; SCIg: Subcutaneous immunoglobulin; SF-36: 36-item short-form health survey; SLE: Systemic lupus erythematosus; TSQM: Treatment satisfaction questionnaire for medication.
A total of ten PID studies reported pooled data for adults and children or adolescents [21,22,32,34,35,43–46,49,50,55,58,62]. The remaining 27 studies included only adult patients [7,14,20,23–31,33,36–42,47,48,51–54,56,57,59–61,63].
Three of the included studies used a cross-over design [24,32,41]; the remainder were treatment switching studies [7,14,20–23,25–31,33–40,42–63].
Results of individual studies: patient preferences
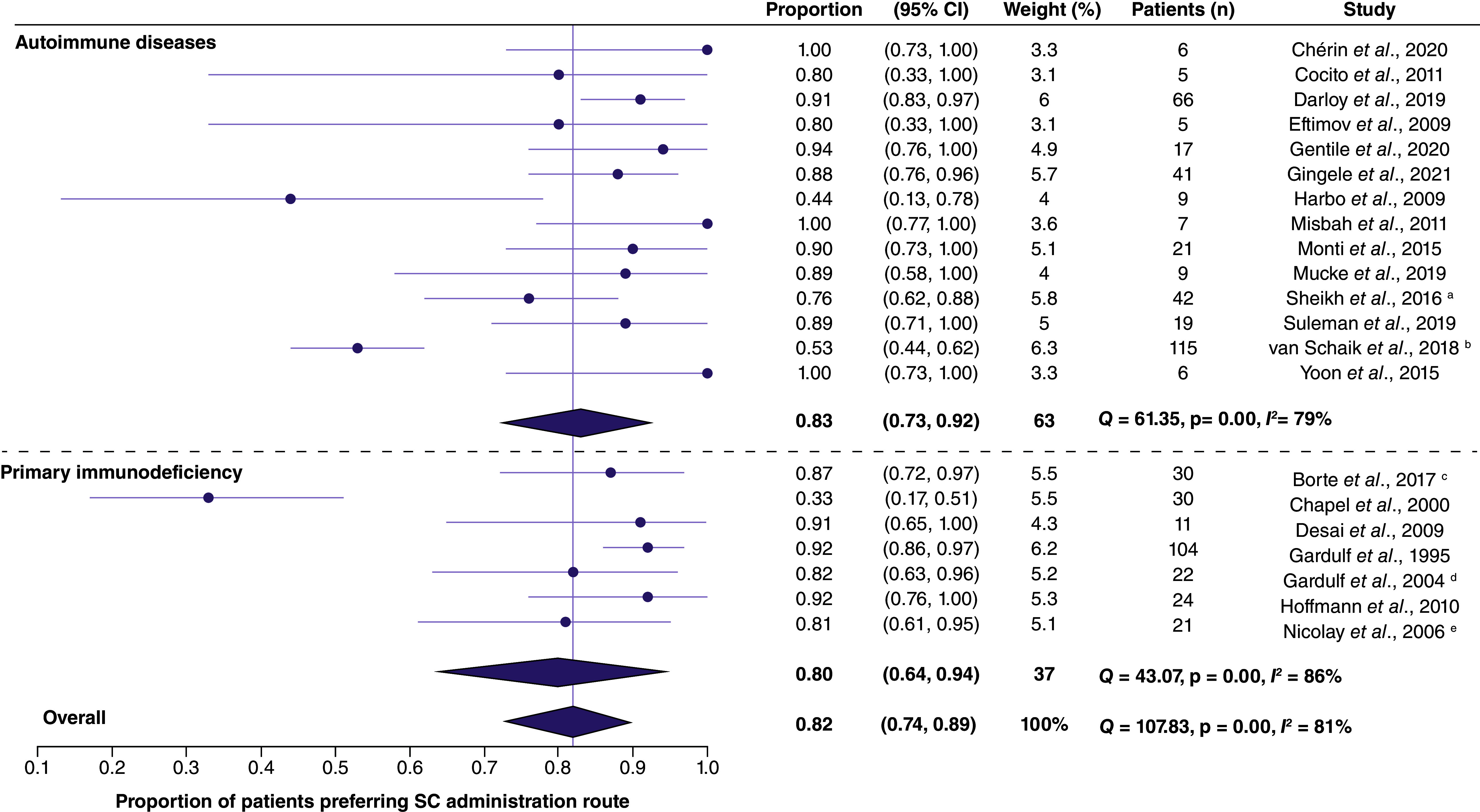
Patient preferences for SC and IV administration are shown in Figure 2.
Figure 2. . Meta-analysis of patient preferences for SC administration at home, compared with IV administration in hospital.
a Data from this study were reported in Sheikh et al. (2016) and in Dashiell-Aje et al. (2018).
b Data from this study were reported in van Schaik et al. (2018 and 2019) and in Hartung et al. (2020).
c Data from this study were reported in Borte et al. (2017) and in Meckley et al. (2020).
d Data from this study were reported in Gardulf et al. (2004 and 2008).
e Data from this study were reported in Nicolay et al. (2006) and in Gardulf et al. (2008).
CI: Confidence interval; RoA: Route of administration; SC: Subcutaneous.
In total, 21 studies reported the proportion of patients who preferred SC administration [7,14,22,24,25,29–38,41–43,50–53,55,57,59,60,63]. Among 14 studies of patients with autoimmune diseases [7,25,29–31,33,36,38,41,42,51–53,57,59,60,63], all except one small study (n = 9) [41] found the majority of patients to prefer SC administration. A further study assessed patients' preferences using a visual analogue scale (VAS; prefer IVIg = 0, prefer SCIg = 100) – the mean reported score among seven adults with autoimmune diseases was 93 (standard deviation [SD], 12) [40].
Preferences among patients with PID were investigated in seven studies [14,22,24,32,34,35,43,50,55]. Of these, all but one study [24] found an overall preference for SC therapy.
The three studies investigating patient preferences for home versus hospital administration found a consistently strong preference for a home setting (range: 82–90%) [34,35,43,55].
Results of synthesis: patient preferences
In the meta-analysis, the proportion of patients in studies of autoimmune diseases who preferred SC administration to IV treatment was 83% (95% confidence interval [CI], 73–92%). Among those with PID, 80% of patients preferred SC treatment (95% CI: 64–94%). Across all indications, the overall proportion of patients preferring SC administration routes was 82% (95% CI: 74–89%). In all three meta-analysis of patient preferences for SC versus IV therapies, Q and I2 statistics indicated substantial heterogeneity among the included studies.
The meta-analysis of patient preferences for home versus hospital administration found that overall, 84% of patients [95% CI: 75–92%] preferred the home setting (Supplementary Figure 1) [34,35,43,55].
Results of individual studies: treatment satisfaction
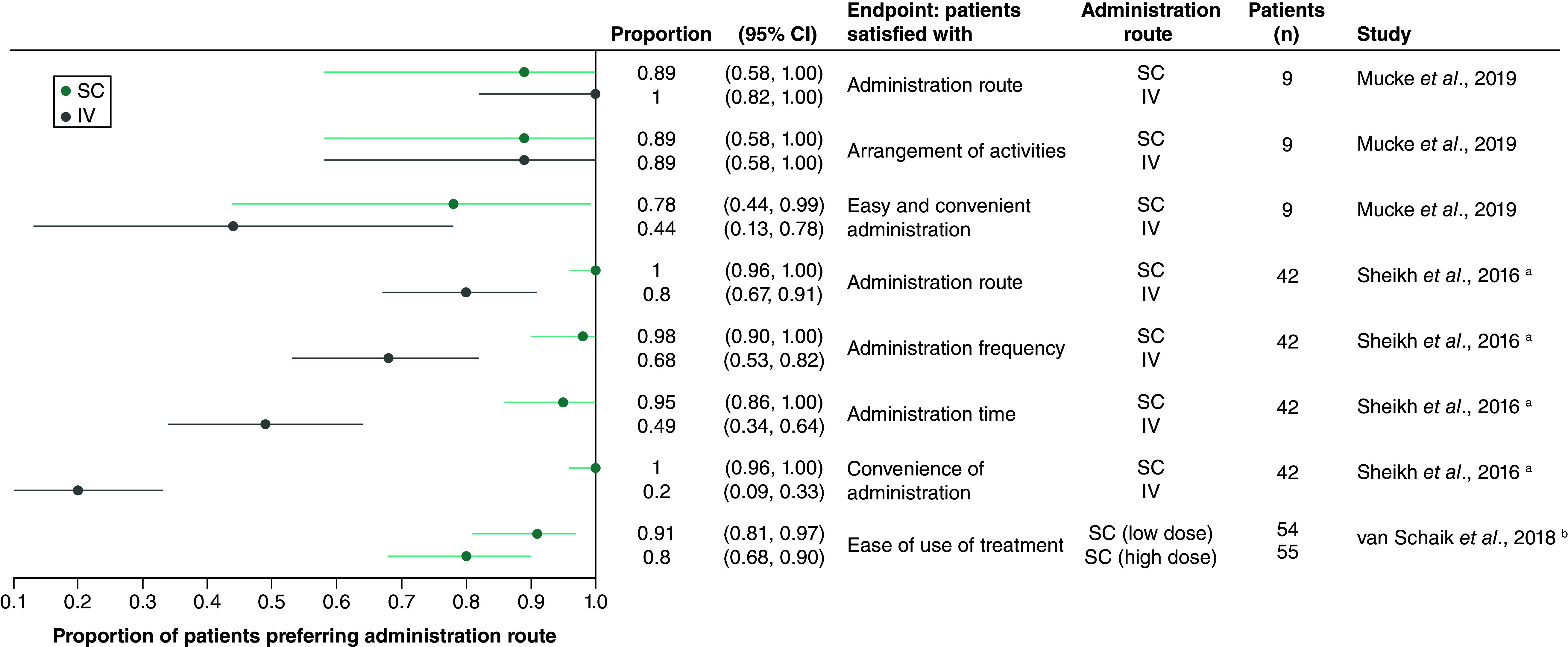
The proportion of patients satisfied with SC administration was reported in three studies (Figure 3) [7,31,42,53,57,60].
Figure 3. . Proportion of patients reporting satisfaction with their treatment.
No p-values were reported for any of the studies reporting treatment satisfaction.
a Data from this study were reported in Sheikh et al. (2016) and in Dashiell-Aje et al. (2018).
b Data from this study were reported in van Schaik et al. (2018 and 2019) and in Hartung et al. (2020).
CI: Confidence interval; IV: Intravenous; SC: Subcutaneous.
In the larger of two studies of patients with SLE, 100% of participants were satisfied with the SC administration route and the convenience of SC belimumab administration [31,57]. By contrast, 79% of patients were satisfied with the IV administration route, and 19% with the convenience of IV administration [31,57]. In the smaller SLE study, results for SC and IV belimumab were mixed, with significantly more patients finding SC administration to be simple, compared with IV (7 of 9 vs 5 of 9 patients), but fewer being satisfied with SC medication in general (8 of 9 vs 9 of 9 patients) [53]. In a third study, conducted among 57 adult patients with CIDP, most participants reported finding either high- or low-dose SCIg to be easy to use [7,42,60].
Treatment satisfaction was assessed using the life quality index (LQI) [14,54] in eight studies [21,22,27,28,34,44,46,49–51,54,55,58], with the Treatment Satisfaction Questionnaire for Medication (TSQM) [64] in four studies [7,42,45,49,50,58,60,61] and with rating scales (0–10 or 0–100) in five studies [23,34,38,40,47,55,56]. In all studies the comparison was between SCIg and IVIg.
The LQI was developed to compare treatment satisfaction in adults and children/caregivers switching from IVIg infusions in a medical setting to IVIg or SCIg self-infusions at home, and encompasses four domains of treatment satisfaction: scale 1, treatment interference with daily life activity; scale 2, therapy-related problems; scale 3, therapy setting (comfortable, pleasant atmosphere); and scale 4, treatment costs [14,54]. Higher scores indicate greater satisfaction with treatment.
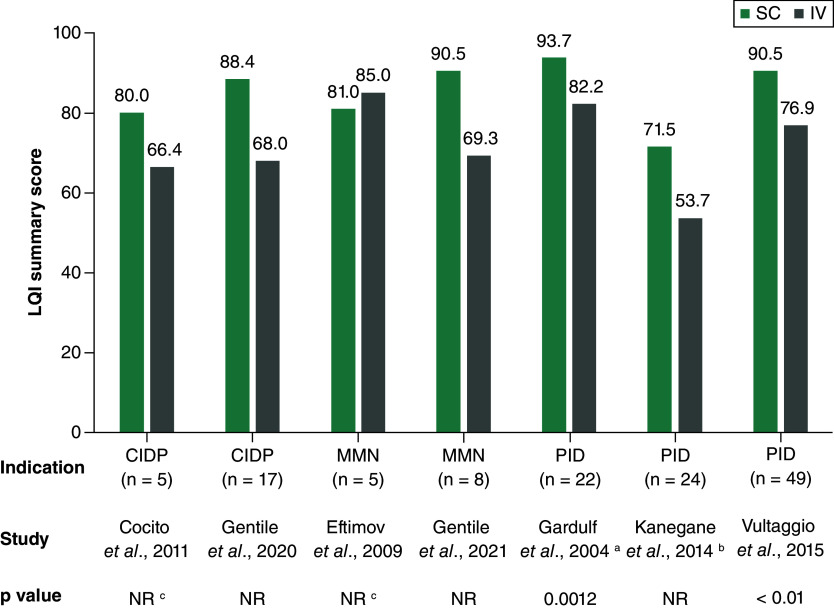
Overall LQI summary scores were consistently higher for SCIg than for IVIg in three studies of patients with PID [34,35,44,46,49,62], with statistically significant differences reported in two of these (Figure 4) [34,35,62]. No significant differences in LQI summary scores between SC and IV administration were reported in four studies of patients with CIDP or MMN [29,33,36,37].
Figure 4. . Life quality index summary score.
In all included studies the investigated compound was immunoglobulin. Higher scores indicate greater satisfaction with treatment.
a Data from this study were reported in Gardulf et al. (2004 and 2008).
b Data from this study were reported in Kanegane et al. (2014), in Igarashi et al. (2014) and in Mallick et al. (2018).
c p-value NR, but no significant difference between SC and IV.
CIDP: Chronic inflammatory demyelinating polyneuropathy; IV: Intravenous administration in hospital; LQI: Life quality index; MMN: Multifocal motor neuropathy; NR: Not reported; PID: Primary immunodeficiency; SC: Subcutaneous administration at home.
LQI scale scores are shown in Supplementary Figure 2. LQI treatment interference scores were consistently statistically significantly higher for SCIg than for IVIg in the five studies of patients with PID that reported these outcomes [22,34,44,46,49,50,54,55,58]; a sixth PID study did not report detailed LQI results, but indicated that the mean treatment interference score was statistically significantly higher with SCIg than with IVIg [21]. Compared with IVIg, SCIg was associated with numerically higher LQI treatment interference scores in two studies of patients with MMN [27,28,51], and statistically significantly higher scores in one study of patients with CIDP [27,28].
LQI therapy setting scores were statistically significantly higher for SCIg than for IVIg in five of the six studies of patients with PID [21,22,34,44,46,49,50,54,55,58]. Similar results favoring SCIg were seen in CIDP and MMN studies, with statistically significant differences seen in two of three populations [27,28,51].
The results for the LQI therapy-related problems and treatment cost scales also favored SCIg administration, with consistently higher scores reported for SCIg than for IVIg (Supplementary Figure 2).
Among seven studies that reported treatment satisfaction using the TSQM or rating scales, satisfaction was either similar for SCIg and IVIg or statistically significantly higher with SCIg (Supplementary Table 3) [7,23,31,34,38,42,45,49–51,53,55,57,58,60,61].
Results of individual studies: health-related quality of life
A total of eight studies reported HRQL data, using the five dimension, five-level EuroQol questionnaire (EQ-5D-5L) [26], VAS instruments [7,20,42,51,60,62], SF-36 [21,33,39,48,62], MG Impairment Index (MGII) [20], 15-item MG quality of life questionnaire (MG-QOL 15) [23] and a 7-point scale [51]. SF-36 results are shown in Supplementary Figure 3; results using the other instruments are summarized in Table 2. Overall, HRQL appeared generally similar with SC or IV administration. However, one small (n = 6) study of patients with MG reported statistically significantly better MG-QOL 15 scores with SCIg than with IVIg; otherwise, there were no significant differences in HRQL outcomes between IV and SC administration [23].
Table 2. . HRQL results for SC or IV treatment administration.
| Study | Indication | Compound | Design | Patients (n) | Age group | Instrument | SC | IV | p-value | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Alcantara et al., 2021 | MG | Ig | Switch study | 30 | Adults | Percentage of normal (0–100%) | 72.3% | 72.4% | 1.00 | [14] |
| MGII | 19.5 | 22 | 0.07 | |||||||
| Bourque et al., 2016 | MG | Ig | Switch study | 6 | Adults | MG-QOL 15 | 8.2 | 13.8 | 0.008 | [17] |
| Christiansen et al., 2018 | CIDP, MMN | Ig | Switch study | 17 | Adults | EQ-5D-5L | 0.8 | 0.7 | 0.16 | [20] |
| Eftimov et al., 2009 | MMN | Ig | Switch study | 5 | Adults | SF-36 summary score | 95 | 92.2 | 0.5 | [27] |
| Katzberg et al., 2016 Rasutis et al., 2017 |
MMN | Ig | Switch study | 15 | Adults | HUI-QoL | NR | NR | NR† | [42,51] |
| Misbah et al., 2011 | MMN | Ig | Switch study | 7 | Adults | VAS (0–100) | 73.9 | 72.1 | NR | [46] |
| Quality of life questionnaire (1–7) | 1.1 | 2.6 | NR | |||||||
| van Schaik et al., 2018 van Schaik et al., 2019 Hartung et al., 2020 [NCT01545076 PATH study] |
CIDP | Ig | Switch study | 54 (LD) 53 (HD) |
Adults | EQ-VAS (0–100) | 62.4 (LD) 68.6 (HD) |
67.4 (LD) 68.6 (HD) |
NR | [10,37,55] |
| Vu et al., 2011 | CIDP | Ig | Switch study | 15 | Adults | CAP-PRI | 12.2 | 16.5 | 0.02 | [56] |
| Vultaggio et al., 2015 | PID | Ig | Switch study | 37 | Adults and children | VAS (0–100) | 68.3 | 65.3 | NR† | [57] |
| 44 | Adults and adolescents | SF-36 summary score | 121 | 130 | NR† |
p-value NR, but no significant difference between SC and IV.
Higher CAP-PRI, MGII and MG-QOL 15 scores indicate greater impairment of HRQL; for all other scales higher scores indicate higher HRQL.
CAP-PRI: Chronic acquired polyneuropathy patient-reported index; CIDP: Chronic inflammatory demyelinating polyneuropathy; EQ-5D-5L: 5-dimension: 5-level EuroQol questionnaire; EQ-VAS: EuroQol visual analogue scale; HD: High dose; HUI-QoL: Health utility index - quality of life; Ig: Immunoglobulin; IV: Intravenous; LD: Low dose; MG: Myasthenia gravis; MGII: Myasthenia gravis impairment index; MG-QOL 15: 15-item myasthenia gravis quality of life questionnaire; MMN: Multifocal motor neuropathy; PID: Primary immunodeficiency; SC: Subcutaneous; SF-36: 36-item short-form health survey; VAS: Visual analogue scale.
Discussion
This SLR concentrated on preferences, treatment satisfaction and HRQL among patients receiving treatment via SC administration at home or IV administration at hospital.
Among the studies including patients with autoimmune diseases, the SLR showed that there was a strong preference for SC parenteral administration, with SC administration also associated with high treatment satisfaction. In addition, SC administration of immunoglobulins was associated with a high level of treatment satisfaction. SCIg gave statistically significantly higher LQI treatment interference and treatment setting scores and significantly higher TSQM convenience scores, with other measures of treatment satisfaction consistently favoring SCIg over IVIg.
Notably, there was little difference in PROs between SC and IV administration for end points that are likely to be influenced by treatment effectiveness or patients' general health, such as the EQ-5D and SF-36. This is consistent with recent studies, including the PATH trial, which have found SCIg to have similar clinical effectiveness to IVIg in the prevention of relapse among patients with CIDP [7,65]. Instead, the SLR found that patients reported significant advantages for SC over IV administration in PROs measuring aspects of their treatment such as interference, setting and convenience.
The findings of this study are consistent with previous SLRs comparing SC and IV administration of therapies for specific conditions, for example PID [66] and RA [67]. Jones et al. found that patients with PID tended to prefer SCIg at home to IVIg in hospital, although differences in HRQL were not always statistically significant [66]. Durand et al. found that among patients with RA SC therapy was often (but not always) preferred to IV administration [67]. An SLR of patient preferences across multiple indications (which did not include any meta-analysis) also found that patients were more likely to prefer the SC route than IV administration [68].
Patient preferences and circumstances can be very individual, and self-administration may not be suitable for all patients. SC administration at home is likely to be most suitable for patients who are confident in the effectiveness of their treatment, who wish to have greater autonomy, and who are both comfortable with carrying out self-infusion and willing to take on the responsibility of the practical handling of the treatment at home [69]. Patients must also have undergone education and shown good understanding of their disease and treatment, including identification and handling of adverse reactions [69].
Matching treatment administration to patients' individual needs may be particularly important for those who need long-term treatment, as is the case for PID and autoimmune disorders. In addition to improving HRQL, selection of the optimal treatment route for each patient is likely to improve adherence and treatment satisfaction [70,71].
A strength of this study is the similarity of results seen for multiple therapies and indications, with a strong overall preference found for SC administration at home over IV therapy at hospital. In general, the use of SC therapies has become more common in recent years. This reflects the extensive, well-established use of SCIg for PID and autoimmune disorders and the growing number of biological therapies in multiple disease areas, for example anti-tumor necrosis factor therapies for RA and interferon-beta for multiple sclerosis [4]. The results of this SLR show a high level of consistency across PID and multiple autoimmune indications, and the findings may be applicable to other chronic diseases and therapies. For example, the higher treatment satisfaction associated with SCIg than IVIg among patients with MG [23] may be expected to translate to new treatments, including monoclonal antibodies and small peptide therapies, in development for this indication. Similarly, SC administration of newer therapies in other indications for which SCIg can be used – for example immune thrombocytopenia and myelin oligodendrocyte glycoprotein antibody disease – is likely to be generally well received by patients.
This SLR has some limitations. First, most (34 of 37) of the included studies followed patients who were switching route of administration for their treatment, most commonly from IV to SC. Accordingly, as is typical of adult switching studies, there is potential for some self-selection of study participants, who may therefore not be representative of the wider patient population. Second, most (33 of 37) of the included studies compared SCIg with IVIg, with few studies of other therapies identified. Third, the SLR was limited to studies published in English; the existence of relevant evidence published in other languages cannot be excluded.
Conclusion
SC and IV administration of drugs for PID and autoimmune conditions have shown similar clinical effectiveness. Consequently, patients' individual preferences and the impact of each administration route on their treatment satisfaction and HRQL are important considerations. The results of this SLR show that, compared with IV infusions in hospital, patients prefer and are more satisfied with SC administration of the same drug at home, primarily due to the greater convenience. Analyzing and compiling the results of the 37 studies that were included in the SLR regarding patient preference, treatment satisfaction and HRQL contributes to evidence-based care of patients with autoimmune diseases or primary immunodeficiencies.
Summary points
Several studies have found subcutaneous (SC) and intravenous (IV) administration of similar drugs for long-lasting immunological and autoimmune diseases to have similar clinical effectiveness.
Accordingly, what patients report they prefer is a major factor in treatment choices.
This systematic literature review and meta-analysis was conducted to compile evidence regarding patient preferences, treatment satisfaction and health-related quality of life (HRQL) for SC administration at home or IV administration of the same drug in hospital.
Across the 37 included studies, there was a strong overall preference for SC administration at home over IV administration in hospital, with similar results seen for PID and autoimmune diseases.
Analysis of treatment satisfaction using the life quality index found consistently better treatment interference and treatment setting scores with SC administration than with IV administration.
Notably, HRQL outcomes likely to be influenced by treatment effectiveness or patients' general health were not significantly different between SC and IV administration.
Overall, the results of this SLR show that, compared with IV infusions in hospital, patients prefer and are more satisfied with SC administration of the same drug at home, primarily due to the greater convenience.
This study contributes to evidence-based care of patients with autoimmune diseases or primary immunodeficiencies.
Supplementary Material
Acknowledgments
The authors acknowledge AM Gross (Lampe & Company, Berlin, Germany) for support in the compilation of these analyses. The authors acknowledge V Porkess of UCB Pharma, for publication and editorial support.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2023-0171
Author contributions
P Kiessling and J Lampe designed the study. J Lampe carried out the analyses. All authors reviewed the list of included studies and analyzed the SLR results. Further, they revised the manuscript critically for important intellectual content. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial disclosure
This study was funded by UCB Pharma. The funder was involved in the study design, data analysis and the preparation of the manuscript. V Bril reports consultancy fees from Grifols, CSL, UCB Pharma, Argenx, Takeda, Alnylam Octapharma, Pfizer, Powell Mansfield Inc., Akcea, Ionis Immunovant, Sanofi, Momenta (J&J), Roche, Janssen, AZ-Alexion and NovoNordisk; and research support from AZ-Alexion, Grifols, CSL, UCB Pharma, Argenx, Takeda, Octapharma, Akcea, Momenta (J&J), Immunovant and Ionis. N Cooper reports consultancy fees from Novartis, Amgen, Rigel, UCB, Argenyx, Sanofi and Principia; and research support from Novartis and Rigel. A Gardulf reports consultancy fees from UCB Pharma. P Kiessling is an employee and shareholder of UCB Pharma. J Lampe reports consultancy fees from UCB Pharma paid to Lampe & Company, Berlin, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with anyorganization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
Editorial assistance was provided by P Overton (Beacon Medical Communications Ltd, Brighton, UK), funded by UCB Pharma, in accordance with the Good Publication Practice (GPP 2022) guidelines.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Perez EE, Orange JS, Bonilla F et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 139(Suppl. 3), S1–S46 (2017). [DOI] [PubMed] [Google Scholar]; •• Reviews indications for immunoglobulin use, including a comparison of subcutaneous (SC) immunoglobulins (Ig) and intravenous (IV) immunoglobulins (Ig).
- 2.Šedivá A, Chapel H, Gardulf A; European Immunoglobulin Map Group for European Society for Immunodeficiencies Primary Immunodeficiencies Care in Development Working Party. Europe immunoglobulin map. Clin. Exp. Immunol. 178(Suppl. 1), 141–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caporali R, Allanore Y, Alten R et al. Efficacy and safety of subcutaneous infliximab versus adalimumab, etanercept and intravenous infliximab in patients with rheumatoid arthritis: a systematic literature review and meta-analysis. Expert Rev. Clin. Immunol. 17(1), 85–99 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Turner MR, Balu-Iyer SV. Challenges and opportunities for the subcutaneous delivery of therapeutic proteins. J. Pharm. Sci. 107(5), 1247–1260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauper K, Mongin D, Iannone F et al. Comparative effectiveness of subcutaneous tocilizumab versus intravenous tocilizumab in a pan-European collaboration of registries. RMD Open 4(2), e000809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates similar clinical efficacy for subcutaneous and intravenous formulations of tocilizumab in the treatment of rheumatoid arthritis.
- 6.Stohl W, Schwarting A, Okada M et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 69(5), 1016–1027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Schaik IN, Bril V, van Geloven N et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 17(1), 35–46 (2018). [DOI] [PubMed] [Google Scholar]; • Demonstrates similar clinical efficacy for subcutaneous and intravenous formulations of tocilizumab in the treatment of rheumatoid arthritis.
- 8.Borte M, Quinti I, Soresina A et al. Efficacy and safety of subcutaneous vivaglobin® replacement therapy in previously untreated patients with primary immunodeficiency: a prospective, multicenter study. J. Clin. Immunol. 31(6), 952–961 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Fu LW, Song C, Isaranuwatchai W, Betschel S. Home-based subcutaneous immunoglobulin therapy vs hospital-based intravenous immunoglobulin therapy: A prospective economic analysis. Ann. Allergy Asthma Immunol. 120(2), 195–199 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Alsina L, Montoro JB, Moral PM et al. Cost-minimization analysis of immunoglobulin treatment of primary immunodeficiency diseases in Spain. Eur. J. Health Econ. 23(3), 551–558 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perraudin C, Bourdin A, Vicino A, Kuntzer T, Bugnon O, Berger J. Home-based subcutaneous immunoglobulin for chronic inflammatory demyelinating polyneuropathy patients: a Swiss cost-minimization analysis. PLOS ONE 15(11), e0242630 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windegger TM, Nghiem S, Nguyen KH, Fung YL, Scuffham PA. Cost-utility analysis comparing hospital-based intravenous immunoglobulin with home-based subcutaneous immunoglobulin in patients with secondary immunodeficiency. Vox Sang 114(3), 237–246 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Gardulf A, Nicolay U. Replacement IgG therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr. Opin. Allergy Clin. Immunol. 6(6), 434–442 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Gardulf A, Björvell H, Andersen V et al. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients' experiences of subcutaneous self-infusions and home therapy. J. Adv. Nurs. 21(5), 917–927 (1995). [DOI] [PubMed] [Google Scholar]; • Large treatment-switching study which investigated preferences among 112 patients with primary immunodeficiencies (PIDs).
- 15.Gardulf A, Möller G, Jonsson E. A comparison of the patient-borne costs of therapy with gamma globulin given at the hospital or at home. Int. J. Technol. Assess. Health Care 11(2), 345–353 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Heald A, Bramham-Jones S, Davies M. Comparing cost of intravenous infusion and subcutaneous biologics in COVID-19 pandemic care pathways for rheumatoid arthritis and inflammatory bowel disease: a brief UK stakeholder survey. Int. J. Clin. Pract. 75(9), e14341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böckenholt U, Tsai R-C. 14 Random-effects models for preference data. In: Handbook of Statistics. Rao CR, Sinharay S (Eds). Elsevier, The Netherlands, 447–468 (2006). [Google Scholar]
- 19.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11(2), 193–206 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Alcantara M, Sarpong E, Barnett C, Katzberg H, Bril V. Chronic immunoglobulin maintenance therapy in myasthenia gravis. Eur. J. Neurol. 28(2), 639–646 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Bienvenu B, Cozon G, Hoarau C et al. Does the route of immunoglobin replacement therapy impact quality of life and satisfaction in patients with primary immunodeficiency? Insights from the French cohort “Visages”. Orphanet J. Rare Dis. 11(1), 83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borte M, Kriván G, Derfalvi B et al. Efficacy, safety, tolerability and pharmacokinetics of a novel human immune globulin subcutaneous, 20%: a Phase 2/3 study in Europe in patients with primary immunodeficiencies. Clin. Exp. Immunol. 187(1), 146–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourque PR, Pringle CE, Cameron W, Cowan J, Chardon JW. Subcutaneous immunoglobulin therapy in the chronic management of myasthenia gravis: a retrospective cohort study. PLOS ONE 11(8), e0159993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J. Clin. Immunol. 20(2), 94–100 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Chérin P, Pindi Sala T, Clerson P et al. Recovering autonomy is a key advantage of home-based immunoglobulin therapy in patients with myositis: a qualitative research study. Medicine 99(7), e19012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christiansen I, Markvardsen LH, Jakobsen J. Comparisons in fluctuation of muscle strength and function in patients with immune-mediated neuropathy treated with intravenous versus subcutaneous immunoglobulin. Muscle Nerve. 57(4), 610–614 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Cocito D, Merola A, Peci E et al. Subcutaneous immunoglobulin in CIDP and MMN: a short-term nationwide study. J. Neurol. 261(11), 2159–2164 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Cocito D, Merola A, Romagnolo A et al. Subcutaneous immunoglobulin in CIDP and MMN: a different long-term clinical response? J. Neurol. Neurosurg. Psychiatry 87(7), 791–793 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Cocito D, Serra G, Falcone Y, Paolasso I. The efficacy of subcutaneous immunoglobulin administration in chronic inflammatory demyelinating polyneuropathy responders to intravenous immunoglobulin. J. Peripher. Nerv. Syst. 16(2), 150–152 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Darloy J, Segaud N, Salmon J-H et al. Tocilizumab effectiveness after switching from intravenous to subcutaneous route in patients with rheumatoid arthritis: the RoSwitch Study. Rheumatol. Ther. 6(1), 61–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Observational study of patients with rheumatoid arthritis switching from intravenous to subcutaneous tocilizumab.
- 31.Dashiell-Aje E, Harding G, Pascoe K, DeVries J, Berry P, Ramachandran S. Patient evaluation of satisfaction and outcomes with an autoinjector for self-administration of subcutaneous belimumab in patients with systemic lupus erythematosus. Patient 11(1), 119–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai SH, Chouksey A, Poll J, Berger M. A pilot study of equal doses of 10% IGIV given intravenously or subcutaneously. J. Allergy Clin. Immunol. 124(4), 854–856 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Eftimov F, Vermeulen M, de Haan RJ, van den Berg LH, van Schaik IN. Subcutaneous immunoglobulin therapy for multifocal motor neuropathy. J. Peripher. Nerv. Syst. 14(2), 93–100 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Gardulf A, Borte M, Ochs HD, Nicolay U. Prognostic factors for health-related quality of life in adults and children with primary antibody deficiencies receiving SCIG home therapy. Clin. Immunol. 126(1), 81–88 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Gardulf A, Nicolay U, Math D et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J. Allergy Clin. Immunol. 114(4), 936–942 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Gentile L, Mazzeo A, Russo M, Arimatea I, Vita G, Toscano A. Long-term treatment with subcutaneous immunoglobulin in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a follow-up period up to 7 years. Sci. Rep. 10(1), 7910 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gentile L, Russo M, Rodolico C et al. Long-term treatment with subcutaneous immunoglobulin in multifocal motor neuropathy. Sci. Rep. 11(1), 9216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingele S, Koch M, Saparilla AC et al. Switch from intravenous to subcutaneous immunoglobulin IgPro20 in CIDP patients: a prospective observational study under real-world conditions. Ther. Adv. Neurol. Disord. 14, 17562864211009100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hachulla E, Benveniste O, Hamidou M et al. High dose subcutaneous immunoglobulin for idiopathic inflammatory myopathies and dysimmune peripheral chronic neuropathies treatment: observational study of quality of life and tolerance. Int. J. Neurosci. 127(6), 516–523 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Hadden RDM, Marreno F. Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: improved tolerability and patient satisfaction. Ther. Adv. Neurol. Disord. 8(1), 14–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harbo T, Andersen H, Hess A, Hansen K, Sindrup SH, Jakobsen J. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. Eur. J. Neurol. 16(5), 631–638 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Hartung HP, Mallick R, Bril V et al. Patient-reported outcomes with subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy: the PATH study. Eur. J. Neurol. 27(1), 196–203 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann F, Grimbacher B, Thiel J, Peter HH, Belohradsky BH, Vivaglobin Study G. Home-based subcutaneous immunoglobulin G replacement therapy under real-life conditions in children and adults with antibody deficiency. Eur. J. Med. Res. 15(6), 238–245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Igarashi A, Kanegane H, Kobayashi M, Miyawaki T, Tsutani K. Cost-minimization Analysis of IgPro20, a Subcutaneous Immunoglobulin, in Japanese Patients With Primary Immunodeficiency. Clin. Ther. 36(11), 1616–1624 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Jolles S, Bernatowska E, de Gracia J et al. Efficacy and safety of Hizentra® in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin. Immunol. 141(1), 90–102 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Kanegane H, Imai K, Yamada M et al. Efficacy and safety of IgPro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency diseases. J. Clin. Immunol. 34(2), 204–211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katzberg HD, Rasutis V, Bril V. Subcutaneous immunoglobulin for treatment of multifocal motor neuropathy. Muscle Nerve. 54(5), 856–863 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Latysheva E, Rodina Y, Sizyakina L, Totolian A, Tuzankina I. Efficacy and safety of octanorm (cutaquig®) in adults with primary immunodeficiencies with predominant antibody deficiency: a prospective, open-label study. Immunotherapy. 12(5), 299–309 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Mallick R, Jolles S, Kanegane H, Agbor-Tarh D, Rojavin M. Treatment Satisfaction with Subcutaneous Immunoglobulin Replacement Therapy in Patients with Primary Immunodeficiency: a Pooled Analysis of Six Hizentra® Studies. J. Clin. Immunol. 38(8), 886–897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meckley LM, Wu Y, Ito D, Berner T, McCoy B, Yel L. Patient experience with subcutaneous immunoglobulin 20%, Ig20Gly, for primary immunodeficiency diseases: a prespecified post hoc analysis of combined data from 2 pivotal trials. BMC Immunol. 21(1), 24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misbah SA, Baumann A, Fazio R et al. A smooth transition protocol for patients with multifocal motor neuropathy going from intravenous to subcutaneous immunoglobulin therapy: an open-label proof-of-concept study. J. Peripher. Nerv. Syst. 16(2), 92–97 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Monti S, Breda S, Grosso V, Todoerti M, Montecucco C, Caporali R. Switching from intravenous to subcutaneous formulation of abatacept: different results in a series of 21 patients. J. Rheumatol. 42(10), 1993–1994 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Mucke J, Brinks R, Fischer-Betz R et al. Patient satisfaction and disease control in patients with systemic lupus erythematosus is not affected by switching from intravenous belimumab to subcutaneous injections. Patient Prefer. Adherence 13, 1889–1894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolay U, Haag S, Eichmann F, Herget S, Spruck D, Gardulf A. Measuring treatment satisfaction in patients with primary immunodeficiency diseases receiving lifelong immunoglobulin replacement therapy. Qual. Life Res. 14(7), 1683–1691 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Nicolay U, Kiessling P, Berger M et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J. Clin. Immunol. 26(1), 65–72 (2006). [DOI] [PubMed] [Google Scholar]; • Study of HRQL and treatment satisfaction which also reports patients' preferences for treatment at home or in hospital.
- 56.Rasutis VM, Katzberg HD, Bril V. High-dose subcutaneous immunoglobulin in patients with multifocal motor neuropathy. J. Infus. Nurs. 40(5), 305–312 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Sheikh SZ, Hammer AE, Fox NL et al. Evaluation of a novel autoinjector for subcutaneous self-administration of belimumab in systemic lupus erythematosus. Int. J. Clin. Pharmacol. Ther. 54(11), 914–922 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suez D, Stein M, Gupta S et al. Efficacy, safety, and pharmacokinetics of a novel human immune globulin subcutaneous, 20% in patients with primary immunodeficiency diseases in North America. J. Clin. Immunol. 36(7), 700–712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suleman A, Theoret L, Bourque P, Pringle E, Cameron DW, Cowan J. Evaluation of a personalized subcutaneous immunoglobulin treatment program for neurological patients. Can. J. Neurol. Sci. 46(1), 38–43 (2019). [DOI] [PubMed] [Google Scholar]
- 60.van Schaik IN, Mielke O, Bril V et al. Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study. Neurol. Neuroimmunol. Neuroinflamm. 6(5), e590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vu T, Anthony N, Alsina R et al. Impact of subcutaneous immunoglobulin on quality of life in patients with chronic inflammatory demyelinating polyneuropathy previously treated with intravenous immunoglobulin. Muscle Nerve 64(3), 351–357 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Vultaggio A, Azzari C, Milito C et al. Subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency in routine clinical practice: the VISPO prospective multicenter study. Clin. Drug. Investig. 35(3), 179–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon M-S, Gold R, Kerasnoudis A. Subcutaneous immunoglobulin in treating inflammatory neuromuscular disorders. Ther. Adv. Neurol. Disord. 8(4), 153–159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atkinson MJ, Sinha A, Hass SL et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual. Life Outcomes. 2, 12 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Racosta JM, Sposato LA, Kimpinski K. Subcutaneous versus intravenous immunoglobulin for chronic autoimmune neuropathies: a meta-analysis. Muscle Nerve 55(6), 802–809 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Jones GL, Vogt KS, Chambers D, Clowes M, Shrimpton A. What is the burden of immunoglobulin replacement therapy in adult patients with primary immunodeficiencies? A systematic review. Front. Immunol. 9, 1308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durand C, Eldoma M, Marshall DA, Bansback N, Hazlewood GS. Patient preferences for disease-modifying antirheumatic drug treatment in rheumatoid arthritis: a systematic review. J. Rheumatol. 47(2), 176–187 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Overton PM, Shalet N, Somers F, Allen JA. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer. Adherence 15, 811–834 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports reasons given by patients for preferring SC or IV administration.
- 69.Menon D, Sarpong E, Bril V. Practical aspects of transitioning from intravenous to subcutaneous immunoglobulin therapy in neuromuscular disorders. Can. J. Neurol. Sci. 49(2), 161–167 (2022). [DOI] [PubMed] [Google Scholar]; • Describes practical aspects of SC treatment use for patients with neuromuscular disorders.
- 70.Epland K, Suez D, Paris K. A clinician's guide for administration of high-concentration and facilitated subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency diseases. Allergy Asthma Clin. Immunol. 18(1), 87 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin JF, Zhu LL, Chen M et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 9, 923–942 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.