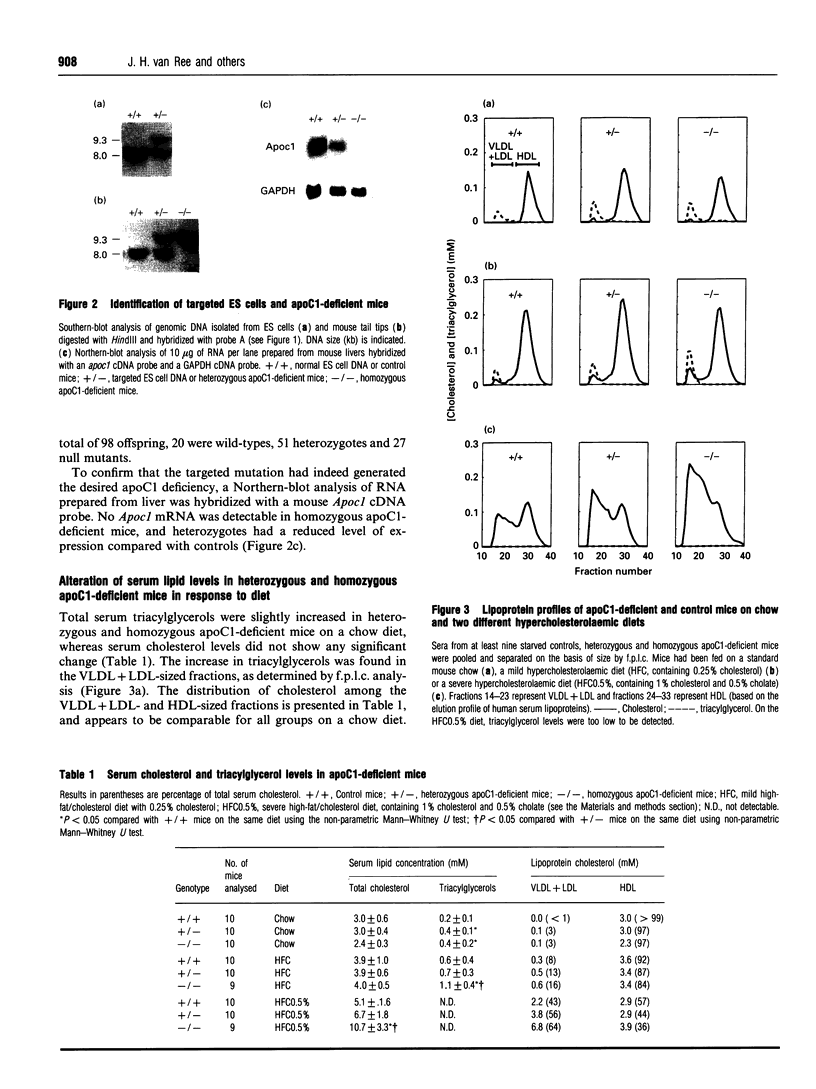
Abstract
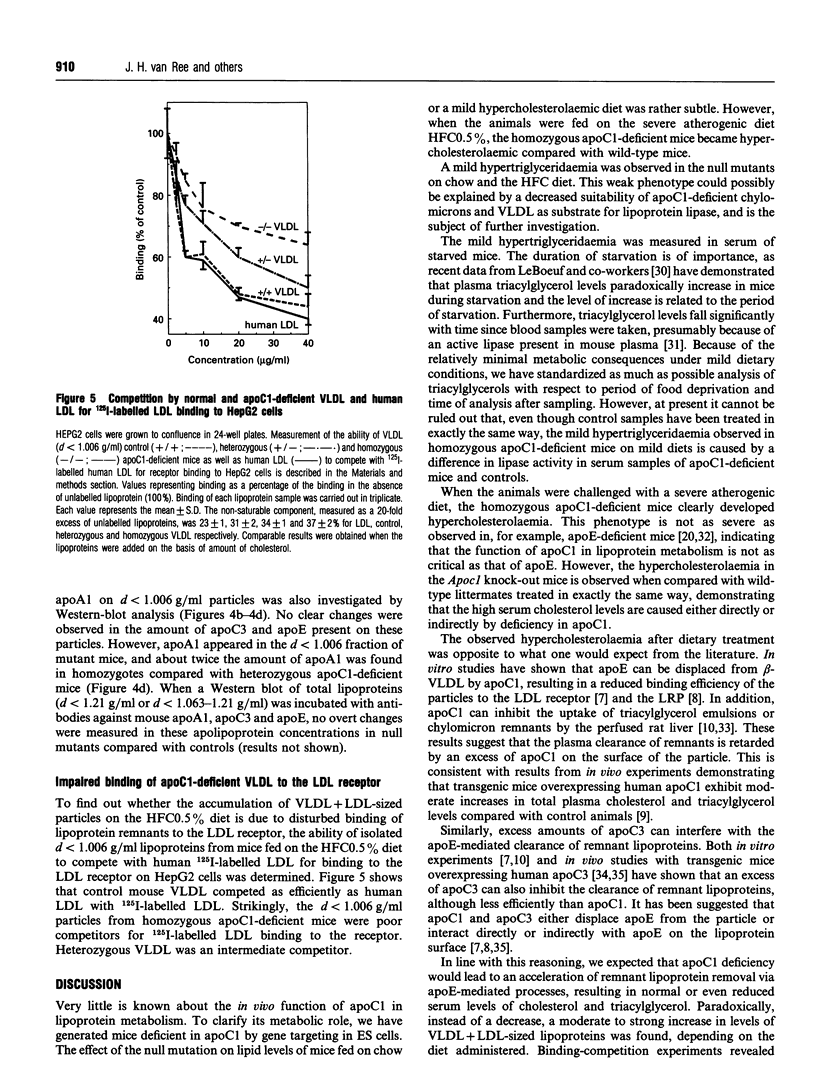
The function of apolipoprotein (apo) C1 in vivo is not well understood. From in vitro studies it has been reported that an excess of apoC1 relative to apoE inhibits receptor-mediated uptake of remnant lipoproteins [Sehayek and Eisenberg (1991) J. Biol. Chem. 266, 22453-22459]. In order to gain a better understanding of the role of apoC1 in lipoprotein metabolism in vivo, we have generated apoC1-deficient mice by gene targeting in embryonic stem cells. Homozygous mutant mice are viable and do not show overt abnormalities. Serum triacylglycerol levels are increased by 60% on both a standard mouse diet and a mild hypercholesterolaemic diet compared with controls. Total serum cholesterol levels are similar to controls on the two diets. However, the level of high-density lipoprotein cholesterol in the apoC1-deficient mice fed on the mild hypercholesterolaemic diet is slightly decreased, which is accompanied by a 3-fold increase in very-low-density plus low-density lipoprotein (VLDL+LDL) cholesterol. On a severe atherogenic diet, the homozygous apoC1-deficient mice become hypercholesterolaemic, with a serum cholesterol level of 10.7 +/- 3.3 mM compared with 6.7 +/- 1.8 mM and 5.1 +/- 1.6 mM in heterozygous and control mice respectively. The increase in cholesterol is mainly confined to the VLDL+LDL-sized fractions. Binding experiments revealed that lipoproteins lacking apoC1 with d < 1.006 g/ml are poor competitors for 125I-labelled LDL binding to the LDL receptor on HepG2 cells. This suggests that total apoC1 deficiency leads to impaired receptor-mediated clearance of remnant lipoproteins rather than enhanced uptake, as was expected from data reported in the literature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto-Setälä K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992 Nov;90(5):1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Hoffer M. J., Hofker M. H., van Eck M. M., Havekes L. M., Frants R. R. Evolutionary conservation of the mouse apolipoprotein e-c1-c2 gene cluster: structure and genetic variability in inbred mice. Genomics. 1993 Jan;15(1):62–67. doi: 10.1006/geno.1993.1010. [DOI] [PubMed] [Google Scholar]
- Hoffer M. J., van Eck M. M., Havekes L. M., Hofker M. H., Frants R. R. The mouse apolipoprotein C1 gene: structure and expression. Genomics. 1993 Oct;18(1):37–42. doi: 10.1006/geno.1993.1424. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Robertson M. E., Priestley L. M., Urdea M., Wallis S., Scott J. Characterisation of mRNAs encoding the precursor for human apolipoprotein CI. Nucleic Acids Res. 1984 May 11;12(9):3909–3915. doi: 10.1093/nar/12.9.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauer S. J., Walker D., Elshourbagy N. A., Reardon C. A., Levy-Wilson B., Taylor J. M. Two copies of the human apolipoprotein C-I gene are linked closely to the apolipoprotein E gene. J Biol Chem. 1988 May 25;263(15):7277–7286. [PubMed] [Google Scholar]
- LeBoeuf R. C., Caldwell M., Kirk E. Regulation by nutritional status of lipids and apolipoproteins A-I, A-II, and A-IV in inbred mice. J Lipid Res. 1994 Jan;35(1):121–133. [PubMed] [Google Scholar]
- Lusis A. J., Taylor B. A., Quon D., Zollman S., LeBoeuf R. C. Genetic factors controlling structure and expression of apolipoproteins B and E in mice. J Biol Chem. 1987 Jun 5;262(16):7594–7604. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Mulder M., de Wit E., Havekes L. M. The binding of human lipoprotein lipase treated VLDL by the human hepatoma cell line HepG2. Biochim Biophys Acta. 1991 Feb 5;1081(3):308–314. doi: 10.1016/0005-2760(91)90287-r. [DOI] [PubMed] [Google Scholar]
- Nishina P. M., Lowe S., Verstuyft J., Naggert J. K., Kuypers F. A., Paigen B. Effects of dietary fats from animal and plant sources on diet-induced fatty streak lesions in C57BL/6J mice. J Lipid Res. 1993 Aug;34(8):1413–1422. [PubMed] [Google Scholar]
- Nishina P. M., Verstuyft J., Paigen B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res. 1990 May;31(5):859–869. [PubMed] [Google Scholar]
- Paigen B., Morrow A., Brandon C., Mitchell D., Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985 Oct;57(1):65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Quarfordt S. H., Michalopoulos G., Schirmer B. The effect of human C apolipoproteins on the in vitro hepatic metabolism of triglyceride emulsions in the rat. J Biol Chem. 1982 Dec 25;257(24):14642–14647. [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Sehayek E., Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991 Sep 25;266(27):18259–18267. [PubMed] [Google Scholar]
- Shulman R. S., Herbert P. N., Wehrly K., Fredrickson D. S. Thf complete amino acid sequence of C-I (apoLp-Ser), an apolipoprotein from human very low density lipoproteins. J Biol Chem. 1975 Jan 10;250(1):182–190. [PubMed] [Google Scholar]
- Simonet W. S., Bucay N., Pitas R. E., Lauer S. J., Taylor J. M. Multiple tissue-specific elements control the apolipoprotein E/C-I gene locus in transgenic mice. J Biol Chem. 1991 May 15;266(14):8651–8654. [PubMed] [Google Scholar]
- Smit M., van der Kooij-Meijs E., Frants R. R., Havekes L., Klasen E. C. Apolipoprotein gene cluster on chromosome 19. Definite localization of the APOC2 gene and the polymorphic Hpa I site associated with type III hyperlipoproteinemia. Hum Genet. 1988 Jan;78(1):90–93. doi: 10.1007/BF00291243. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Garner C. W., Baker H. N., Sparrow J. T., Jackson R. L., Gotto A. M., Smith L. C. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: cholesterol acyltransferase. Biochemistry. 1975 Jul 15;14(14):3057–3064. doi: 10.1021/bi00685a003. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W., Kowal R. C., Herz J., Goldstein J. L., Brown M. S. Apolipoprotein C-I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein. J Biol Chem. 1990 Dec 25;265(36):22453–22459. [PubMed] [Google Scholar]
- Windler E., Havel R. J. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985 May;26(5):556–565. [PubMed] [Google Scholar]
- de Silva H. V., Lauer S. J., Mahley R. W., Weisgraber K. H., Taylor J. M. Apolipoproteins E and C-III have opposing roles in the clearance of lipoprotein remnants in transgenic mice. Biochem Soc Trans. 1993 May;21(2):483–487. doi: 10.1042/bst0210483. [DOI] [PubMed] [Google Scholar]
- van Deursen J., Heerschap A., Oerlemans F., Ruitenbeek W., Jap P., ter Laak H., Wieringa B. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell. 1993 Aug 27;74(4):621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]
- van Deursen J., Lovell-Badge R., Oerlemans F., Schepens J., Wieringa B. Modulation of gene activity by consecutive gene targeting of one creatine kinase M allele in mouse embryonic stem cells. Nucleic Acids Res. 1991 May 25;19(10):2637–2643. doi: 10.1093/nar/19.10.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J., Wieringa B. Targeting of the creatine kinase M gene in embryonic stem cells using isogenic and nonisogenic vectors. Nucleic Acids Res. 1992 Aug 11;20(15):3815–3820. doi: 10.1093/nar/20.15.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vlijmen B. J., van den Maagdenberg A. M., Gijbels M. J., van der Boom H., HogenEsch H., Frants R. R., Hofker M. H., Havekes L. M. Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J Clin Invest. 1994 Apr;93(4):1403–1410. doi: 10.1172/JCI117117. [DOI] [PMC free article] [PubMed] [Google Scholar]




