In 2021, the American Thoracic Society (ATS)/European Respiratory Society (ERS) statements were revised, and the criteria for bronchodilator response (BDR) test were changed.1 The 2005 criteria (old criteria) were ≥12% and ≥200 mL in forced expiratory volume in 1 second (FEV1) and/or forced vital capacity (FVC) from baseline;2 however, the 2021 criteria (new criteria) are >10% of the predicted value in FEV1 and/or FVC. The major limitation of the old criteria is that the absolute and relative changes in FEV1 and FVC are inversely proportional to baseline lung function and are associated with height, age, and sex in both health and disease.1 The change was to reduce the association of baseline lung function (sex, age, and height differences) in assessing BDR by using the change in FEV1 and/or FVC relative to predicted values. It is unclear how changing from the old to the new criteria would change the patient population with BDR in clinical practice. This study compared cases meeting the old and new criteria in asthma patients in clinical practice. The subjects included 190 asthma patients who underwent BDR tests using short-acting β2 agonist (30 μg procaterol) inhalation according to a previous methods3 at our hospital from April 2014 to March 2018. Bronchodilator response tests were accumulated and compared for cases meeting the old and new criteria. The Shizuoka General Hospital ethics committee approved this study (approval number: SGHIRB#2021035, date: August 26, 2021) and permitted the use of the information in the database. The data acquired were kept anonymized. Since this was a retrospective study, the Board waived patient approval or informed consent.
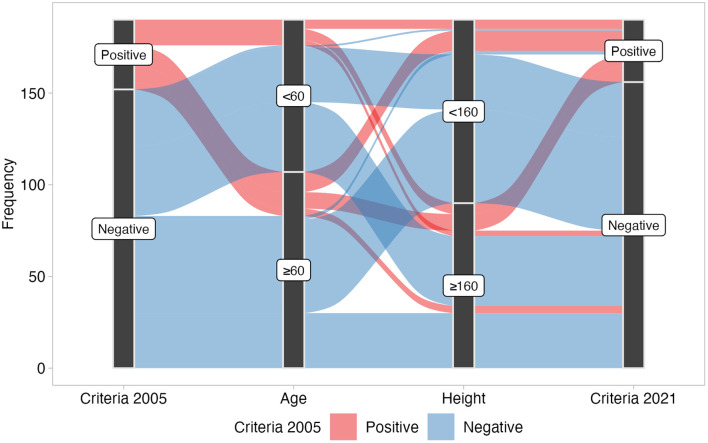
Among 190 patients (mean age ± standard deviation 60 ± 15 years; 106 females; median (interquartile range) body mass index, 22.9 (21.0-26.8) kg/m2) who underwent the BDR test, 38 (20.0%) were positive by the old criteria and 34 (17.9%) by the new criteria. Thirty-one patients were positive by both old and new criteria. Table 1 and Figure 1 show the patients with negative or positive conversions between 2005 and 2021 ATS/ERS BDR criteria, that is, 2005, ≥12% and 200 mL in FEV1 and/or FVC from baseline, and 2021, >10% of the predicted value in FEV1 and/or FVC, respectively. Seven patients who were positive by the old criteria but negative by the new criteria were taller and younger than the overall mean. Three patients who were negative by the old criteria but positive by the new criteria were shorter and older than the overall mean. The new criteria are based on predicted values as denominators, which become smaller in lower-height and older subjects, leading to larger BDRs.4 A positive conversion from the old criteria to the new criteria in 3 patients was caused by this effect.
Table 1.
Patients with Negative or Positive Conversions Between 2005 and 2021 American Thoracic Society /European Respiratory Society Bronchodilator Response Criteria
| Age, years | Sex | Height, cm | Weight, kg | BMI, kg/m2 | 2005 BDR | 2022 BDR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Changes in FVC | Changes in FEV1 | ||||||||||
| mL | % | mL | % | FVC, % | FEV1, % | ||||||
| Negative conversion | |||||||||||
| 1 | 53 | Male | 173 | 60.4 | 20.2 | 10 | 0.2 | 300 | 12.6 | 0.2 | 8.5 |
| 2 | 65 | Male | 168 | 76.0 | 27.1 | 270 | 13.6 | 240 | 24.7 | 7.3 | 7.9 |
| 3 | 62 | Male | 165 | 79.3 | 29.2 | 60 | 2.0 | 240 | 12.5 | 1.6 | 8.0 |
| 4 | 59 | Male | 169 | 58.1 | 20.5 | −30 | −1.3 | 230 | 14.1 | −0.8 | 7.1 |
| 5 | 70 | Male | 165 | 98.8 | 36.3 | 200 | 9.3 | 200 | 12.7 | 5.8 | 7.1 |
| 6 | 16 | Male | 176 | 93.0 | 30.1 | 420 | 17.9 | 320 | 19.3 | 9.2 | 7.6 |
| 7 | 75 | Male | 162 | 55.6 | 21.2 | 310 | 21.2 | 20 | 2.2 | 9.7 | 0.8 |
| Mean, n = 7 | 57 | NA | 168* | 74.5 | 26.4 | 177 | 9.0 | 221 | 14.0 | 4.7 | 6.7 |
| Positive conversion | |||||||||||
| 1 | 70 | Female | 156 | 49.7 | 20.4 | 250 | 10.0 | 190 | 13.9 | 10.4 | 10.1 |
| 2 | 69 | Female | 150 | 59.2 | 26.5 | 330 | 11.1 | 140 | 5.0 | 11.1 | 5.5 |
| 3 | 63 | Male | 157 | 49.3 | 19.9 | 140 | 8.0 | 190 | 16.4 | 6.0 | 10.0 |
| Mean, n = 3 | 67 | NA | 154 | 52.7 | 22.4 | 240 | 9.7 | 173 | 11.8 | 9.2 | 8.5 |
| Overall mean, n = 190 | 60 | 84/106 | 160 | 62.6 | 24.4 | 79 | 2.8 | 65 | 4.9 | 2.5 | 2.6 |
The bold values meet positive criteria.
ATS, American Thoracic Society; BDR, bronchodilator response; BMI, body mass index; ERS, European Respiratory Society; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NA, not applicable.
*P < .007 compared to the overall mean value.
Figure 1.
River plot of the relationship between positive or negative bronchodilator response, age (years), and height (cm).
The new criteria are more stringent because the denominator is the predicted FEV1 (L), which considers height and age. Some cases went from positive to negative and vice versa. Some were negative in the FEV1 evaluation but positive in the FVC evaluation. Bronchodilator response in FVC, rather than FEV1, has been shown to better reflect the physiological processes of air trapping.5-8 It is essential to evaluate BDR not only by FEV1 but also by FVC.
The new criteria are affected by height and age, so caution should be taken in interpreting the results. The ability of an acute response to bronchodilators to predict future clinical status other than survival is unclear, and BDR does not accurately differentiate between types of airway diseases.9 Further evidence is needed to determine whether BDR is associated with outcomes other than survival.
Funding Statement
This study received no funding.
Footnotes
Ethics Committee Approval: This study was approved by Ethics Committee of Sizuoka General Hospital, (SGHIRB#2021035, Date: August 26, 2021).
Informed Consent: Since this was a retrospective study, the Board waived patient approval or informed consent.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.S.; Design – K.O., T.S., T.H., T.A.; Supervision – K.O., T.S., T.H., T.A.; Resources – K.O., T.S., T.H., T.A.; Materials – K.O., T.S., T.H., T.A.; Data Collection and/or Processing – K.O., T.A.; Analysis and/or Interpretation – S.T., T.S., T.A.; Literature Search – K.O., T.S., T.H., T.A.; Writing – K.O., T.S., T.H.; Critical Review – K.O., T.S., T.H.
Declaration of Interests: The authors have no conflicts of interest to declare.
References
- 1. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. ( 10.1183/13993003.01499-2021) [DOI] [PubMed] [Google Scholar]
- 2. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319 338. ( 10.1183/09031936.05.00034805) [DOI] [PubMed] [Google Scholar]
- 3. Ito S, Uchida A, Isobe Y, Hasegawa Y. Responsiveness to bronchodilator Procaterol in COPD as assessed by forced oscillation technique. Respir Physiol Neurobiol. 2017;240:41 47. ( 10.1016/j.resp.2017.02.012) [DOI] [PubMed] [Google Scholar]
- 4. Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig. 2014;52(4):242 250. ( 10.1016/j.resinv.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 5. Rodríguez-Carballeira M, Heredia JL, Rué M, Quintana S, Almagro P. The bronchodilator test in chronic obstructive pulmonary disease: interpretation methods. Respir Med. 2007;101(1):34 42. ( 10.1016/j.rmed.2006.04.018) [DOI] [PubMed] [Google Scholar]
- 6. Han MK, Wise R, Mumford J, et al. Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J. 2010;35(5):1048 1056. ( 10.1183/09031936.00052509) [DOI] [PubMed] [Google Scholar]
- 7. Lee JS, Huh JW, Chae EJ, et al. Response patterns to bronchodilator and quantitative computed tomography in chronic obstructive pulmonary disease. Clin Physiol Funct Imaging. 2012;32(1):12 18. ( 10.1111/j.1475-097X.2011.01046.x) [DOI] [PubMed] [Google Scholar]
- 8. Chen C, Jian W, Gao Y, Xie Y, Song Y, Zheng J. Early COPD patients with lung hyperinflation associated with poorer lung function but better bronchodilator responsiveness. Int J Chron Obstruct Pulmon Dis. 2016;11:2519 2526. ( 10.2147/COPD.S110021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker PP, Calverley PM. The volumetric response to bronchodilators in stable chronic obstructive pulmonary disease. COPD. 2008;5(3):147 152. ( 10.1080/15412550802092928) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a