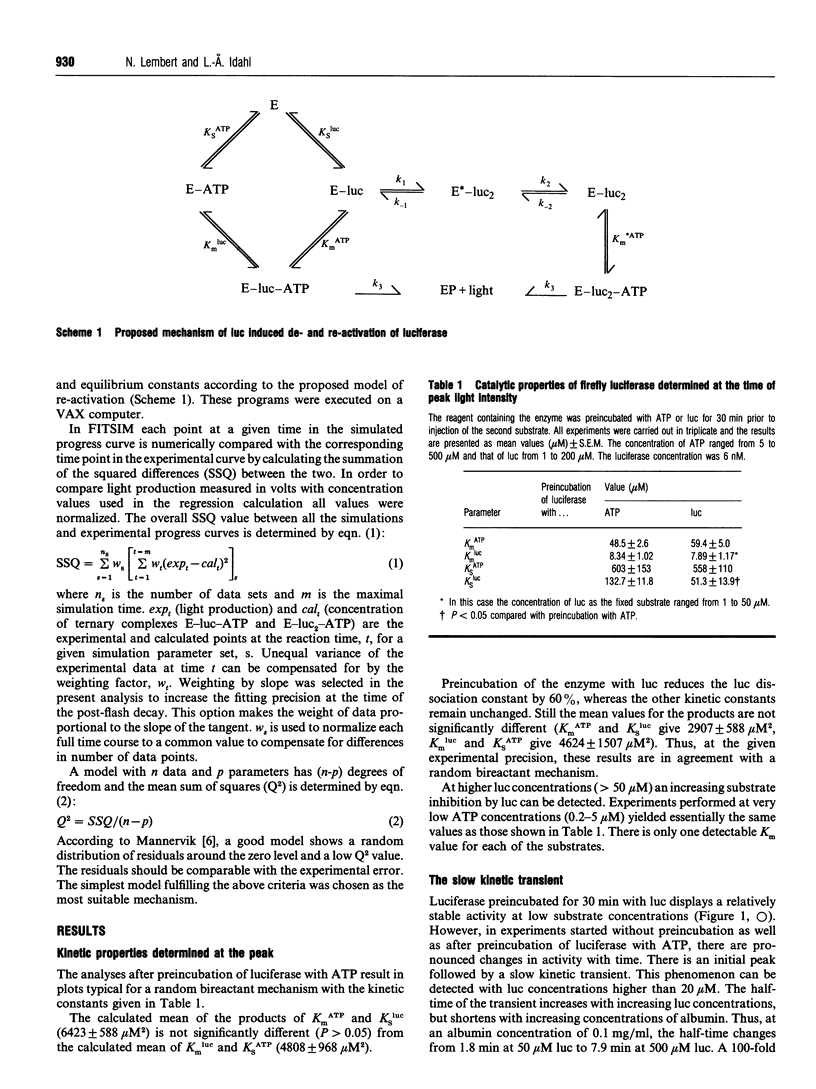
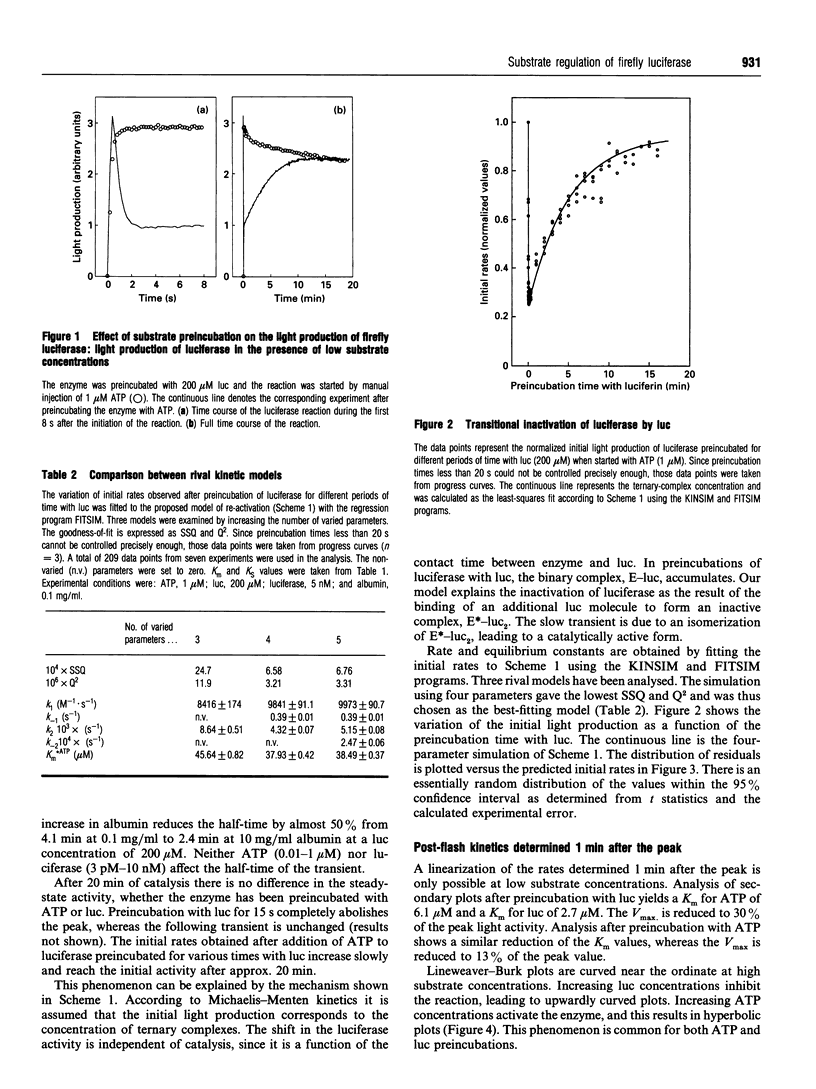
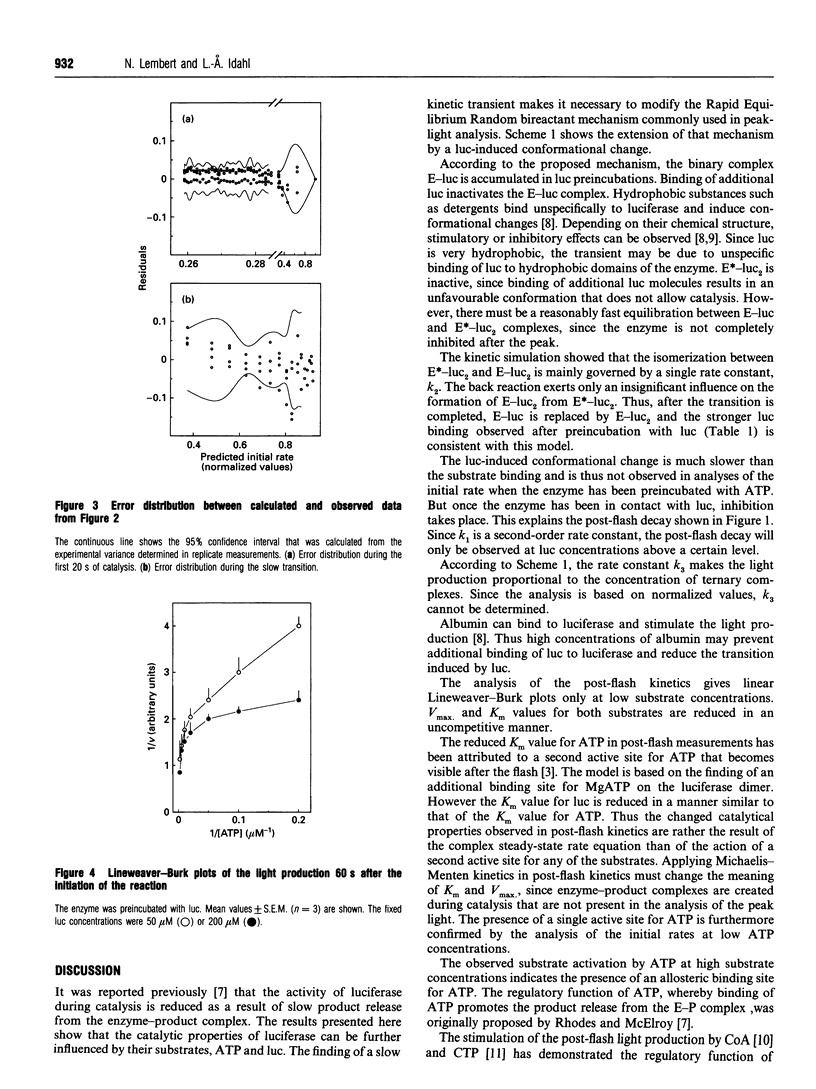
Abstract
ATP and luciferin are not only substrates of firefly luciferase, but can, in addition, modulate its activity. High concentrations of luciferin induce a conformational change of the enzyme that temporarily reduces the catalytic rate. Re-activation takes approx. 20 min and is independent of variation in the concentration of enzyme or ATP, but lengthens with increasing luciferin concentration. High concentrations of albumin reduce this luciferin effect. The kinetic properties of firefly luciferase determined from initial rates and at steady state after 1 min of catalysis have been analysed according to Michaelis-Menten kinetics. There is only one active site for each of the substrates. At steady state the Km and Vmax. values for both substrates are reduced in an uncompetitive manner. Hyperbolic Lineweaver-Burk plots indicate an activation by ATP probably by binding to an allosteric site. A model is presented which incorporates luciferin induced de- and re-activation effects. Experimental conditions to avoid the regulatory effects of substrates during ATP monitoring are proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIRTH R. L., RHODES W. C., McELROY W. D. The function of coenzyme A in luminescence. Biochim Biophys Acta. 1958 Mar;27(3):519–532. doi: 10.1016/0006-3002(58)90381-0. [DOI] [PubMed] [Google Scholar]
- Barshop B. A., Wrenn R. F., Frieden C. Analysis of numerical methods for computer simulation of kinetic processes: development of KINSIM--a flexible, portable system. Anal Biochem. 1983 Apr 1;130(1):134–145. doi: 10.1016/0003-2697(83)90660-7. [DOI] [PubMed] [Google Scholar]
- DeLuca M., McElroy W. D. Two kinetically distinguishable ATP sites in firefly luciferase. Biochem Biophys Res Commun. 1984 Sep 17;123(2):764–770. doi: 10.1016/0006-291x(84)90295-x. [DOI] [PubMed] [Google Scholar]
- Denburg J. L., Lee R. T., McElroy W. D. Substrate-binding properties of firefly luciferase. I. Luciferin-binding site. Arch Biochem Biophys. 1969 Nov;134(2):381–394. doi: 10.1016/0003-9861(69)90297-5. [DOI] [PubMed] [Google Scholar]
- Ford S. R., Hall M. S., Leach F. R. Enhancement of firefly luciferase activity by cytidine nucleotides. Anal Biochem. 1992 Aug 1;204(2):283–291. doi: 10.1016/0003-2697(92)90239-4. [DOI] [PubMed] [Google Scholar]
- Ford S. R., Leach F. R. Firefly luciferase-luciferin-ATP mixtures respond to etheno-ATP with an increased and steady production of light: a partial explanation of the Limulus ventral photoreceptor controversy. Am J Physiol. 1991 Dec;261(6 Pt 1):C1210–C1211. doi: 10.1152/ajpcell.1991.261.6.C1210. [DOI] [PubMed] [Google Scholar]
- Koop A., Cobbold P. H. Continuous bioluminescent monitoring of cytoplasmic ATP in single isolated rat hepatocytes during metabolic poisoning. Biochem J. 1993 Oct 1;295(Pt 1):165–170. doi: 10.1042/bj2950165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kricka L. J., De Luca M. Effect of solvents on the catalytic activity of firefly luciferase. Arch Biochem Biophys. 1982 Sep;217(2):674–681. doi: 10.1016/0003-9861(82)90549-5. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Denburg J. L., McElroy W. D. Substrate-binding properties of firefly luciferase. II. ATP-binding site. Arch Biochem Biophys. 1970 Nov;141(1):38–52. doi: 10.1016/0003-9861(70)90103-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. Regression analysis, experimental error, and statistical criteria in the design and analysis of experiments for discrimination between rival kinetic models. Methods Enzymol. 1982;87:370–390. doi: 10.1016/s0076-6879(82)87023-7. [DOI] [PubMed] [Google Scholar]
- RHODES W. C., McELROY W. D. The synthesis and function of luciferyl-adenylate and oxyluciferyl-adenylate. J Biol Chem. 1958 Dec;233(6):1528–1537. [PubMed] [Google Scholar]
- Rubin L. J., Brown J. E. [ATP]i in Limulus photoreceptors: no correlation with responsiveness or discrete event rate. Am J Physiol. 1988 Jan;254(1 Pt 1):C27–C36. doi: 10.1152/ajpcell.1988.254.1.C27. [DOI] [PubMed] [Google Scholar]
- Simpson W. J., Hammond J. R. The effect of detergents on firefly luciferase reactions. J Biolumin Chemilumin. 1991 Apr-Jun;6(2):97–106. doi: 10.1002/bio.1170060207. [DOI] [PubMed] [Google Scholar]
- Zhang Z. X., Fein A. Luminescence of luciferin-luciferase microinjected into Limulus ventral photoreceptors may not reflect the intracellular ATP level. Am J Physiol. 1991 Jan;260(1 Pt 1):C181–C182. doi: 10.1152/ajpcell.1991.260.1.C181. [DOI] [PubMed] [Google Scholar]
- Zimmerle C. T., Frieden C. Analysis of progress curves by simulations generated by numerical integration. Biochem J. 1989 Mar 1;258(2):381–387. doi: 10.1042/bj2580381. [DOI] [PMC free article] [PubMed] [Google Scholar]


