Background
In 2016, an International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Special Task Force (STF) was formed to evaluate a number of frameworks to value pharmaceuticals that were being developed within the US by several different stakeholders, including from the second panel on cost–effectiveness in health and medicine, from a health economics perspective [1,2]. To carry out its mission, the STF assembled a core group of experts in health economics, including, non-US experts. The health economics perspective adopted by the STF led to an emphasis on considering patients as both purchasers of health insurance and recipients of healthcare services. This perspective also had implications for the STF's conceptualization of the value of pharmaceuticals [3].
In terms of defining elements of value for a medicine, the STF acknowledged the merits of the traditional cost per quality-adjusted life-year (QALY) approach as a foundation for discussions concerning value assessment. However, it also recognized the limitations inherent in this metric [4]. The STF identified and considered 12 potential elements of value, including two core elements of QALYs and net costs, two common but inconsistently used elements (productivity and adherence improvement) and eight potentially ‘novel’ elements (reduction in uncertainty, fear of contagion, insurance value, severity of disease, value of hope, real option value, equity and scientific spillovers). The ‘ISPOR value flower’, a figure found in the 2018 report of the ISPOR STF, visually highlights all these aspects of value. Modified versions of the value flower have since replaced adherence improvement with family spillover (Figure 1). The idea of the flower was to help broaden the view of value in healthcare and spur new research on incorporating additional elements of value in traditional cost–effectiveness analysis (CEA).
Figure 1. . The International Society for Pharmacoeconomics and Outcomes Research value flower.
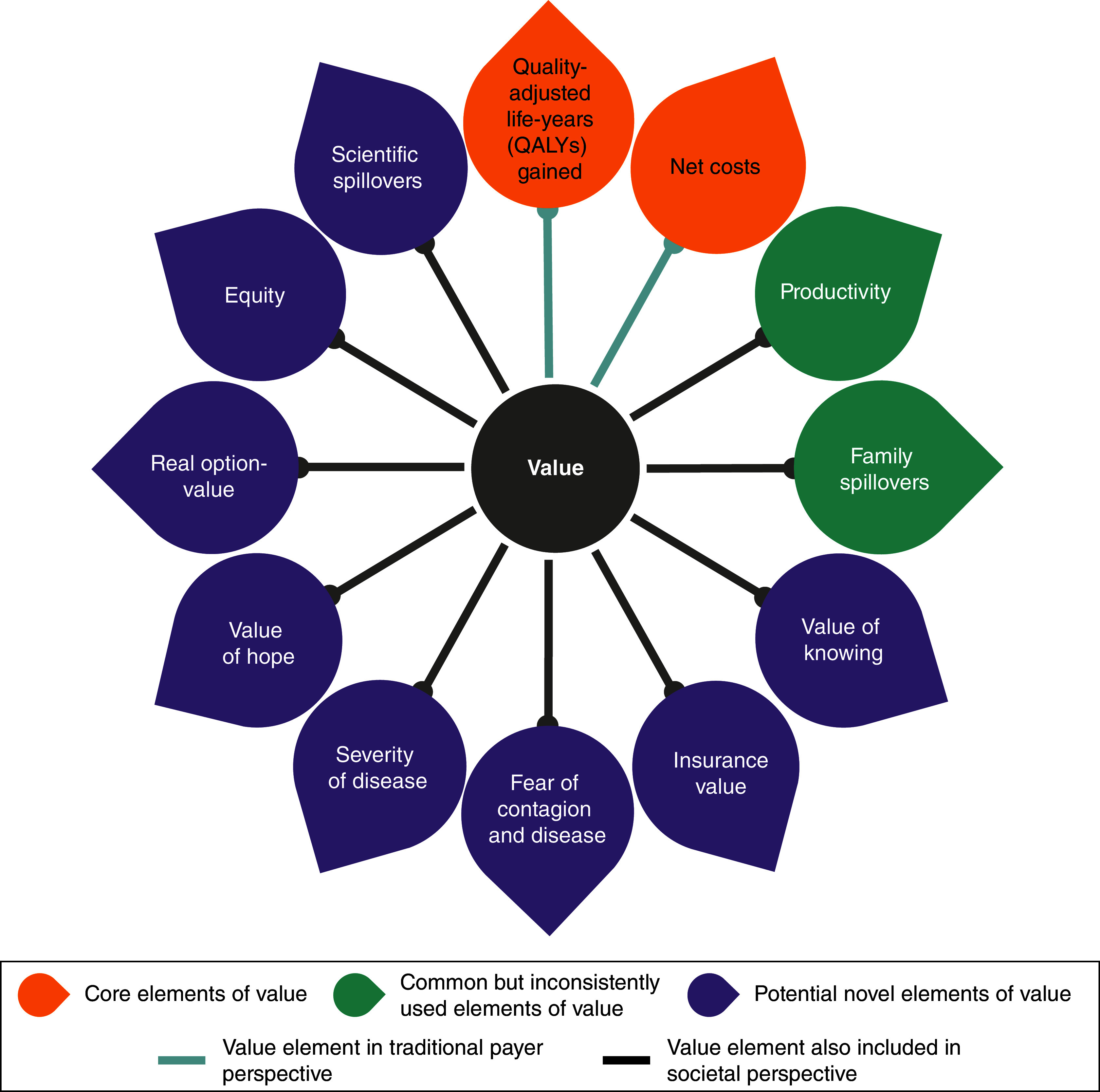
Adapted from Lakdawalla et al. [4].
The report has been cited over 400-times since it was published. Although it has received significant attention, no major US public or private insurer has adopted it explicitly as a comprehensive evaluation framework, and therefore it is an open question as to how practically useful it has been. The impact of the report on how health technology assessment (HTA) agencies globally appraise medicines has also been limited to date. Breslau and colleagues reviewed 53 HTA guidelines representing 52 countries and collected data on whether each guideline mentioned elements of value [5]. Breslau et al. evaluated 21 value elements in total; looking at the ten elements of value from the original ISPOR value flower (all elements apart from QALYs and net costs and considering adherence instead of family spillover); HTA guidelines on average mentioned only three out of ten of these elements. Importantly, mention of value elements in guidelines does not necessarily mean that HTA bodies use these elements in their ultimate decision making; indeed, these elements were rarely suggested to be included in base-case analyses.
The motivation for the emerging value frameworks in the US were related to concerns about the rising price of new medicines, and the STF focused on the issue of value as an incentive for innovation. HTA considerations are broader, being pertinent for a series of related health system decisions for medicines (e.g., incorporation into guidelines). In thinking about how the ISPOR value flower might be useful in countries outside of the US, it is perhaps useful to compare two alternatives: a non-US archetype with the pluralistic (or fragmented) US insurance system. Arguably, the major distinction is that non-US systems provide universal health coverage (UHC): all citizens are in the same health plan and lifetime risk-sharing pool. Although there is usually private sector supplemental insurance available, for the large majority of health spending, the government is a single-payer operating with an annual budget. This archetype most represents jurisdictions in Europe, as well as Australia and Canada, where mature HTA processes exist to evaluate health technologies for single-payers. Therefore, it is possible that HTA agencies are not considering some elements of value from the flower because they are not as applicable to a non-US archetype. This paper reflects on the elements of value which were defined in the ISPOR value flower and critiques their relevance in the context of jurisdictions in Europe, Australia and Canada from the perspective of both patients and (largely) single payers who have decisions informed by HTA. In doing so, we hope to further the routine consideration of elements of value by HTA agencies and drive research into the best approaches to incorporate them into HTA.
Assessment of value element relevance to Europe, Australia & Canada
Table 1 shows our assessment of whether each of the ISPOR value elements are relevant from a single-payer or non-US patient perspective from a targeted literature review. We also noted whether European, Canadian or Australian HTA guidelines consider these value elements using data from Breslau et al., with the acknowledgement that inclusion in guidelines does not mean they are actually used in decision making. An additional caveat is that while the non-US archetype may reflect a ‘single-payer’, currently payers generally only consider impact on healthcare budgets, whereas we have considered an ideal situation where a government budget is considered holistically. The only elements currently not mentioned in any European, Australian or Canadian HTA guideline is insurance value and the fear of contagion. Given their lack of inclusion in any HTA guideline, we discuss next the potential reasons for this.
Table 1. . The relevance of the International Society for Pharmacoeconomics and Outcomes Research value flower elements from a patient and single-payer perspective in Europe, Australia and Canada and their inclusion in European, Australian or Canadian HTA guidelines.
| Value element | Relevant in Europe, Australia and Canada-single-payer perspective? | Relevant in Europe, Australia and Canada-patient perspective? | Countries currently mentioning in HTA guidelines (data from Breslau et al.) | Ref. |
|---|---|---|---|---|
| Reduction in uncertainty | Yes – relevant in terms of more efficient use of technologies | Yes – knowledge of who would benefit would be valued | Australia, Canada, Denmark | [23,24] |
| Fear of contagion | Not applicable | Yes – people would value a technology that reduces their fear that they may catch an infectious disease | None | [25] |
| Insurance value | Potentially. In the case of anti-microbials it has been classified as the societal value of having a treatment available in case of a future major or rapidly escalating health problem | Yes- physical risk protection is relevant as healthy individuals would value knowing there is a treatment available for them should they fall sick, however this has not been well quantified empirically. No for financial risk- with universal healthcare coverage this is less relevant | None | [6,9] |
| Severity of disease | Not applicable | Yes – evidence exists that society places additional value on treatments for those with severe disease | Australia, Belgium, Canada, Czech Republic, France, Germany, Ireland, Norway, Scotland, Slovenia, Switzerland | [26] |
| Value of hope | Not applicable | Yes – potentially relevant, however the risk appetite for EU patients has not been well quantified | Denmark | [27] |
| Real option-value | Not applicable | Yes – patients would value an extension of life to benefit from future medical innovations | Denmark | [28] |
| Equity | Yes – society is willing to trade efficiency against inequality | Yes – society is willing to trade efficiency against inequality | Australia, Belgium, Canada, Croatia, Czech Republic, England, France, Germany, Hungary, Ireland, Norway, Poland, Slovenia, Spain, Switzerland | [29] |
| Scientific spillovers | Yes – pharmaceutical innovation should enable other organizations to generate new value for payers in the future | Yes – the potential of knowledge spillovers leading to better drugs in the future would be valued | England, Scotland | [30,31] |
| Family spillover | Yes – relevant in terms of humanistic and economic cost of diseases impacting caregivers | Yes – relevant in terms of humanistic impact on caregivers | Baltics, Belgium, Croatia, Czech Republic, Denmark, England, Finland, France, Germany, Ireland, Italy, The Netherlands, Norway, Poland, Portugal, Scotland, Spain, Sweden, Switzerland | [32,33] |
| Adherence improving factors | Yes – relevant in terms of more efficient use of technologies | Yes – relevant as it should increase a technology's benefit | Australia, Canada, Germany, Hungary, Ireland, The Netherlands, Norway, Scotland, Slovakia, Slovenia, Spain | [34,35] |
| Productivity | Yes – relevant in terms of tax revenues | Yes – relevant given financial and quality of life benefits | Austria, Australia, Belgium, Canada, Czech Republic, Denmark, England, Finland, France, Germany, Hungary, Ireland, Italy, The Netherlands, Norway, Poland, Spain, Sweden | [36,37] |
Relevance was assessed using data from a targeted literature review.
Insurance value-lost in translation?
Perhaps the primary reason for the lack of inclusion of ‘insurance value’ in European, Australian or Canadian HTA guidelines reflects the differing healthcare systems between these countries and the USA. The ISPOR STF described the element of insurance value as having two separate contributing elements. The first element is that of physical risk, in other words, the risk of a healthy individual becoming sick. With the availability of a novel intervention for a particular disease or condition, the risk for a healthy person getting sick is reduced and/or the ‘sick’ state is less unpleasant. This element of insurance value is undoubtedly important to people regardless of the nature of the healthcare system in their country, and perhaps best exemplified by COVID-19 vaccines reducing the risk of getting sick or experiencing severe complications [6,7]. Vaccines meant that we all had less to fear from it, even though not all of us would develop COVID-19. The second element of insurance value is financial risk protection which considers the importance of medical insurance covering novel interventions to protect healthy individuals from paying for the medical costs that the new intervention would avert. The financial risk protection aspect of insurance value is, however, not as relevant for single-payer/universal healthcare countries where there is little financial risk to a patient for the costs of medical care (although private insurance does exist in these countries for those who want to access services faster). As such, it is notable how for countries outside of the USA, the concept of insurance value has been used differently. For example, the UK Office for Health Economics has used insurance value in the context of assessing the value of novel antibiotics [8]. In this setting, it is seen as the value associated with avoiding potential costs to health systems and society through making a treatment such as a novel antimicrobial available, or keeping it in reserve, for a range of adverse scenarios where anti-microbial resistance could become substantially worse. The potential insurance value of a novel antimicrobial for the UK, under the assumption that antimicrobial resistance levels remain at the levels seen today, was calculated to be £718 million over a decade, reflecting the increase in operational healthcare costs needed, as well as the broader societal impact involving population disease transmission, productivity loss and informal care [9]. Therefore, while physical risk protection should be appraised from a patient perspective in Europe, Australia and Canada, for a more relevant value flower, financial risk protection could be seen from the perspective of the payer, specifically as the societal costs of not having a treatment in place (for example, the costs of additional nursing homes for patients with dementia). In summary, while there is currently no common definition of insurance value, it appears to be relevant in all countries regardless of payer archetype.
No fear of (European) contagion?
The ISPOR STF described the ‘fear of contagion’ as the anxiety associated with the risk of a future spread of an infectious disease, such as Ebola, and the value an intervention can bring in reducing this anxiety. Despite not being explicitly mentioned in European HTA guidelines, it has nevertheless been evaluated in non-US HTA. Yuasa et al. investigated appraisals of hepatitis C drugs and found that the Australian HTA agency, the Pharmaceutical Benefits Advisory Committee (PBAC) and the English HTA body, the National Institute for Health and Care Excellence (NICE) both recognized how hepatitis C treatments can reduce transmission and therefore fear of disease spread [10]. Perhaps the reason why it is not explicitly mentioned in HTA guidelines is due to the limited instances of epidemics broadly afflicting European countries (and Western European mature HTA agency guidelines influence HTA guidelines across the globe) and relatedly, vaccines only represent a small fraction of technologies assessed by HTA bodies. However, as we emerge from the COVID-19 pandemic, perhaps this will change in preparation for future infectious disease outbreaks. Empirical estimates for the fear of contagion in Europe, Australia and Canada are also lacking – a general problem for many value elements which we will discuss next.
Where's the non-US data?
The value elements ‘value of hope’, ‘real option value’, ‘reduction in uncertainty’ and ‘scientific spillovers’ have had limited uptake in European, Australian or Canadian HTA guidelines being seen in guidelines for one to three countries. It is likely that the limited consideration of these value elements is because the inherent value assigned to these elements by patients or society has not been quantified well in Europe, Australia or Canada. For example, the value of hope describes the value that a patient may place on the chance (however small) that a treatment which offers a wider range of potential outcomes, gives them a significant benefit. This is well demonstrated in oncology, where advanced cancer patients in the USA value the hope of a large survival gain from a treatment, independent of the therapy's average mortality improvement [11]. No study has yet been conducted to investigate this preference from a non-US patient perspective [12,13]. The same is also true for real option value and reduction in uncertainty. The concept of real option value suggests that value may be generated when a medical technology extends life for a patient long enough so that they may potentially benefit from the next treatment innovation. An example using US real-world data showed that for patients with advanced or metastatic melanoma, 50% receiving first-line ipilimumab survived long enough to receive a more efficacious immunotherapy (pembrolizumab) after its FDA approval as compared with only 18% who received first-line chemotherapy [14]. Similar studies could easily be performed using non-US real-world data but have not been done to date. For reduction in uncertainty there is also the fact that diagnostics generally have different HTA processes than those used for medicines [15], so it is plausible that the ISPOR value flower has been slower to permeate into the area of diagnostics assessment as compared with medicines. Scientific spillovers, referring to positive knowledge externalities arising from the development of a product, has received little attention globally in terms of attempts to quantify. Delandistrogene moxeparvovec, a newly approved gene therapy for Duchenne muscular dystrophy, can serve as an example of scientific spillovers. Learnings from the development of this therapy around adeno-associated virus gene delivery are already being used to inform development of other gene-based treatments in areas ranging from efficiency of viral vectors and ability to scale manufacturing volumes [16]. There is limited research in this area perhaps due to the difficulties in robustly enumerating the positive externalities arising from innovation. There may also be reluctance from agencies to factor in elements like scientific spillovers and option value, as they depend on as yet undiscovered innovations. There is also the issue that HTA agencies have a narrow remit and do not consider spillovers outside of healthcare. To conclude, a number of value elements have limited quantification in non-US settings, and attempts need to be made to quantify these elements for HTA decision makers to deliberate upon.
What methods to use?
There are two general challenges when it comes to novel value elements and HTA. First, how to measure these elements and second, once measured how to incorporate them into decision making. For elements such as severity of disease, equity, family spillovers, adherence improving factors and productivity, while they have been included in many HTA guidelines, there is not clear methodological guidance as to how to best incorporate these factors into CEA, and importantly also whether or not CEA is the most optimal way to incorporate such evidence, and as such they are not routinely considered in HTA decision making. For example, for family spillovers there are questions around how to incorporate caregiver quality of life into health economic models due to the lack of consensus on aggregating QALYs across multiple individuals [17]. For equity, while there is increasing awareness of health equity across HTA bodies, it is not yet routinely considered by them. Potential approaches to do so range from quantifying inequalities in the unmet need of the label patient population through to more specific assessments such as distributional cost–effectiveness analyses which can help to provide information about the equity impacts of new health technologies as well as trade-offs that might arise between equity and efficiency [18]. This method however requires further demonstration studies including country-specific considerations such as domains of equity of most relevance to enable adoption routinely by HTA agencies. There still remains the challenge of how to translate results into greater rewards for innovation. In Europe, not all countries have a distinct role for CEA in decision making, for example Germany. For these non-cost–effectiveness HTA systems, there is a need to understand how these value elements could be considered in decision making, including transparency in how these elements are weighted and prioritized.
Do we need more petals?
The US second panel on cost–effectiveness in health and medicine took a public health perspective when determining what additional aspects of value to include in societal CEA [2]. These aspects included social services, education, criminal justice, housing and the environment. Given the governmental single-payers in Europe, Australia and Canada for healthcare are also responsible for all these other societal costs or issues, it is perhaps sensible to consider these in an international value flower to foster decision making based on total costs to governments rather than a siloed healthcare-only perspective.
These elements again all need quantification but there are some data already to support their inclusion. For example, a study in Denmark has shown that parental cancer can negatively impact children's educational outcomes, especially if the cancer is one that has a poor prognosis (e.g., limited treatment options) [19] and an investigation in Sweden demonstrated that treatment for mental health disorders significantly reduces the rate of crime [20].
Moreover, healthcare is responsible for up to 8% of a country's carbon dioxide equivalent emissions annually [21]. The emissions are primarily derived from the healthcare supply chain through the production, transport and disposal of goods and services, such as pharmaceuticals, medical devices, hospital equipment and instruments. By incorporating environmental impact in decision-making, HTA has the potential to influence the impact of carbon footprint of new technologies. Crucially, this will not only impact current global health outcomes, but those of future generations [22].
Conclusion
The limited adoption of the ISPOR value flower by HTA agencies in Europe, Australia and Canada may be attributed to various factors. The relatively recent release of the STF report may contribute to the current lack of widespread use. Additionally, there are undoubted challenges associated with altering established HTA agency practices, and the lack of compelling incentives for HTA agencies to incorporate non-health effects into their recommendations for spending healthcare budgets. In UHC settings, legislation underpins value assessments and therefore this likely will need a wider consultation.
We contend that the ISPOR value elements should matter both in UHC systems outside the USA and in the USA. However, their impact will vary depending on the degree of risk aversion and baseline severity of the population as well as health system productivity and costs. Our call for action is to ensure that the single-payer and non-US patient perspective is considered. This may influence how some value elements are quantified (e.g., insurance value), and also lead to the inclusion of additional petals. We need more collaborative research that engages HTA bodies to generate European, Canadian and Australian value element estimates as well as to decide upon the best modelling approach to drive enhanced inclusion of these estimates into CEA. For example, by definition, a health system with UHC places a higher weight on equity than one without UHC. Methodology to transparently use value elements for decision making in HTA systems not using CEA must also be established. This is perhaps all the more important as we move to a EU HTA and a separate value flower tailored specifically to Europe, Australia and Canada may help to stimulate all of this.
To conclude, we argue that the ISPOR value flower has been useful both in the USA and outside the USA in stimulating discussion and research about the broader impact of new medicines on societal well-being. The standard cost–effectiveness framework of health economics has been and remains a useful starting point. But if we begin to recognize and address some of its limitations, we should be able to better steer innovation in the direction of global dynamic efficiency, thereby also providing an opportunity to improve global health equity as well.
Footnotes
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Goring S, Garrison LP, Jansen JP, Briggs AH. Novel elements of the value flower: fake or truly novel? Value Outcomes Spotlight 7(2), 34–39 (2021). [Google Scholar]
- 2.Sanders GD, Neumann PJ, Basu A et al. Recommendations for conduct, methodological practices, and reporting of cost–effectiveness analyses: second panel on cost–effectiveness in health and medicine. JAMA 316(10), 1093–1103 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Neumann PJ, Garrison LP, Willke RJ. The history and future of the “ISPOR Value Flower”: addressing limitations of conventional cost–effectiveness analysis. Value Health 25(4), 558–565 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care-a health economics approach: an ISPOR Special Task Force Report [3]. Value Health 21(2), 131–139 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Breslau RM, Cohen JT, Diaz J, Malcolm B, Neumann PJ. A review of HTA guidelines on societal and novel value elements. Int. J. Technol. Assess. Health Care 39(1), e31 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakdawalla D, Malani A, Reif J. The insurance value of medical innovation. J. Public Econ. 145, 94–102 (2017). [Google Scholar]
- 7.Kamal-Bahl S, Willke RJ, Puckett JT, Doshi JA. The case for using novel value elements when assessing COVID-19 vaccines and therapeutics. Health Aff. Forefr. (2020). [Google Scholar]
- 8.Schaffer SK, West P, Towse A, Henshall C, Mestre-Ferrandiz J, Fischer A. Assessing the value of new antibiotics: additional elements of value for health technology assessment decisions. Office for Health Economics (2017). https://www.ohe.org/news/assessing-value-new-antibiotics-additional-elements-value-health-technology-assessment/ [Google Scholar]
- 9.Chan MS, Holloway R, King R et al. An insurance value modeling approach that captures the wider value of a novel antimicrobial to health systems, patients, and the population. J. Health Econ. Outcomes Res. 10(2), 1–9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuasa A, Yonemoto N, Demiya S, Foellscher C, Ikeda S. Investigation of factors considered by health technology assessment agencies in eight countries. PharmacoEconomics Open 5(1), 57–69 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost–effectiveness assessments of high-cost cancer therapies. Health Aff. Proj. Hope 31(4), 676–682 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Peasgood T, Mukuria C, Rowen D, Tsuchiya A, Wailoo A. Should we consider including a value for “hope” as an additional benefit within health technology assessment? Value Health 5(9), 1619–1623 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Garrison LP, Zamora B, Li M, Towse A. Augmenting cost–effectiveness analysis for uncertainty: the implications for value assessment-rationale and empirical support. J. Manag. Care Spec. Pharm. 26(4), 400–406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong WB, To TM, Li M, Lee W, Veenstra DL, Garrison LP. Real-world evidence for option value in metastatic melanoma. J. Manag. Care Spec. Pharm. 27(11), 1546–1555 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.di Ruffano LF, Harris IM, Zhelev Z et al. Health technology assessment of diagnostic tests: a state of the art review of methods guidance from international organisations. Int. J. Technol. Assess. Health Care 39(1), e14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher D, Dai D, Klimchak AC, Sedita LE, Gooch KL, Rodino-Klapac L. Paving the way for future gene therapies: a case study of scientific spillover from delandistrogene moxeparvovec. Mol. Ther. Methods Clin. Dev. 30, 474–483 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittenberg E, James LP, Prosser LA. Spillover effects on caregivers' and family members' utility: a systematic review of the literature. Pharmacoeconomics 37(4), 475–499 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Cookson R, Griffin S, Norheim OF, Culyer AJ. Distributional cost–effectiveness Analysis: Quantifying Health Equity Impacts and Trade-Offs. Oxford University Press, UK: (2020). [Google Scholar]
- 19.Aaskoven MS, Kjaer T, Gyrd-Hansen D. Effects of parental health shocks on children's school achievements: a register-based population study. J. Health Econ. 81, 102573 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Fazel S, Zetterqvist J, Larsson H, Långström N, Lichtenstein P. Antipsychotics, mood stabilisers, and risk of violent crime. Lancet 384(9949), 1206–1214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Shift Project. Décarboner la santé pour soigner durablement (2023). https://theshiftproject.org/article/decarboner-sante-rapport-2023/
- 22.McAlister S, Morton RL, Barratt A. Incorporating carbon into health care: adding carbon emissions to health technology assessments. Lancet Planet. Health 6(12), e993–e999 (2022). [DOI] [PubMed] [Google Scholar]
- 23.World Economic Forum. An economic analysis of the value of genetic testing. (2021). https://www3.weforum.org/docs/WEF_An_Economic_Analysis_of_the_Value_of_Genetic_Testing_2021.pdf
- 24.Neumann PJ, Cohen JT, Hammitt JK et al. Willingness-to-pay for predictive tests with no immediate treatment implications: a survey of US residents. Health Econ. 21(3), 238–251 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Poudel N, Ngorsuraches S. A preference-based value assessment of the fear of COVID-19 contagion. Patient Prefer. Adherence 17, 3435–3448 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor M, Chilton S, Ronaldson S, Metcalf H, Nielsen JS. Comparing increments in utility of health: an individual-based approach. Value Health 20(2), 224–229 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Mulligan K, Baid D, Doctor JN, Phelps CE, Lakdawalla DN. Risk preferences over health: empirical estimates and implications for healthcare decision making. Natl Bureau Economic Res. 94, 102857 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Li M, Basu A, Bennette CS, Veenstra DL, Garrison LP. Do cancer treatments have option value? Real-world evidence from metastatic melanoma. Health Econ. 28(7), 855–867 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Cadham CJ, Prosser LA. Eliciting trade-offs between equity and efficiency: a methodological scoping review. Value Health 26(6), 943–952 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Xie RZ, Towse A, Garrison LP. Should we pay for scientific knowledge spillovers? The underappreciated value of “failed” R&D efforts. Int. J. Technol. Assess. Health Care 38(1), e31 (2022). [DOI] [PubMed] [Google Scholar]
- 31.“Our policy on cancer drugs” (2017). https://www.cancerresearchuk.org/about-us/we-develop-policy/our-policy-on-access-to-cancer-treatments/our-policy-on-cancer-drugs
- 32.Park JY, Marcum ZA, Garrison LP. Toward a broader concept of societal value: family spillovers in Alzheimer's disease. Int. J. Technol. Assess. Health Care 38(1), e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Janabi H, Wittenberg E, Donaldson C, Brouwer W. The relative value of carer and patient quality of life: a person trade-off (PTO) study. Soc. Sci. Med. 1982 292, 114556 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 8(1), e016982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson SH, Eurich DT, Majumdar SR et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 333(7557), 15 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Public Health England. Cost of Ill Health (2016). https://www.gov.uk/government/publications/health-matters-health-and-work/health-matters-health-and-work [Google Scholar]
- 37.Maksymowych WP, Gooch KL, Wong RL, Kupper H, Heijde DVD. Impact of age, sex, physical function, health-related quality of life, and treatment with adalimumab on work status and work productivity of patients with ankylosing spondylitis. J. Rheumatol. 37(2), 385–392 (2010). [DOI] [PubMed] [Google Scholar]


