Abstract
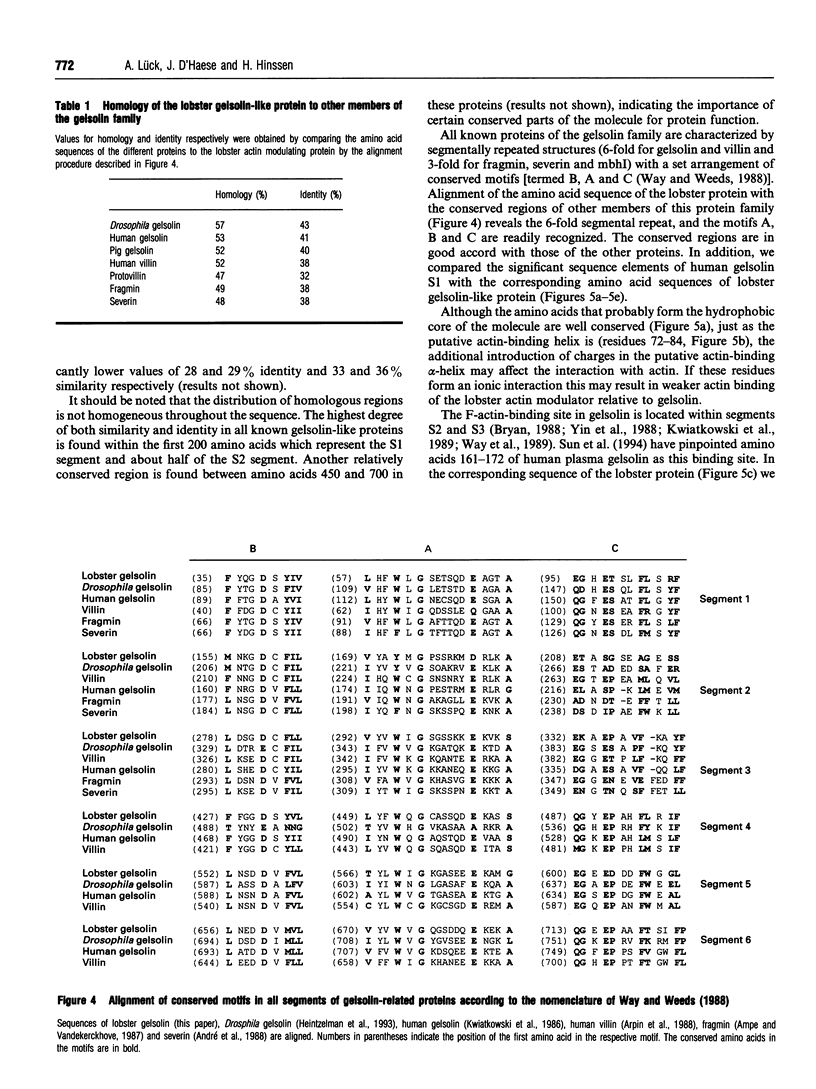
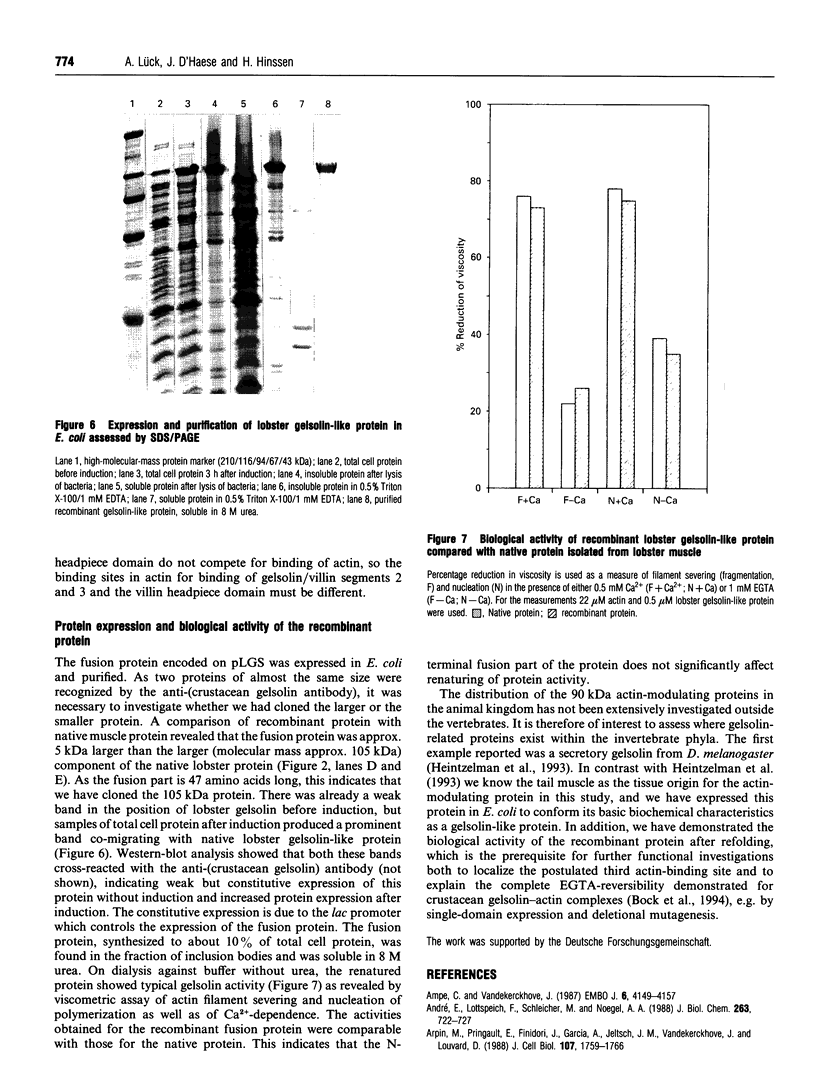
The tail muscle of the lobster Homarus americanus contains an actin-binding protein with an apparent molecular mass of 105 kDa determined by SDS/PAGE and gelsolin-like properties. We isolated this protein and peptide sequences were obtained after limited proteolysis with chymotrypsin. A tail-muscle-specific cDNA library was constructed in a lambda expression vector and a full-length clone was obtained by screening with a polyclonal anti-(crustacean gelsolin) antibody. The cDNA insert of approx. 3.2 kb length was sequenced. The cDNA contained an open reading frame of 2.265 kb, and the deduced amino acid sequence of 754 residues (83,469 Da) identified the protein as a cytoplasmic member of the gelsolin/villin protein family. Comparison of the lobster gelsolin amino acid sequence with other members of this protein family revealed the characteristic 6-fold repeated segmental structure as well as the three conserved sequence motifs typical of each segment [Way and Weeds (1988) J. Mol. Biol. 203, 1127-1133]. Strong homologies were found with Drosophila gelsolin, human gelsolin, villin core, Dictyostelium severin and Physarum fragmin. In addition, the gelsolin-like protein from lobster muscle revealed motifs that were clearly similar to the actin-bundling region of human villin headpiece although it did not itself contain a distinct headpiece domain. The recombinant lobster gelsolin-like protein, expressed in Escherichia coli as a fusion protein, was purified from inclusion bodies and renatured as a functional protein. There were no significant differences in the biological activity tested between the recombinant and the native protein isolated from lobster muscle.
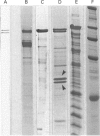
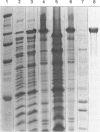
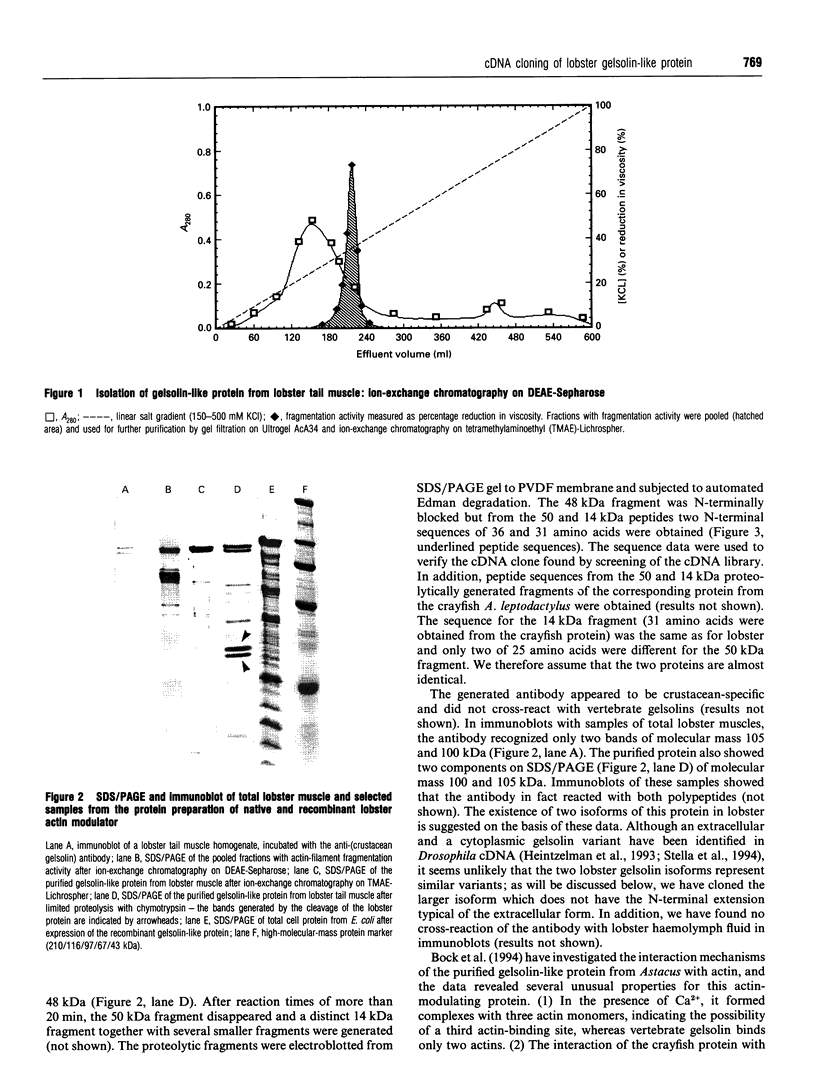
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampe C., Vandekerckhove J. The F-actin capping proteins of Physarum polycephalum: cap42(a) is very similar, if not identical, to fragmin and is structurally and functionally very homologous to gelsolin; cap42(b) is Physarum actin. EMBO J. 1987 Dec 20;6(13):4149–4157. doi: 10.1002/j.1460-2075.1987.tb02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E., Lottspeich F., Schleicher M., Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988 Jan 15;263(2):722–727. [PubMed] [Google Scholar]
- Arpin M., Pringault E., Finidori J., Garcia A., Jeltsch J. M., Vandekerckhove J., Louvard D. Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J Cell Biol. 1988 Nov;107(5):1759–1766. doi: 10.1083/jcb.107.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazari W. L., Matsudaira P., Wallek M., Smeal T., Jakes R., Ahmed Y. Villin sequence and peptide map identify six homologous domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4986–4990. doi: 10.1073/pnas.85.14.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock D., Hinssen H., D'Haese J. A gelsolin-related actin-severing protein with fully reversible actin-binding properties from the tail muscle of crayfish, Astacus leptodactylus. Eur J Biochem. 1994 Oct 15;225(2):727–735. doi: 10.1111/j.1432-1033.1994.00727.x. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Osborn M., Wehland J., Weber K. Villin associates with specific microfilamentous structures as seen by immunofluorescence microscopy on tissue sections and cells microinjected with villin. Exp Cell Res. 1981 Sep;135(1):213–219. doi: 10.1016/0014-4827(81)90313-x. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci U S A. 1979 May;76(5):2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Coluccio L. M. Kinetic analysis of F-actin depolymerization in the presence of platelet gelsolin and gelsolin-actin complexes. J Cell Biol. 1985 Oct;101(4):1236–1244. doi: 10.1083/jcb.101.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. Gelsolin has three actin-binding sites. J Cell Biol. 1988 May;106(5):1553–1562. doi: 10.1083/jcb.106.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Kurth M. C. Actin-gelsolin interactions. Evidence for two actin-binding sites. J Biol Chem. 1984 Jun 25;259(12):7480–7487. [PubMed] [Google Scholar]
- Dissmann E., Hinssen H. Immunocytochemical localization of gelsolin in fibroblasts, myogenic cells, and isolated myofibrils. Eur J Cell Biol. 1994 Apr;63(2):336–344. [PubMed] [Google Scholar]
- Finidori J., Friederich E., Kwiatkowski D. J., Louvard D. In vivo analysis of functional domains from villin and gelsolin. J Cell Biol. 1992 Mar;116(5):1145–1155. doi: 10.1083/jcb.116.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E., Vancompernolle K., Huet C., Goethals M., Finidori J., Vandekerckhove J., Louvard D. An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell. 1992 Jul 10;70(1):81–92. doi: 10.1016/0092-8674(92)90535-k. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Geisler N., Kaulfus P., Weber K. Demonstration of at least two different actin-binding sites in villin, a calcium-regulated modulator of F-actin organization. J Biol Chem. 1981 Aug 10;256(15):8156–8161. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Kaulfus P., Weber K. F actin assembly modulated by villin: Ca++-dependent nucleation and capping of the barbed end. Cell. 1981 May;24(2):471–480. doi: 10.1016/0092-8674(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Takahashi S., Hayashi H., Hatano S. Fragmin: a calcium ion sensitive regulatory factor on the formation of actin filaments. Biochemistry. 1980 Jun 10;19(12):2677–2683. doi: 10.1021/bi00553a021. [DOI] [PubMed] [Google Scholar]
- Heintzelman M. B., Frankel S. A., Artavanis-Tsakonas S., Mooseker M. S. Cloning of a secretory gelsolin from Drosophila melanogaster. J Mol Biol. 1993 Apr 5;230(3):709–716. doi: 10.1006/jmbi.1993.1191. [DOI] [PubMed] [Google Scholar]
- Hinssen H. An actin-modulating protein from Physarum polycephalum. I. Isolation and purification. Eur J Cell Biol. 1981 Feb;23(2):225–233. [PubMed] [Google Scholar]
- Hinssen H., Small J. V., Sobieszek A. A Ca2+-dependent actin modulator from vertebrate smooth muscle. FEBS Lett. 1984 Jan 23;166(1):90–95. doi: 10.1016/0014-5793(84)80051-4. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Eichinger L., André E., Rieger D., Schleicher M. Cap100, a novel phosphatidylinositol 4,5-bisphosphate-regulated protein that caps actin filaments but does not nucleate actin assembly. Cell Motil Cytoskeleton. 1992;23(2):133–144. doi: 10.1002/cm.970230206. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Noegel A. A., Bomblies L., Lottspeich F., Schleicher M. The 100 kDa F-actin capping protein of Dictyostelium amoebae is a villin prototype ('protovillin'). FEBS Lett. 1993 Aug 9;328(1-2):71–76. doi: 10.1016/0014-5793(93)80968-z. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C., Lind S. E., Zaner K. S., Stossel T. P., Yin H. L. Interactions of gelsolin and gelsolin-actin complexes with actin. Effects of calcium on actin nucleation, filament severing, and end blocking. Biochemistry. 1985 Jul 2;24(14):3714–3723. doi: 10.1021/bi00335a046. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Lamb J., Allen P. G., Matsudaira P. T. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem. 1992 Jun 15;267(17):11818–11823. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Janmey P. A., Yin H. L. Identification of critical functional and regulatory domains in gelsolin. J Cell Biol. 1989 May;108(5):1717–1726. doi: 10.1083/jcb.108.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Stossel T. P., Orkin S. H., Mole J. E., Colten H. R., Yin H. L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986 Oct 2;323(6087):455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993 Aug 19;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Sakurai T., Nonomura Y. Differential expression of bovine adseverin in adrenal gland revealed by in situ hybridization. Cloning of a cDNA for adseverin. J Biol Chem. 1994 Feb 25;269(8):5890–5896. [PubMed] [Google Scholar]
- Pope B., Way M., Matsudaira P. T., Weeds A. Characterisation of the F-actin binding domains of villin: classification of F-actin binding proteins into two groups according to their binding sites on actin. FEBS Lett. 1994 Jan 24;338(1):58–62. doi: 10.1016/0014-5793(94)80116-9. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. Mbh 1: a novel gelsolin/severin-related protein which binds actin in vitro and exhibits nuclear localization in vivo. EMBO J. 1991 Apr;10(4):757–766. doi: 10.1002/j.1460-2075.1991.tb08007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine S., Huet C., Moll R., Sahuquillo-Merino C., Coudrier E., Zweibaum A., Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci U S A. 1985 Dec;82(24):8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Ohmi K., Kurokawa H., Nonomura Y. Distribution of a gelsolin-like 74,000 mol. wt protein in neural and endocrine tissues. Neuroscience. 1990;38(3):743–756. doi: 10.1016/0306-4522(90)90067-e. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella M. C., Schauerte H., Straub K. L., Leptin M. Identification of secreted and cytosolic gelsolin in Drosophila. J Cell Biol. 1994 May;125(3):607–616. doi: 10.1083/jcb.125.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. Q., Wooten D. C., Janmey P. A., Yin H. L. The actin side-binding domain of gelsolin also caps actin filaments. Implications for actin filament severing. J Biol Chem. 1994 Apr 1;269(13):9473–9479. [PubMed] [Google Scholar]
- Way M., Gooch J., Pope B., Weeds A. G. Expression of human plasma gelsolin in Escherichia coli and dissection of actin binding sites by segmental deletion mutagenesis. J Cell Biol. 1989 Aug;109(2):593–605. doi: 10.1083/jcb.109.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Weeds A. G. Are the conserved sequences in segment 1 of gelsolin important for binding actin? J Cell Biol. 1992 Mar;116(5):1135–1143. doi: 10.1083/jcb.116.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Weeds A. G. Evidence for functional homology in the F-actin binding domains of gelsolin and alpha-actinin: implications for the requirements of severing and capping. J Cell Biol. 1992 Nov;119(4):835–842. doi: 10.1083/jcb.119.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Weeds A. Nucleotide sequence of pig plasma gelsolin. Comparison of protein sequence with human gelsolin and other actin-severing proteins shows strong homologies and evidence for large internal repeats. J Mol Biol. 1988 Oct 20;203(4):1127–1133. doi: 10.1016/0022-2836(88)90132-5. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Iida K., Janmey P. A. Identification of a polyphosphoinositide-modulated domain in gelsolin which binds to the sides of actin filaments. J Cell Biol. 1988 Mar;106(3):805–812. doi: 10.1083/jcb.106.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Yu F. X., Sun H. Q., Janmey P. A., Yin H. L. Identification of a polyphosphoinositide-binding sequence in an actin monomer-binding domain of gelsolin. J Biol Chem. 1992 Jul 25;267(21):14616–14621. [PubMed] [Google Scholar]