Abstract
Protein synthesis initiation factor 5A (eIF-5A) from human erythrocytes was found to be a substrate for both plasma transglutaminase (Factor XIIIa) and guinea pig liver transglutaminase (GPLTG). When purified eIF-5A was incubated with GPLTG or Factor XIIIa in the presence of succinylated beta-casein, a covalent complex was identified. By isolating and analysing the product of the transglutaminases (TGases) reaction, the site of modification on eIF-5A has been identified as the unique amino acid hypusine. The complex beta-casein.eIF-5A was enzymatically digested with proteinases and the predicted covalent cross-link of gamma-glutamyl-omega-hypusine was isolated from the digests by ion-exchange chromatography and purified by reversed-phase h.p.l.c. Acid hydrolysis of the purified dipeptide yielded equimolar amounts of hypusine and glutamic acid. Furthermore, fast atom bombardment m.s. analysis confirmed the isomer assignment to be gamma-glutamyl-omega-hypusine. These data indicate that hypusine-50 of the eIF-5A chain functions as acyl acceptor substrate for TGases, and reveal that eIF-5A may be cross-linked to intracellular proteins by TGases. Because the precise function of eIF-5A is still unknown, our results appear particularly stimulating in the light of the recent finding of a new biological role for this protein as a cellular factor binding specifically to the human immunodeficiency virus-1 Rev activation domain [Ruhl, Himmelspach, Bahr, Hammerschmid, Jaksche, Wolff, Auschauer, Farrington, Probst, Bevec and Hauber (1993) J. Cell Biol. 123, 1309-1320].
Full text
PDF
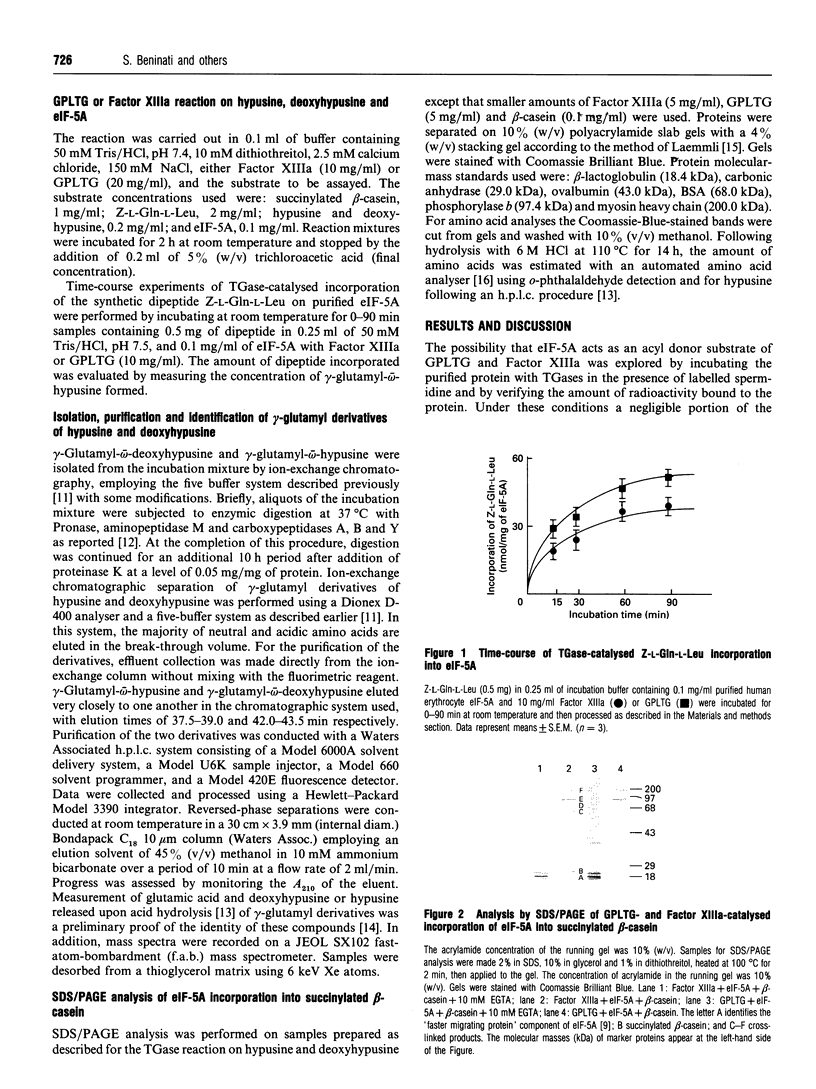
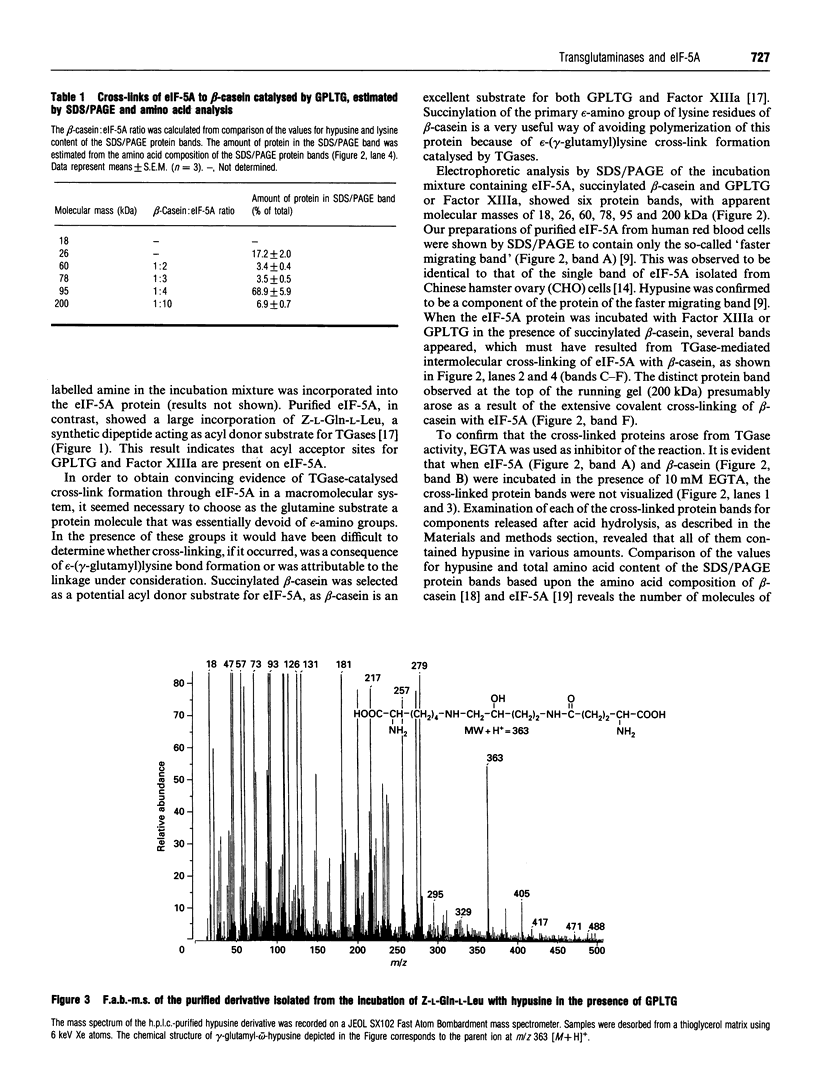

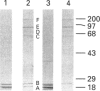
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbruzzese A., Park M. H., Folk J. E. Deoxyhypusine hydroxylase from rat testis. Partial purification and characterization. J Biol Chem. 1986 Mar 5;261(7):3085–3089. [PubMed] [Google Scholar]
- Adams S. L., Safer B., Anderson W. F., Merrick W. C. Eukaryotic initiation complex formation. Evidence for two distinct pathways. J Biol Chem. 1975 Dec 10;250(23):9083–9089. [PubMed] [Google Scholar]
- Beninati S., Abbruzzese A., Folk J. E. High-performance liquid chromatographic method for determination of hypusine and deoxyhypusine. Anal Biochem. 1990 Jan;184(1):16–20. doi: 10.1016/0003-2697(90)90004-s. [DOI] [PubMed] [Google Scholar]
- Beninati S., Piacentini M., Argento-Cerù M. P., Russo-Caia S., Autuori F. Presence of di- and polyamines covalently bound to protein in rat liver. Biochim Biophys Acta. 1985 Jul 26;841(1):120–126. doi: 10.1016/0304-4165(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Beninati S., Piacentini M., Cocuzzi E. T., Autuori F., Folk J. E. Covalent incorporation of polyamines as gamma-glutamyl derivatives into CHO cell protein. Biochim Biophys Acta. 1988 Feb 10;952(3):325–333. doi: 10.1016/0167-4838(88)90134-3. [DOI] [PubMed] [Google Scholar]
- Connellan J. M., Chung S. I., Whetzel N. K., Bradley L. M., Folk J. E. Structural properties of guinea pig liver transglutaminase. J Biol Chem. 1971 Feb 25;246(4):1093–1098. [PubMed] [Google Scholar]
- Cooper H. L., Park M. H., Folk J. E., Safer B., Braverman R. Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1854–1857. doi: 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Kemper W. M., Berry K. W., Merrick W. C. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem. 1976 Sep 25;251(18):5551–5557. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinet N., Beninati S., Nigra T. P., Folk J. E. N1N8-bis(gamma-glutamyl)spermidine cross-linking in epidermal-cell envelopes. Comparison of cross-link levels in normal and psoriatic cell envelopes. Biochem J. 1990 Oct 15;271(2):305–308. doi: 10.1042/bj2710305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981 May;78(5):2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. The biosynthesis of protein-bound hypusine (N epsilon -(4-amino-2-hydroxybutyl)lysine). Lysine as the amino acid precursor and the intermediate role of deoxyhypusine (N epsilon -(4-aminobutyl)lysine). J Biol Chem. 1982 Jun 25;257(12):7217–7222. [PubMed] [Google Scholar]
- Park M. H., Liu T. Y., Neece S. H., Swiggard W. J. Eukaryotic initiation factor 4D. Purification from human red blood cells and the sequence of amino acids around its single hypusine residue. J Biol Chem. 1986 Nov 5;261(31):14515–14519. [PubMed] [Google Scholar]
- Ribadeau Dumas B., Brignon G., Grosclaude F., Mercier J. C. Structure primaire de la caséine beta bovine. Séquence complète. Eur J Biochem. 1972 Feb;25(3):505–514. doi: 10.1111/j.1432-1033.1972.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Ruhl M., Himmelspach M., Bahr G. M., Hammerschmid F., Jaksche H., Wolff B., Aschauer H., Farrington G. K., Probst H., Bevec D. Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J Cell Biol. 1993 Dec;123(6 Pt 1):1309–1320. doi: 10.1083/jcb.123.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Smit-McBride Z., Dever T. E., Hershey J. W., Merrick W. C. Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein. J Biol Chem. 1989 Jan 25;264(3):1578–1583. [PubMed] [Google Scholar]