Abstract
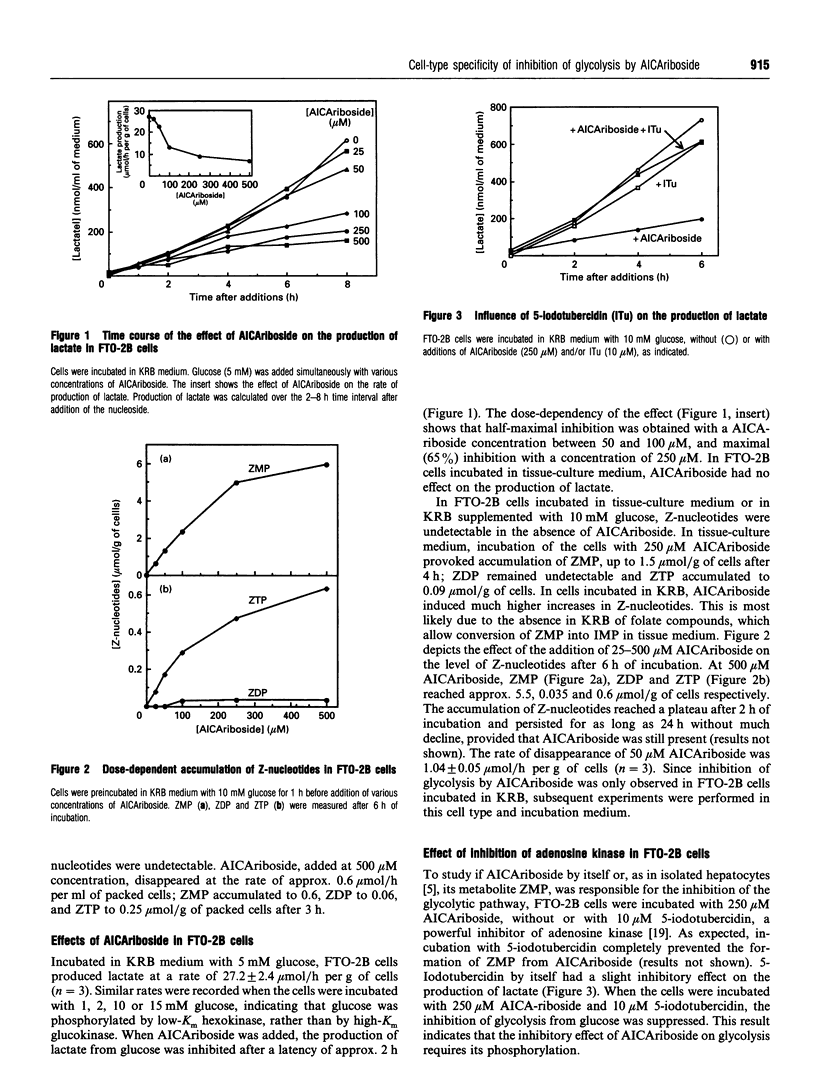
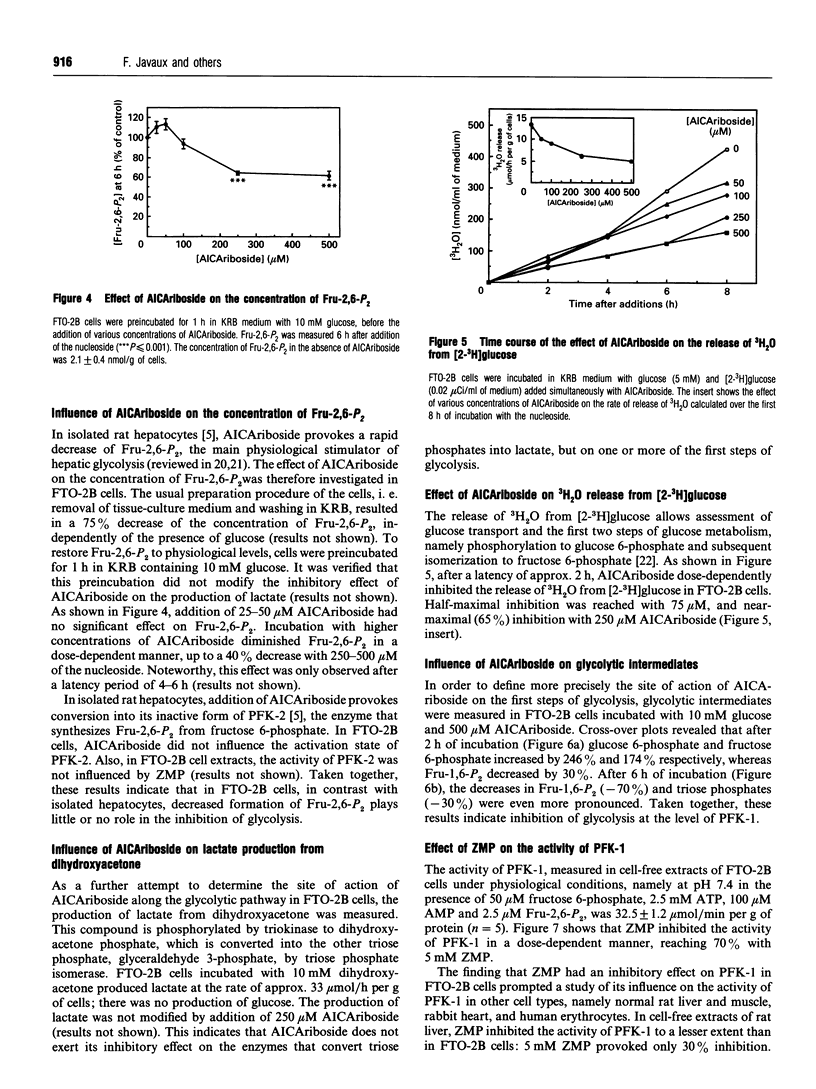
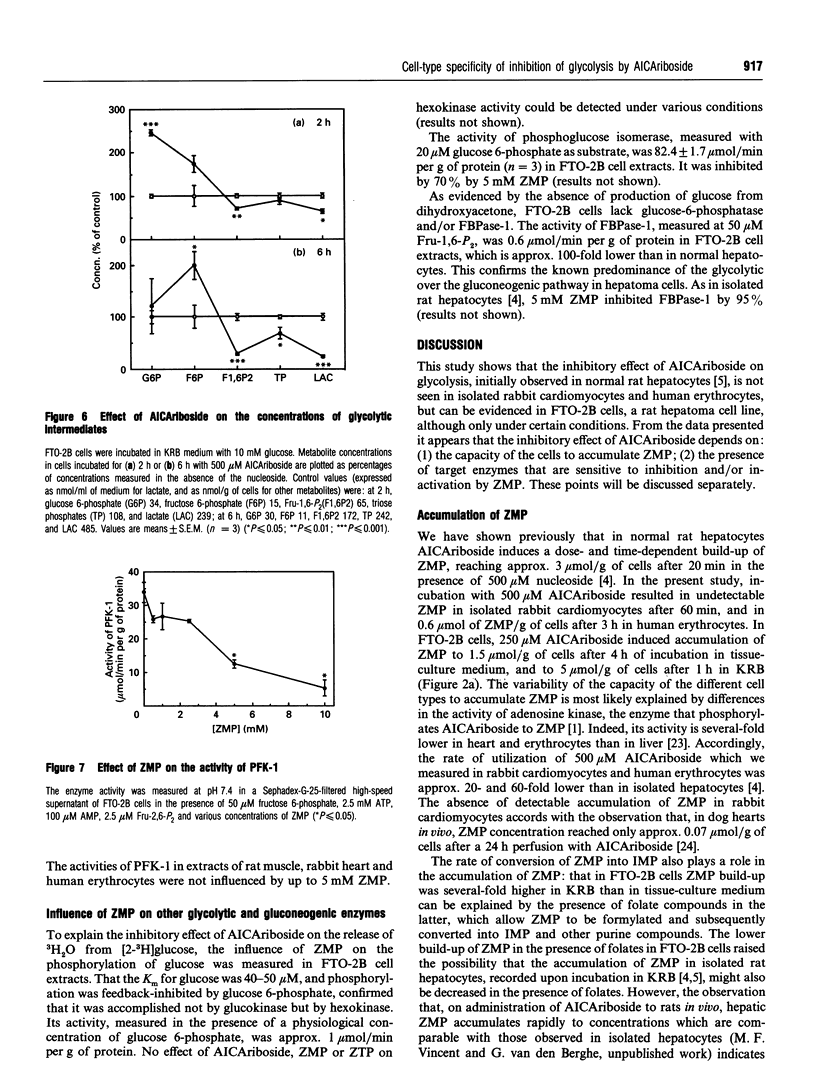
The nucleoside AICAriboside (5-amino-4-imidazolecarboxamide riboside) has been shown to inhibit glycolysis in isolated rat hepatocytes [Vincent, Bontemps and Van den Berghe (1992) Biochem. J. 281, 267-272]. The effect is mediated by AICA-ribotide (ZMP), the product of the phosphorylation of AICA-riboside by adenosine kinase. To assess the cell-type specificity of the effect, studies were conducted in rabbit cardiomyocytes, human erythrocytes and rat hepatoma FTO-2B cells. AICA-riboside had no effect on glycolysis in cardiomyocytes, and a slight stimulatory effect in erythrocytes, but inhibited glycolysis by 65% at 250 microM concentration in FTO-2B cells, although only when tissue-culture medium was replaced by Krebs-Ringer bicarbonate buffer. At 500 microM AICAriboside, ZMP remained undetectable in cardiomyocytes, but reached 0.65 mM in erythrocytes and 5 mM in FTO-2B cells. In the latter, AICAriboside provoked up to 2-fold elevations of glucose 6-phosphate and fructose 6-phosphate, accompanied by a decrease in fructose 1,6-bisphosphate. This indicated inhibition of 6-phosphofructo-1-kinase (PFK-1). Accordingly, in FTO-2B cell-free extracts, the activity of PFK-1, measured under physiological conditions, was inhibited by approx. 70% by 5 mM ZMP. ZMP had a less pronounced effect on the activity of PFK-1 in normal rat liver; it did not influence the activity of PFK-1 in rat muscle, rabbit heart and human erythrocytes. It is concluded that the inhibitory effect of AICAriboside on glycolysis is dependent on both (1) the capacity of the cells to accumulate ZMP and (2) the presence of target enzymes which are sensitive to ZMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Gamelin L. M., Kelley R. E., Lambert M. R., Apel L. E., Brierley G. P. Degradation and resynthesis of adenine nucleotides in adult rat heart myocytes. J Biol Chem. 1987 Oct 5;262(28):13527–13533. [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrons R., Hue L., Van Schaftingen E., Hers H. G. Hormonal control of fructose 2,6-bisphosphate concentration in isolated rat hepatocytes. Biochem J. 1983 Sep 15;214(3):829–837. doi: 10.1042/bj2140829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Hue L., Hers H. G. Phosphorylation of glucose in isolated rat hepatocytes. Sigmoidal kinetics explained by the activity of glucokinase alone. Biochem J. 1978 Aug 15;174(2):603–611. doi: 10.1042/bj1740603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Pathways of adenine nucleotide catabolism in erythrocytes. J Clin Invest. 1986 Mar;77(3):824–830. doi: 10.1172/JCI112379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cifuentes M. E., Espinet C., Lange A. J., Pilkis S. J., Hod Y. Hormonal control of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression in rat hepatoma cells. J Biol Chem. 1991 Jan 25;266(3):1557–1563. [PubMed] [Google Scholar]
- Dunaway G. A. A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol Cell Biochem. 1983;52(1):75–91. doi: 10.1007/BF00230589. [DOI] [PubMed] [Google Scholar]
- Dupriez V. J., Darville M. I., Antoine I. V., Gegonne A., Ghysdael J., Rousseau G. G. Characterization of a hepatoma mRNA transcribed from a third promoter of a 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-encoding gene and controlled by ets oncogene-related products. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8224–8228. doi: 10.1073/pnas.90.17.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. F., Paterson A. R., Caldwell I. C., Paul B., Chan M. C., Lau K. F. Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 2. 1972 Nov;3(1):71–85. [PubMed] [Google Scholar]
- Hers H. G., Van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982 Jul 15;206(1):1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nissim I., Yudkoff M., Segal S. Effect of 5-amino-4-imidazolecarboxamide riboside on renal ammoniagenesis. Study with [15N]aspartate. J Biol Chem. 1986 May 15;261(14):6509–6514. [PubMed] [Google Scholar]
- Rousseau G. G., Hue L. Mammalian 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a bifunctional enzyme that controls glycolysis. Prog Nucleic Acid Res Mol Biol. 1993;45:99–127. doi: 10.1016/s0079-6603(08)60868-5. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Patterson D., Holmes E. W. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J Biol Chem. 1985 May 25;260(10):6107–6114. [PubMed] [Google Scholar]
- Swain J. L., Hines J. J., Sabina R. L., Holmes E. W. Accelerated repletion of ATP and GTP pools in postischemic canine myocardium using a precursor of purine de novo synthesis. Circ Res. 1982 Jul;51(1):102–105. doi: 10.1161/01.res.51.1.102. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Jett M. F., Hue L., Hers H. G. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. F., Bontemps F., Van den Berghe G. Inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside in isolated rat hepatocytes. Biochem J. 1992 Jan 1;281(Pt 1):267–272. doi: 10.1042/bj2810267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. F., Marangos P. J., Gruber H. E., Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991 Oct;40(10):1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Deeprose R. D. Metabolism of 5-amino-1-beta-D-ribofuranosylimidazole-4-carboxamide and related five-membered heterocycles to 5'-triphosphates in human blood and L5178Y cells. Biochem Pharmacol. 1978 Mar 1;27(5):709–716. doi: 10.1016/0006-2952(78)90508-7. [DOI] [PubMed] [Google Scholar]


