Abstract
Background
Supraventricular tachycardia is the most common dysrhythmia in children. Initial vagal maneuvers are successful less than half of the time. Adenosine, a potent AV nodal blocker with a short half-life, is recommended as first line pharmacotherapy. Minor side effects from adenosine are common, but report of serious side effects such as sustained ventricular tachycardia, torsades de pointes, syncope or hypotension are confined to small case series or studies greater than 20 years old. We aimed to specifically identify the incidence of serious side effects of adenosine in children in the emergency department.
Methods
Between 2002 and 2022, all children less than 18 years old who received adenosine for tachyarrhythmia treatment in two emergency departments were included. The electronic record was reviewed for demographic information, patient history, treatments given, and side effects or complications were observed. Electrocardiograms before, during and after adenosine administration were reviewed.
Results
77 patients met inclusion criteria. There were 74 patients with an initial rhythm of typical SVT. The other three patients included one with a junctional rhythm, one with atrial fibrillation, and one with an undetermined narrow complex tachycardia. 49 patients had cardiac rhythm monitoring during adenosine administration. 17 of these patients had three or more consecutive ventricular beats following adenosine, however no patients required treatment. No patients had syncope. One patient had brief hypotension after adenosine that normalized without intervention. Four patients were electrically cardioverted after adenosine, all for persistent dysrhythmias: two for persistent SVT with hypotension, one for atrial fibrillation and one for an undetermined rhythm. Twelve patients were placed on continuous antiarrhythmic medication for persistent SVT. Age, gender, prior SVT history, initial adenosine dose, and need for additional doses were not significant risk factors for a prolonged sinus pause or greater than two ventricular beats.
Conclusions
Adenosine treatment in typical supraventricular tachycardia in pediatric patients is safe.
Keywords: Adenosine, Supraventricular tachycardia, Tachydysrhythmia, Arrhythmia
Background
Supraventricular tachycardia (SVT) is the most common symptomatic dysrhythmia in children. It can be defined as a group of tachydysrhythmias that originate above or at the level of the atrioventricular (AV) node. The ventricular rate is greater than what is normal for age, usually more than 220 beats per minute (BPM) in infants and more than 180 BPM in children and adolescents. There is usually a narrow QRS complex, measuring less than 120 milliseconds [1–4].
In children younger than 12 years of age, accessory pathways are the most common cause of SVT. Patients with congenital heart disease confer the highest risk of occurrence [5, 6]. The most common presenting symptoms of SVT are palpitations (96%), anxiety, lightheadedness (75%), chest pain (35%), diaphoresis (17%), fatigue (23%), with less common manifestations of hypotension or syncope (20%). Infants typically manifest with fussiness, irritability, tachypnea, failure-to-thrive, and poor feeding [7, 8].
Vagal maneuvers are non-invasive and recommended as initial treatment for stable pediatric patients with SVT. However, they are successful only 27–53% of the time [7, 9, 10]. If vagal maneuvers fail and the patient is still hemodynamically stable, then adenosine is recommended as first line pharmacotherapy [7, 9]. Adenosine is an endogenous nucleoside that has a wide variety of effects on the cardiovascular system. It has an extremely short half-life and is a potent AV nodal blocker [11, 12]. When treating AV nodal reentrant tachycardia, conversion rates are up to 96% [13].
Minor side effects with rapid adenosine administration are common. These include chest discomfort, flushing, and dyspnea [7, 14–17]. Serious side effects of adenosine requiring critical interventions have been confined to case reports or small case series. These side effects include sustained ventricular tachycardia, hypotension, and torsades de pointes [18–22]. In a number of papers over 20 years ago, adenosine was shown to be effective in children and results indicated that no serious side effects were observed [13, 16, 23, 24]. These studies did not have side effects as the primary outcome. However, despite older evidence of minimal risk, we continue to devote a significant number of staff and resources into preventing these “possible” complications in the emergency department (ED).
With this paper, we aimed to determine the incidence of any serious side effects of adenosine requiring intervention in children over a decade of treatment in the ED. Secondary outcomes included the number of patients that required further intervention including repeat adenosine dosages, other anti-arrhythmic or AV nodal blocking medication, or electrical cardioversion.
Methods
We performed a retrospective multi-hospital series of all patients less than 18 years of age who received intravenous adenosine for tachyarrhythmia treatment in the ED over the 10-year period between 2002 and 2022. The two participating sites were Loma Linda University Children’s Hospital, a tertiary pediatric referral center, with approximately 38,000 ED visits per year and Riverside University Health System, with approximately 15,000 pediatric ED visits per year.
The electronic medical record was queried for all patients that received adenosine in the emergency department. Using a standardized data collection form and trained data collectors not blinded to the study objective, the following data points were collected: demographic information, past medical and cardiac history, vital signs, ED treatments, and disposition.
The standard age-based formula defining hypotension of less than 70 + 2(age in years) in millimeters of mercury (mmHg) was used to determine hypotension before and after adenosine was given. Systolic blood pressure less than 90mmHg was considered hypotensive for patients older than 10 years. While systolic blood pressure is not a perfect surrogate for pediatric hypotension, it was chosen due to being the closest marker available which is reported regularly in the ED. Weight-based dosing of adenosine was recorded, including dosage and number of repeat doses given.
Troponin lab values when performed were recorded. Troponin levels were ordered at the discretion of the ED provider. We reviewed electrocardiogram and cardiac rhythm strips and recorded the length of the cardiac pause after adenosine administration. The treating physician’s determination of initial cardiac rhythm and rhythm after interventions were recorded. We reviewed documented complications immediately after adenosine administration including hypotension, ventricular arrhythmia greater than two consecutive ventricular beats, prolonged cardiac pause, or syncope.
Data analysis was conducted using STATA (STATA 16, StataCorp LLC, College Station, TX). Logistic regression was used to determine the likelihood of multiple risk factors for side effects. Non-normally distributed data were described using medians and interquartile ranges (IQR). Descriptive statistics were also used. This study was approved by the Institutional Review Boards at both hospital sites.
Results
There were 77 patients that met inclusion criteria. These included 43 males and 34 females. The age range was one week old to 17 years old with a median of nine years (IQR 3–13).
37 patients had a previous history of SVT, including two patients with known Wolff-Parkinson-White syndrome. Six patients had previously diagnosed structural cardiac disease including one with aortic stenosis and ventricular septal defect (VSD), one with right atrial isomerism, total anomalous pulmonary venous return, functional single ventricle and pulmonary atresia, one with double inlet left ventricle status post Fontan procedure, one with right atrial dilation and two with VSD alone.
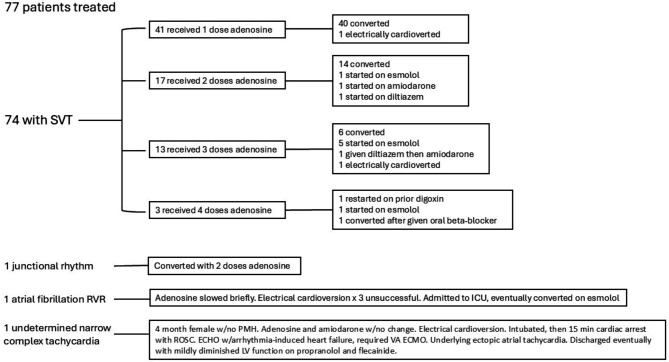
74 patients had an initial rhythm of SVT. One patient had a junctional rhythm, one patient had atrial fibrillation with rapid ventricular rate, and one patient had an undetermined narrow complex tachycardia (Fig. 1).
Fig. 1.
Diagram of Patients Rhythms and Treatments SVT: supraventricular tachycardia RVR: rapid ventricular response ICU: intensive care unit w/: with PMH: past medical history ROSC: return of spontaneous circulation ECHO: echocardiography VA ECMO: veno-arterial extracorporeal membrane oxygenation LV: left ventricle
For the patients weighing greater than 60 kg (kg), initial dose was 6 milligrams (mg) in 88% of patients, and 12 mg in 12%. For patients weighing less than 60 kg, initial dose ranged from 0.07 to 0.38 mg/kg with a median of 0.11 mg/kg (IQR 0.10–0.12). 34 patients required repeated doses of adenosine including 18 patients who received 1 extra dose, 13 patients who received 2 doses, and 3 patients who received 3 doses (Fig. 1).
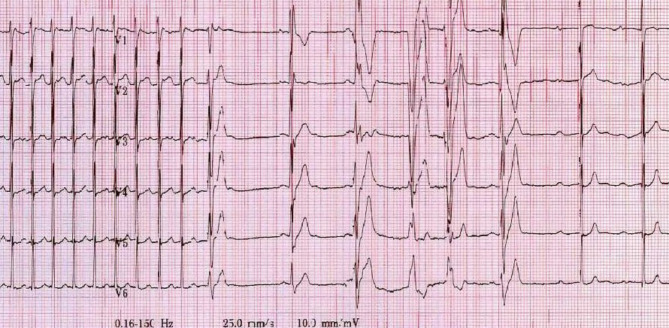
49 patients had cardiac rhythm monitoring during adenosine administration available to review. 28 patients did not. Of the 49 patients with reviewable rhythm, the cardiac pause immediately after adenosine was a range between 0.4 and 4.2 s, with a median of 1 s (IQR 0.8–1.6 s). There were 13 patients that had three or less consecutive ventricular beats immediately after adenosine. Two patients had four ventricular beats, one patient had six and one patient had nine. Figure 2 shows the rhythm strip of a nine-year old female with new-onset SVT who was given 6 mg of adenosine. She had a brief sinus pause, six ventricular beats, then conversion to sinus rhythm. No patients with ventricular beats required treatment for ventricular tachycardia. One patient had documented hypotension after adenosine cardioversion, however blood pressure normalized without intervention.
Fig. 2.
ECG showing ventricular beats after adenosine
Using logistic regression, we calculated the risk of multiple factors including age, gender, prior history of SVT, initial dose and repeat adenosine doses for the complications of sinus pause greater than 3 s or ventricular beats three or greater. None of these factors were statistically significant for either complication (Tables 1 and 2).
Table 1.
Risk for sinus pause > 3 s SVT: supraventricular tachycardia CI: confidence interval
| Odd Ratio | 95% CI | P value | |
|---|---|---|---|
| age | 1.33 | 0.69–2.56 | 0.395 |
| gender | 0.12 | 0.00-7.47 | 0.313 |
| history of SVT | 0.05 | 0.00-3.87 | 0.177 |
| initial dose | 30.94 | 1.42 × 10− 6- 6.74 × 108 | 0.691 |
| repeat dose | 14.24 | 0.09–2281 | 0.305 |
Table 2.
Risk for > 2 ventricular beats SVT: supraventricular tachycardia CI: confidence interval
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| age | 1.08 | 0.90–1.30 | 0.415 |
| gender | 2.26 | 0.34–14.88 | 0.397 |
| history of SVT | 6.08 | 0.15-250.44 | 0.342 |
| initial dose | 0.17 | 8.09 × 10− 10- 3.5 × 107 | 0.855 |
| repeat dose | 0.15 | 0.04–2.37 | 0.256 |
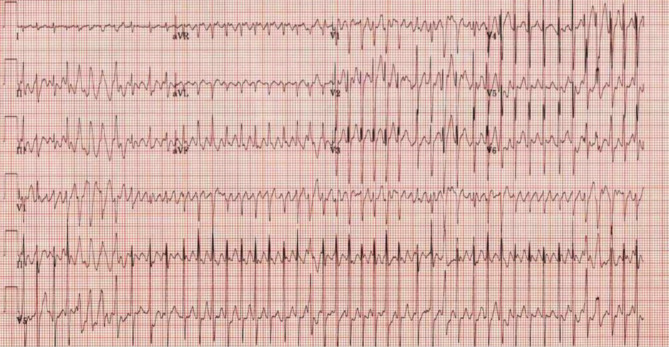
Four patients were electrically cardioverted including two patients that were hypotensive and still in SVT after adenosine. One patient with persistent atrial fibrillation underwent electrical cardioversion three times, but did not convert, and was admitted to the pediatric intensive care unit (ICU). One patient with an undetermined rhythm was electrically cardioverted after adenosine and amiodarone produced no change in rhythm (Fig. 3). The patient then required intubation, immediately after which had 15 min of cardiac arrest with return of spontaneous circulation. The patient’s echocardiography (ECHO) showed arrhythmia-induced heart failure, requiring extracorporeal membrane oxygenation for cardiogenic shock. Subsequently, the rhythm was shown to be an ectopic atrial tachycardia. The patient regained normal cardiac function and was weaned off antiarrhythmic medication completely.
Fig. 3.
Undetermined rhythm ECG
Twelve patients with persistent SVT after adenosine were placed on continuous antiarrhythmic medication including eleven patients on esmolol and one patient on amiodarone. One patient with persistent SVT was admitted and placed back on their home digoxin.
A troponin T level was drawn in five patients. Of these, four were normal. One was significantly elevated at 0.51 nanograms per milliliter (ng/mL) (reference range, <=0.03ng/ml). This increased to 0.77 during admission. The patient’s ECHO showed normal ventricular function with no wall motion abnormality. Per chart review, this patient was likely in SVT for more than 24 h prior to arrival at the hospital.
36 patients were discharged from the ED. 11 patients were admitted to a non-ICU bed, including one patient with acute appendicitis, one patient with a supracondylar humerus fracture, and one patient that was scheduled for cardiac ablation the following day. 29 patients were admitted to the ICU. Two of these patients were admitted to the ICU for bronchiolitis with albuterol-associated SVT. Ultimately, eight patients in the study group were diagnosed with Wolff-Parkinson-White syndrome.
Discussion
In this study, we found that adenosine treatment in pediatric patients with SVT was safe. This is the largest study of side effects of adenosine in children to date. There were no patients with sustained ventricular tachycardia or prolonged cardiac pauses that required intervention. When excluding undetermined rhythms and atrial fibrillation, the only patients that required electrical cardioversion were those persistently in SVT who then became hypotensive. We discovered that 44% of patients required extra doses, and 22% of patients either required electrical cardioversion or continuous antiarrhythmic medication to maintain sinus rhythm. Adenosine is often thought to induce significant cardiac pause and frequent ventricular beats which may be anxiety-inducing for treating physicians and staff. This study supports the fact that these events are transient and do not require intervention.
There are rare times that additional consideration or precautions may be needed when administering adenosine in children. Patients with difficult-to-determine rhythm or ill-appearing on presentation are more likely to have underlying heart failure. These patients are more likely to have life-threatening events requiring immediate intervention. There are very rare cases in the literature of bronchospasm induced by adenosine [25–27]. We had no cases of bronchospasm which reiterated the infrequency of this side effect.
When used in atrial fibrillation, adenosine can precipitate ventricular fibrillation due to increased ventricular irritability [28, 29]. Giving adenosine to a patient with an accessory conduction pathway such as in Wolff-Parkinson-White syndrome can also be dangerous when paired with atrial tachycardia. Adenosine often does not inhibit conduction through an accessory pathway, causing unopposed conduction through it and ventricular fibrillation [29]. Given the low number of pediatric patients with atrial fibrillation, potential for complications, and poor conversion rate with adenosine, we recommend against the use of adenosine for treatment of atrial fibrillation.
Multiple case studies have shown patients with long QT syndrome at risk for developing torsades de pointes [18, 20, 30]. It has been proposed as a test to identify patients with long QT syndrome [22]. There were no patients with long QT syndrome in our cohort, but we advise caution in these patients if the syndrome is known.
Conclusions
The use of adenosine is safe in the vast number of children with typical SVT that is narrow, regular and uniform. More caution is advised if patients have hypotension, signs of heart-failure, long QT syndrome, or other less common atrial rhythms. Without these indications, the authors advise against placing defibrillator pads on the patient. Overall, a practitioner can feel confident when using adenosine in the correct clinical scenario.
Acknowledgements
Not applicable.
Author contributions
MR contributed to the conception of the project, the analysis and interpretation of data, the drafting of the manuscript, and reviewing it critically for content. MR approves of the final version to be published and agrees to be accountable for all aspects of the work. TB contributed to the acquisition and analysis of the data, the drafting of the manuscript, and reviewing it critically for content. TB approves of the final version to be published and agrees to be accountable for all aspects of the work. SC contributed to the acquisition and analysis of the data and reviewing it critically for content. SC approves of the final version to be published and agrees to be accountable for all aspects of the work. MM contributed to the conception of the project and reviewed it critically for content. MM approves of the final version to be published and agrees to be accountable for all aspects of the work. TM contributed to the conception of the project and reviewed it critically for content. TM approves of the final version to be published and agrees to be accountable for all aspects of the work.
Funding
The authors declare they have no source of funding for this project.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Approved by Loma Linda University Medical Center IRB, number 5220426 Approved by Riverside University Health System IRB, number 2018356-6 The authors state that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was waived as this was a retrospective chart review study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brubaker S, Long B, Koyfman A. Alternative Treatment options for atrioventricular-nodal-reentry tachycardia: an Emergency Medicine Review. J Emerg Med. 2018. 10.1016/j.jemermed.2017.10.003. 10.1016/j.jemermed.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Karmegeraj B, Namdeo S, Sudhakar A, Krishnan V, Kunjukutty R, Vaidyanathan B. Clinical presentation, management, and postnatal outcomes of fetal tachyarrhythmias: a 10-year single-center experience. Ann Pediatr Cardiol. 2018. 10.4103/apc.APC_102_17. 10.4103/apc.APC_102_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundqvist CB, Potpara TS, Malmborg H. Supraventricular arrhythmias in patients with adult congenital heart disease. Arrhythm Electrophysiol Rev. 2017. 10.15420/aer.2016:29:3. 10.15420/aer.2016:29:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manole MD, Saladino RA. Emergency department management of the pediatric patient with supraventricular tachycardia. Pediatr Emerg Care. 2007. 10.1097/PEC.0b013e318032904c. 10.1097/PEC.0b013e318032904c [DOI] [PubMed] [Google Scholar]
- 5.Khurshid S, Choi SH, Weng LC, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of Cardiac Rhythm abnormalities in a half million adults. Circ Arrhythm Electrophysiol. 2018. 10.1161/CIRCEP.118.006273. 10.1161/CIRCEP.118.006273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amara W, Montagnier C, Cheggour S, Boursier M, Gully C, Barnay C, et al. Early Detection and Treatment of Atrial Arrhythmias alleviates the arrhythmic Burden in Paced patients: the SETAM Study. Pacing Clin Electrophysiol. 2017. 10.1111/pace.13062. 10.1111/pace.13062 [DOI] [PubMed] [Google Scholar]
- 7.Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/AHA/HRS Guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the Heart Rhythm Society. Circulation. 2016. 10.1161/CIR.0000000000000311. 10.1161/CIR.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 8.Wood KA, Drew BJ, Scheinman MM. Frequency of disabling symptoms in supraventricular tachycardia. Am J Cardiol. 1997. 10.1016/s0002-9149(96)00701-1. 10.1016/s0002-9149(96)00701-1 [DOI] [PubMed] [Google Scholar]
- 9.Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020. 10.1093/eurheartj/ehz467. 10.1093/eurheartj/ehz467 [DOI] [PubMed] [Google Scholar]
- 10.Schlechte EA, Boramanand N, Funk M. Supraventricular tachycardia in the pediatric primary care setting: age-related presentation, diagnosis, and management. J Pediatr Health Care. 2008. 10.1016/j.pedhc.2007.08.013. 10.1016/j.pedhc.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 11.Innes JA. Review article: Adenosine use in the emergency department. Emerg Med Australas. 2008. 10.1111/j.1742-6723.2008.01100.x. 10.1111/j.1742-6723.2008.01100.x [DOI] [PubMed] [Google Scholar]
- 12.Richardson C, Silver ES. Management of supraventricular tachycardia in infants. Paediatr Drugs. 2017. 10.1007/s40272-017-0254-0. 10.1007/s40272-017-0254-0 [DOI] [PubMed] [Google Scholar]
- 13.Sherwood MC, Lau KC, Sholler GF. Adenosine in the management of supraventricular tachycardia in children. J Paediatr Child Health. 1998. 10.1046/j.1440-1754.1998.00153.x. 10.1046/j.1440-1754.1998.00153.x [DOI] [PubMed] [Google Scholar]
- 14.McIntosh-Yellin NL, Drew BJ, Scheinman MM. Safety and efficacy of central intravenous bolus administration of adenosine for termination of supraventricular tachycardia. J Am Coll Cardiol. 1993. 10.1016/0735-1097(93)90185-4. 10.1016/0735-1097(93)90185-4 [DOI] [PubMed] [Google Scholar]
- 15.Riccardi A, Arboscello E, Ghinatti M, Minuto P, Lerza R. Adenosine in the treatment of supraventricular tachycardia: 5 years of experience (2002–2006). Am J Emerg Med. 2008. 10.1016/j.ajem.2007.11.029. 10.1016/j.ajem.2007.11.029 [DOI] [PubMed] [Google Scholar]
- 16.Till J, Shinebourne EA, Rigby ML, Clarke B, Ward DE, Rowland E. Efficacy and safety of adenosine in the treatment of supraventricular tachycardia in infants and children. Br Heart J. 1989. 10.1136/hrt.62.3.204. 10.1136/hrt.62.3.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tednes P, Marquardt S, Kuhrau S, Heagler K, Rech M. Keeping it current: a review of Treatment options for the management of supraventricular tachycardia. Ann Pharmacother. 2023. 10.1177/10600280231199136. 10.1177/10600280231199136 [DOI] [PubMed] [Google Scholar]
- 18.Celiker A, Tokel K, Cil E, Ozkutlu S, Ozme S. Adenosine induced torsades de pointes in a child with congenital long QT syndrome. Pacing Clin Electrophysiol. 1994. 10.1111/j.1540-8159.1994.tb03752.x. 10.1111/j.1540-8159.1994.tb03752.x [DOI] [PubMed] [Google Scholar]
- 19.Rankin AC, Rae AP, Houston A. Acceleration of ventricular response to atrial flutter after intravenous adenosine. Br Heart J. 1993. 10.1136/hrt.69.3.263. 10.1136/hrt.69.3.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington GR, Froelich EG. Adenosine-induced torsades de pointes. Chest. 1993. 10.1378/chest.103.4.1299. 10.1378/chest.103.4.1299 [DOI] [PubMed] [Google Scholar]
- 21.Paixão MR, Ribas FF, Accorsi TAD, Amicis K, Souza JL Jr. Torsades De Pointes and myocardial infarction following reversal of supraventricular tachycardia with adenosine: a case report. Einstein (Sao Paulo). 2024. 10.31744/einstein_journal/2024RC0522. 10.31744/einstein_journal/2024RC0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viskin S, Rosso R, Rogowski O, Belhassen B, Levitas A, Wagshal A, et al. Provocation of sudden heart rate oscillation with adenosine exposes abnormal QT responses in patients with long QT syndrome: a bedside test for diagnosing long QT syndrome. Eur Heart J. 2006. 10.1093/eurheartj/ehi460. 10.1093/eurheartj/ehi460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan HL, Spekhorst HH, Peters RJ, Wilde AA. Adenosine induced ventricular arrhythmias in the emergency room. Pacing Clin Electrophysiol. 2001. 10.1046/j.1460-9592.2001.00450.x. 10.1046/j.1460-9592.2001.00450.x [DOI] [PubMed] [Google Scholar]
- 24.Losek JD, Endom E, Dietrich A, Stewart G, Zempsky W, Smith K. Adenosine and pediatric supraventricular tachycardia in the emergency department: multicenter study and review. Ann Emerg Med. 1999. 10.1016/s0196-0644(99)70392-6. 10.1016/s0196-0644(99)70392-6 [DOI] [PubMed] [Google Scholar]
- 25.DeGroff CG, Silka MJ. Bronchospasm after intravenous administration of adenosine in a patient with asthma. J Pediatr. 1994. 10.1016/s0022-3476(94)70085-0. 10.1016/s0022-3476(94)70085-0 [DOI] [PubMed] [Google Scholar]
- 26.Coli S, Mantovani F, Ferro J, Gonzi G, Zardini M, Ardissino D. Adenosine-induced severe bronchospasm in a patient without pulmonary disease. Am J Emerg Med. 2012. 10.1016/j.ajem.2011.11.005. 10.1016/j.ajem.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 27.Morrow A, Ford TJ, Brogan R. Incidence of acute bronchospasm during systemic adenosine administration for coronary angiography. J R Coll Physicians Edinb. 2019. 10.4997/JRCPE.2019.307. 10.4997/JRCPE.2019.307 [DOI] [PubMed] [Google Scholar]
- 28.Guieu R, Deharo JC, Maille B, Crotti L, Torresani E, Brignole M, et al. Adenosine and the Cardiovascular System: the good and the bad. J Clin Med. 2020. 10.3390/jcm9051366. 10.3390/jcm9051366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Lokhandwala Y, Rai N, Malviya A. Adenosine-A drug with myriad utility in the diagnosis and treatment of arrhythmias. J Arrhythm. 2020. 10.1002/joa3.12453. 10.1002/joa3.12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesley RC Jr, Turnquest P. Torsades De Pointe after intravenous adenosine in the presence of prolonged QT syndrome. Am Heart J. 1992. 10.1016/0002-8703(92)90525-z. 10.1016/0002-8703(92)90525-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.