Abstract
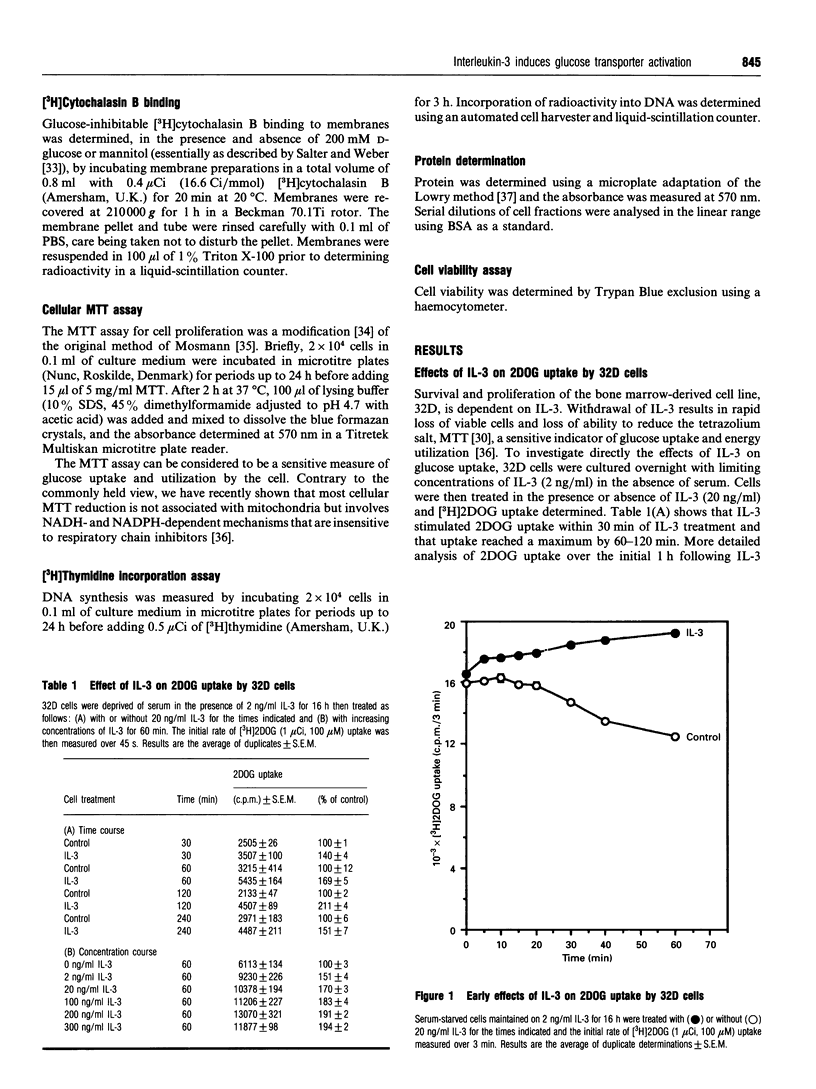
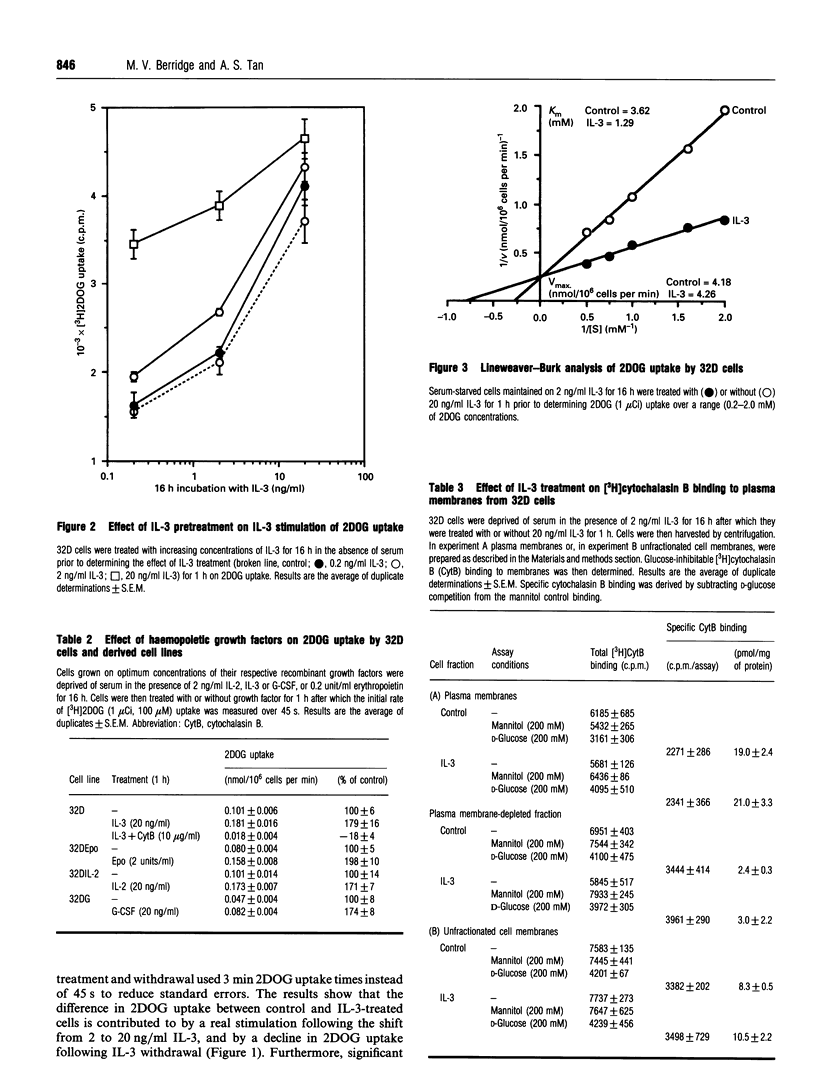
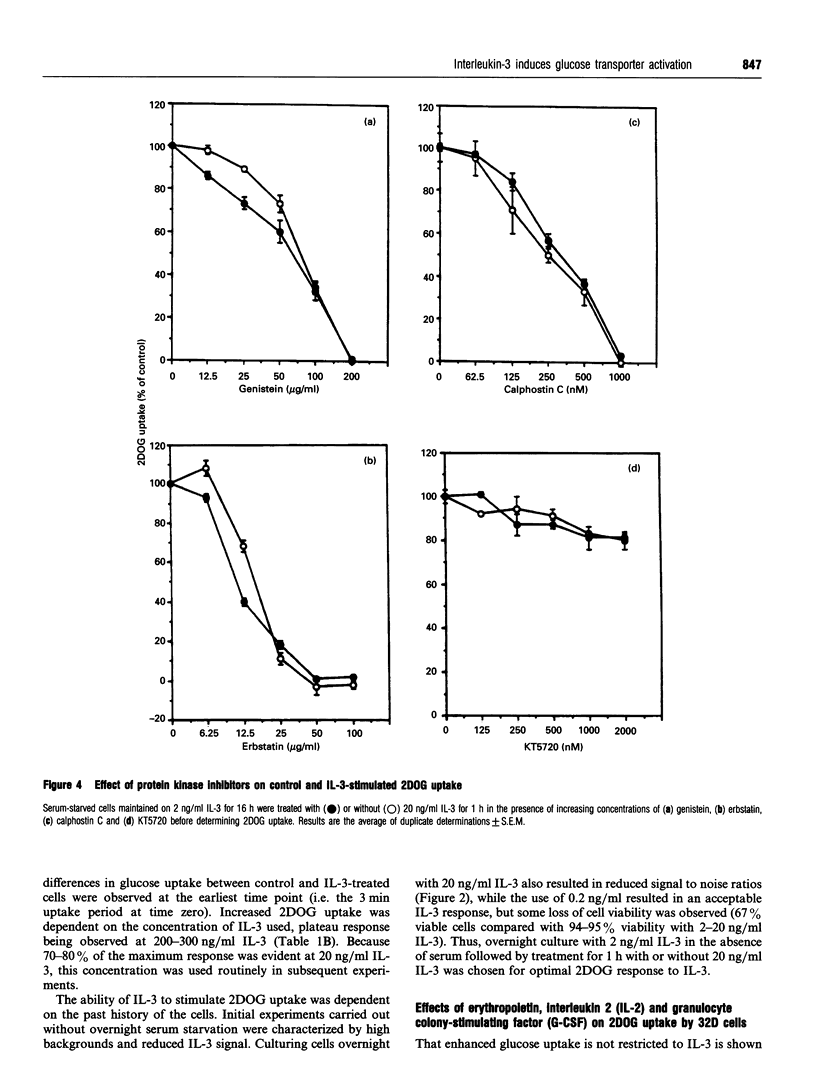
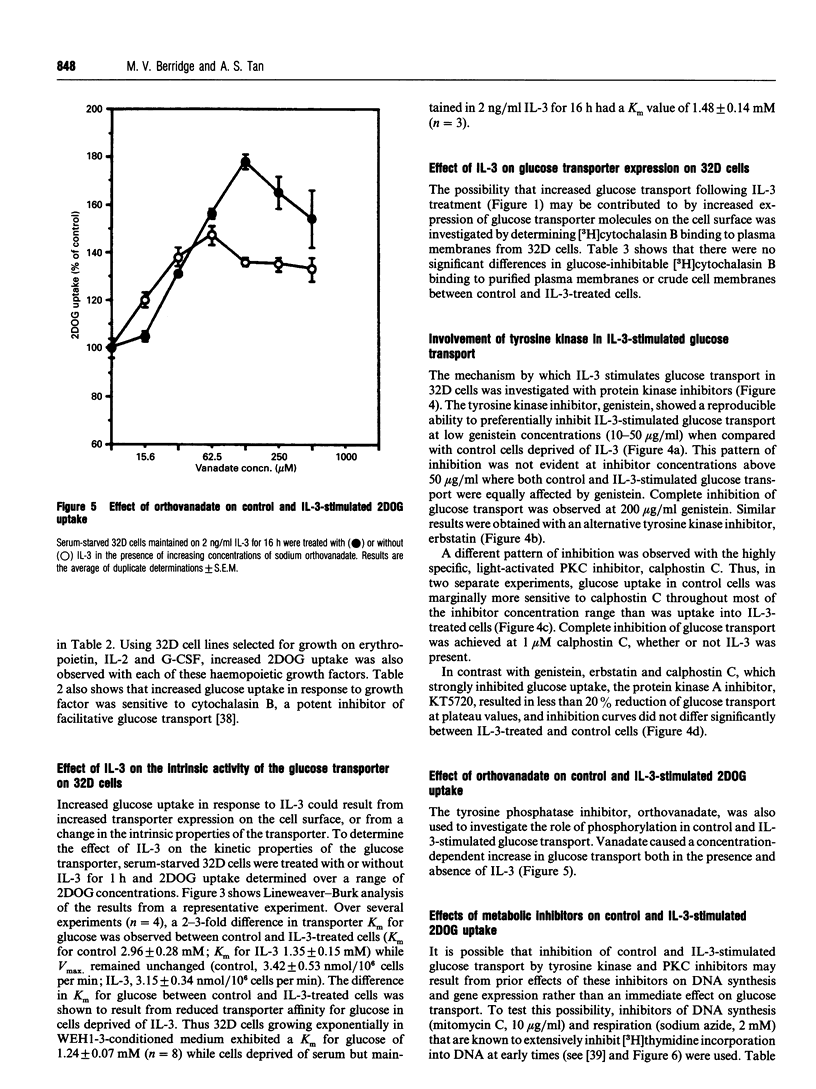
Growth factors promote cell survival and proliferation by activating signal transduction pathways that result in progression through the cell cycle and differential gene expression. Uptake of simple sugars needed for basal cell metabolism, and for macromolecular synthesis necessary for cell growth and proliferation, is thought to follow as a consequence of signal transduction to the nucleus. However, in the presence of inhibitors of DNA synthesis and respiration, growth factors can still promote cell survival responses in the short term, raising the possibility that they may also regulate critical membrane and cytosolic processes necessary for cell survival. We have tested this hypothesis directly by investigating the role of the haemopoietic growth factor, interleukin-3 (IL-3), in the regulation of glucose transport in the bone marrow-derived cell line, 32D. We show that IL-3 promotes glucose transport by actively maintaining the affinity of the plasma membrane, glucose transporter for glucose (Km 1.35 +/- 0.15 mM, n = 4). Withdrawal of IL-3 for 1 h resulted in reduced affinity for glucose (Km 2.96 +/- 0.28 mM, n = 4) without an associated change in Vmax. Furthermore, glucose transporter molecules as the cell surface, as determined by cytochalasin B binding to isolated plasma membranes, did not differ significantly between control and IL-3-treated cells. Inhibition of DNA synthesis with mitomycin C or with the respiratory poison, sodium azide, did not affect the ability of IL-3 to promote glucose transport. In contrast, the tyrosine kinase inhibitors genistein and erbstatin extensively inhibited control and IL-3-stimulated glucose transport, some preference of IL-3-stimulated glucose transport, some preference for IL-3-stimulated responses being observed at low inhibitor concentrations. The light-activated protein kinase C inhibitor, calphostin C, also inhibited control and IL-3-stimulated glucose transport but without preference for IL-3 responses. Additionally, the tyrosine phosphatase inhibitor, orthovanadate, stimulated control and IL-3-dependent glucose transport by 50-80% while the protein kinase A inhibitor, KT5720, inhibited glucose transport by about 20% at plateau values. These results indicate that IL-3 is involved in continuous maintenance of glucose transporter activity by a mechanism that involves tyrosine kinases and protein kinase C, and demonstrate that this activation is not dependent on respiration or signal transduction to the nucleus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard W. J., Gibbs E. M., Witters L. A., Lienhard G. E. The glucose transporter in human fibroblasts is phosphorylated in response to phorbol ester but not in response to growth factors. Biochim Biophys Acta. 1987 Jul 29;929(3):288–295. doi: 10.1016/0167-4889(87)90255-2. [DOI] [PubMed] [Google Scholar]
- Begum N., Draznin B. Effect of streptozotocin-induced diabetes on GLUT-4 phosphorylation in rat adipocytes. J Clin Invest. 1992 Oct;90(4):1254–1262. doi: 10.1172/JCI115988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N., Leitner W., Reusch J. E., Sussman K. E., Draznin B. GLUT-4 phosphorylation and its intrinsic activity. Mechanism of Ca(2+)-induced inhibition of insulin-stimulated glucose transport. J Biol Chem. 1993 Feb 15;268(5):3352–3356. [PubMed] [Google Scholar]
- Berridge M. V., Tan A. S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993 Jun;303(2):474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Tan A. S., Hilton C. J. Cyclic adenosine monophosphate promotes cell survival and retards apoptosis in a factor-dependent bone marrow-derived cell line. Exp Hematol. 1993 Feb;21(2):269–276. [PubMed] [Google Scholar]
- Bramwell M. E., Davies A., Baldwin S. A. Heterogeneity of the glucose transporter in malignant and suppressed hybrid cells. Exp Cell Res. 1990 May;188(1):97–104. doi: 10.1016/0014-4827(90)90282-f. [DOI] [PubMed] [Google Scholar]
- Bushart G. B., Vetter U., Hartmann W. Glucose transport during cell cycle in IM9 lymphocytes. Horm Metab Res. 1993 Apr;25(4):210–213. doi: 10.1055/s-2007-1002078. [DOI] [PubMed] [Google Scholar]
- Carruthers A. Facilitated diffusion of glucose. Physiol Rev. 1990 Oct;70(4):1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S., Jaspers S., Pasceri M. Acute inhibition of insulin-stimulated glucose transport by the phosphatase inhibitor, okadaic acid. J Biol Chem. 1991 May 15;266(14):9271–9275. [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Esko J. D., Gilmore J. R., Glaser M. Use of a fluorescent probe to determine the viscosity of LM cell membranes with altered phospholipid compositions. Biochemistry. 1977 May 3;16(9):1881–1890. doi: 10.1021/bi00628a019. [DOI] [PubMed] [Google Scholar]
- Gibbs E. M., Allard W. J., Lienhard G. E. The glucose transporter in 3T3-L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J Biol Chem. 1986 Dec 15;261(35):16597–16603. [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber R. S., Weinstein S. P., O'Boyle E., Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993 Jun;132(6):2538–2543. doi: 10.1210/endo.132.6.8504756. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Vairo G., Lingelbach S. R. Activation and proliferation signals in murine macrophages: stimulation of glucose uptake by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Mar;134(3):405–412. doi: 10.1002/jcp.1041340311. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Harrington C. R. Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determinations on fractions from brain tissue. Anal Biochem. 1990 May 1;186(2):285–287. doi: 10.1016/0003-2697(90)90081-j. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Clancy B. M., Czech M. P. Evidence that erythroid-type glucose transporter intrinsic activity is modulated by cadmium treatment of mouse 3T3-L1 cells. J Biol Chem. 1991 Oct 15;266(29):19438–19449. [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Czech M. P. Suppressed intrinsic catalytic activity of GLUT1 glucose transporters in insulin-sensitive 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7839–7843. doi: 10.1073/pnas.88.17.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman M. F., Goodyear L. J., Wardzala L. J., Horton E. D., Horton E. S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990 Jan 15;265(2):987–991. [PubMed] [Google Scholar]
- Holman G. D., Kozka I. J., Clark A. E., Flower C. J., Saltis J., Habberfield A. D., Simpson I. A., Cushman S. W. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990 Oct 25;265(30):18172–18179. [PubMed] [Google Scholar]
- James D. E., Hiken J., Lawrence J. C., Jr Isoproterenol stimulates phosphorylation of the insulin-regulatable glucose transporter in rat adipocytes. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8368–8372. doi: 10.1073/pnas.86.21.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J. F., James D. E. Phosphorylation of the glucose transporter in rat adipocytes. Identification of the intracellular domain at the carboxyl terminus as a target for phosphorylation in intact-cells and in vitro. J Biol Chem. 1990 Feb 5;265(4):2324–2332. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Lodish H. F. Post-translational insertion of a fragment of the glucose transporter into microsomes requires phosphoanhydride bond cleavage. Nature. 1986 Aug 7;322(6079):549–552. doi: 10.1038/322549a0. [DOI] [PubMed] [Google Scholar]
- Nefesh I., Bauskin A. R., Alkalay I., Golembo M., Ben-Neriah Y. IL-3 facilitates lymphocyte hexose transport by enhancing the intrinsic activity of the transport system. Int Immunol. 1991 Aug;3(8):827–831. doi: 10.1093/intimm/3.8.827. [DOI] [PubMed] [Google Scholar]
- Piper R. C., James D. E., Slot J. W., Puri C., Lawrence J. C., Jr GLUT4 phosphorylation and inhibition of glucose transport by dibutyryl cAMP. J Biol Chem. 1993 Aug 5;268(22):16557–16563. [PubMed] [Google Scholar]
- Reusch J. E., Begum N., Sussman K. E., Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology. 1991 Dec;129(6):3269–3273. doi: 10.1210/endo-129-6-3269. [DOI] [PubMed] [Google Scholar]
- Reusch J. E., Sussman K. E., Draznin B. Inverse relationship between GLUT-4 phosphorylation and its intrinsic activity. J Biol Chem. 1993 Feb 15;268(5):3348–3351. [PubMed] [Google Scholar]
- Salter D. W., Weber M. J. Glucose-specific cytochalasin B binding is increased in chicken embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1979 May 10;254(9):3554–3561. [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. Reassessment of the translocation hypothesis by kinetic studies on hexose transport in isolated rat adipocytes. J Biol Chem. 1988 Sep 5;263(25):12247–12252. [PubMed] [Google Scholar]
- Toyoda N., Flanagan J. E., Kono T. Reassessment of insulin effects on the Vmax and Km values of hexose transport in isolated rat epididymal adipocytes. J Biol Chem. 1987 Feb 25;262(6):2737–2745. [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Bazill G. W., Dexter T. M. Haemopoietic cell growth factor mediates cell survival via its action on glucose transport. EMBO J. 1984 Feb;3(2):409–413. doi: 10.1002/j.1460-2075.1984.tb01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Kinetic parameters of hexose transport in hybrids between malignant and nonmalignant cells. J Cell Sci. 1983 Jul;62:49–80. doi: 10.1242/jcs.62.1.49. [DOI] [PubMed] [Google Scholar]
- Whitesell R. R., Abumrad N. A. Increased affinity predominates in insulin stimulation of glucose transport in the adipocyte. J Biol Chem. 1985 Mar 10;260(5):2894–2899. [PubMed] [Google Scholar]
- Witters L. A., Vater C. A., Lienhard G. E. Phosphorylation of the glucose transporter in vitro and in vivo by protein kinase C. 1985 Jun 27-Jul 3Nature. 315(6022):777–778. doi: 10.1038/315777a0. [DOI] [PubMed] [Google Scholar]
- Yano H., Seino Y., Inagaki N., Hinokio Y., Yamamoto T., Yasuda K., Masuda K., Someya Y., Imura H. Tissue distribution and species difference of the brain type glucose transporter (GLUT3). Biochem Biophys Res Commun. 1991 Jan 31;174(2):470–477. doi: 10.1016/0006-291x(91)91440-n. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., Pilch P. F. Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J Biol Chem. 1989 Jul 25;264(21):12358–12363. [PubMed] [Google Scholar]


