Abstract
The requirement for a normal insulin response in mediating the starved-to-refed transition, with respect to the partitioning of hepatic fatty acids between beta-oxidation and esterification to glycerol, was studied. Diabetic rats were starved for 24 h and refed ad libitum for various periods of time. There was no increase in plasma insulin in response to the meal. However, the fatty acid oxidation:esterification ratio was very rapidly decreased from the starved to the fed value, most of the transition being achieved within the first hour of refeeding. There was a 2 h lag in the response of hepatic malonyl-CoA concentration, such that this rapid switch from oxidation to esterification could not be explained on the basis of changes in the absolute concentration of this inhibitor of carnitine palmitoyltransferase I (CPT I). Hepatic pyruvate and lactate concentrations both increased by several-fold upon refeeding and peaked after 1 h and 3 h, respectively. The hepatic lactate:pyruvate ratio increased 3.2-fold during the first 3 h of refeeding, suggesting that the cytosolic NAD(+)-NADH couple became much more highly reduced during the lag-period between the onset of inhibition of flux of fatty acids towards oxidation and the rise in malonyl-CoA concentration. This may be indicative of a lowering of intracellular pH, which would amplify greatly the sensitivity of CPT I to the inhibitor. In view of the very rapid and high food intake by these diabetic rats, the possibility is also considered that portal concentrations of amino acids and other metabolites could give rise to an increase in liver cell-volume that would inhibit CPT I acutely by an as yet unknown mechanism [M. Guzman, G. Velasco, J. Castro and V. A. Zammit (1994) FEBS Lett. 344, 239-241].
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Cuthbert C. The hydrogen ion in normal metabolism: a review. Ciba Found Symp. 1982;87:1–19. doi: 10.1002/9780470720691.ch1. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., McCormack J. G., Prentki M., Jeanrenaud B., Denton R. M. Parallel increases in rates of fatty acid synthesis and in pyruvate dehydrogenase activity in isolated rat hepatocytes incubated with insulin. Biochim Biophys Acta. 1982 Jul 16;717(1):86–90. doi: 10.1016/0304-4165(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Baquet A., Gaussin V., Bollen M., Stalmans W., Hue L. Mechanism of activation of liver acetyl-CoA carboxylase by cell swelling. Eur J Biochem. 1993 Nov 1;217(3):1083–1089. doi: 10.1111/j.1432-1033.1993.tb18340.x. [DOI] [PubMed] [Google Scholar]
- Berger W., Keller U. Treatment of diabetic ketoacidosis and non-ketotic hyperosmolar diabetic coma. Baillieres Clin Endocrinol Metab. 1992 Jan;6(1):1–22. doi: 10.1016/s0950-351x(05)80328-3. [DOI] [PubMed] [Google Scholar]
- Borrebaek B. Acylation of carnitine and glycerophosphate in suspensions of rat liver mitochondria at varying levels of palmitate and coenzyme A. Acta Physiol Scand. 1975 Dec;95(4):448–456. doi: 10.1111/j.1748-1716.1975.tb10073.x. [DOI] [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer G. S., Kerbey A. L., Randle P. J. Kinase activator protein mediates longer-term effects of starvation on activity of pyruvate dehydrogenase kinase in rat liver mitochondria. Biochem J. 1986 Oct 15;239(2):347–354. doi: 10.1042/bj2390347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N., Shadid S., Hamlani R., Humphreys S. M., Clark M. L., Fielding B. A., Boland O., Coppack S. W. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol. 1994 Mar;266(3 Pt 1):E308–E317. doi: 10.1152/ajpendo.1994.266.3.E308. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M., Kolodziej M. P., Caldwell A., Corstorphine C. G., Zammit V. A. Evidence against direct involvement of phosphorylation in the activation of carnitine palmitoyltransferase by okadaic acid in rat hepatocytes. Biochem J. 1994 Jun 15;300(Pt 3):693–699. doi: 10.1042/bj3000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán M., Velasco G., Castro J., Zammit V. A. Inhibition of carnitine palmitoyltransferase I by hepatocyte swelling. FEBS Lett. 1994 May 16;344(2-3):239–241. doi: 10.1016/0014-5793(94)00405-6. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F., Bauers K., Gerok W. Interactions between glutamine metabolism and cell-volume regulation in perfused rat liver. Eur J Biochem. 1990 Mar 30;188(3):689–695. doi: 10.1111/j.1432-1033.1990.tb15451.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume and hormone action. Trends Pharmacol Sci. 1992 Oct;13(10):371–373. doi: 10.1016/0165-6147(92)90114-l. [DOI] [PubMed] [Google Scholar]
- Ip M. M., Ip C., Tepperman H. M., Tepperman J. Effect of adaptation to meal-feeding on insulin, glucagon and the cyclic nucleotide-protein kinase system in rats. J Nutr. 1977 May;107(5):746–757. doi: 10.1093/jn/107.5.746. [DOI] [PubMed] [Google Scholar]
- Kaloyianni M., Freedland R. A. Effect of diabetes and time after in vivo insulin administration on ketogenesis and gluconeogenesis in isolated rat hepatocytes. Int J Biochem. 1990;22(2):159–164. doi: 10.1016/0020-711x(90)90178-6. [DOI] [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. Investigations into the direct effects of insulin on hepatic ketogenesis, lipoprotein secretion and pyruvate dehydrogenase activity. Biochim Biophys Acta. 1984 Sep 12;795(2):427–430. doi: 10.1016/0005-2760(84)90094-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk G. M., Helmy I. M., Thampy K. G., Wakil S. J. Acute hormonal control of acetyl-CoA carboxylase. The roles of insulin, glucagon, and epinephrine. J Biol Chem. 1990 Apr 15;265(11):6330–6338. [PubMed] [Google Scholar]
- Marchington D. R., Kerbey A. L., Jones A. E., Randle P. J. Insulin reverses effects of starvation on the activity of pyruvate dehydrogenase kinase in cultured hepatocytes. Biochem J. 1987 Aug 15;246(1):233–236. doi: 10.1042/bj2460233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. L., Levy H. R. Rat mammary acetyl coenzyme A carboxylase. I. Isolation and characterization. J Biol Chem. 1969 May 10;244(9):2334–2342. [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. H., Huang W. Y., Huang W., Venkatachalam K. V., Wakil S. J. Isolation and characterization of a novel acetyl-CoA carboxylase kinase from rat liver. J Biol Chem. 1994 Mar 4;269(9):6859–6865. [PubMed] [Google Scholar]
- Mohan C., Bessman S. P. Effect of insulin on the metabolic distribution of carbons 1, 2, and 3 of pyruvate. Arch Biochem Biophys. 1986 Jul;248(1):190–199. doi: 10.1016/0003-9861(86)90416-9. [DOI] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Effects of insulin treatment of diabetic rats on hepatic partitioning of fatty acids between oxidation and esterification, phospholipid and acylglycerol synthesis, and on the fractional rate of secretion of triacylglycerol in vivo. Biochem J. 1994 Nov 15;304(Pt 1):177–182. doi: 10.1042/bj3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
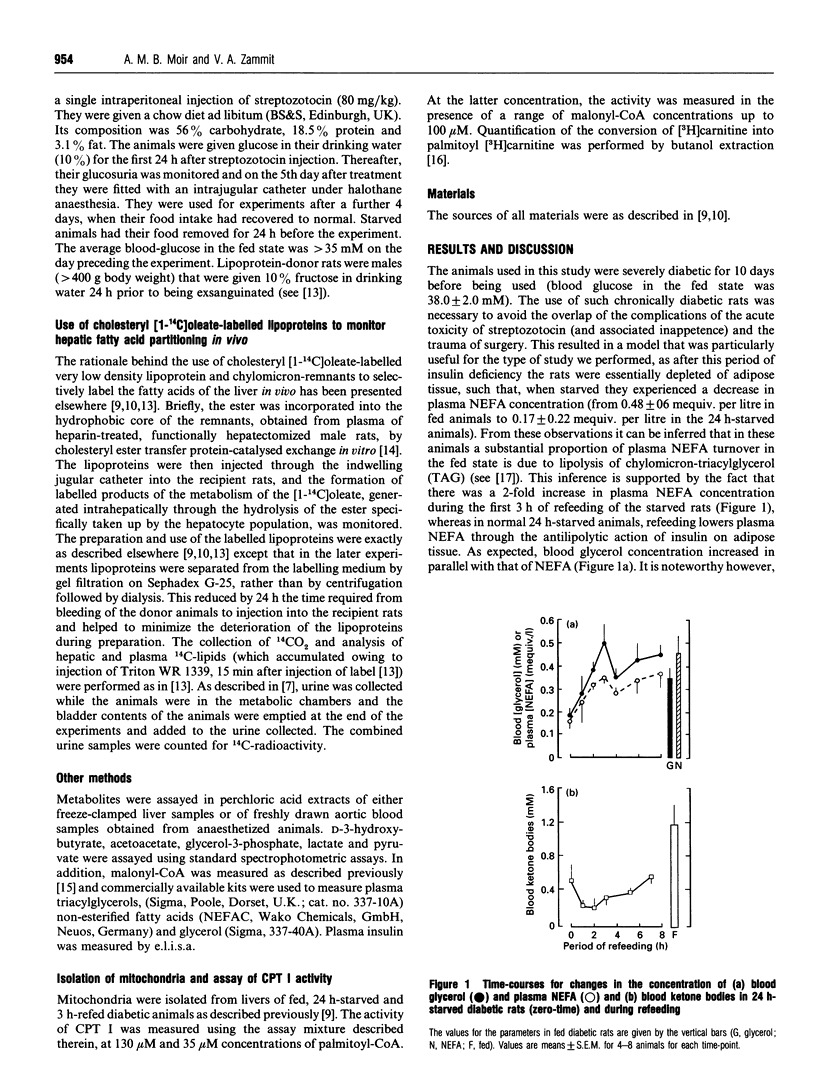
- Moir A. M., Zammit V. A. Monitoring of changes in hepatic fatty acid and glycerolipid metabolism during the starved-to-fed transition in vivo. Studies on awake, unrestrained rats. Biochem J. 1993 Jan 1;289(Pt 1):49–55. doi: 10.1042/bj2890049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Rapid switch of hepatic fatty acid metabolism from oxidation to esterification during diurnal feeding of meal-fed rats correlates with changes in the properties of acetyl-CoA carboxylase, but not of carnitine palmitoyltransferase I. Biochem J. 1993 Apr 1;291(Pt 1):241–246. doi: 10.1042/bj2910241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Selective labelling of hepatic fatty acids in vivo. Studies on the synthesis and secretion of glycerolipids in the rat. Biochem J. 1992 Apr 1;283(Pt 1):145–149. doi: 10.1042/bj2830145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Kerbey A. L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988 Nov;4(7):623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
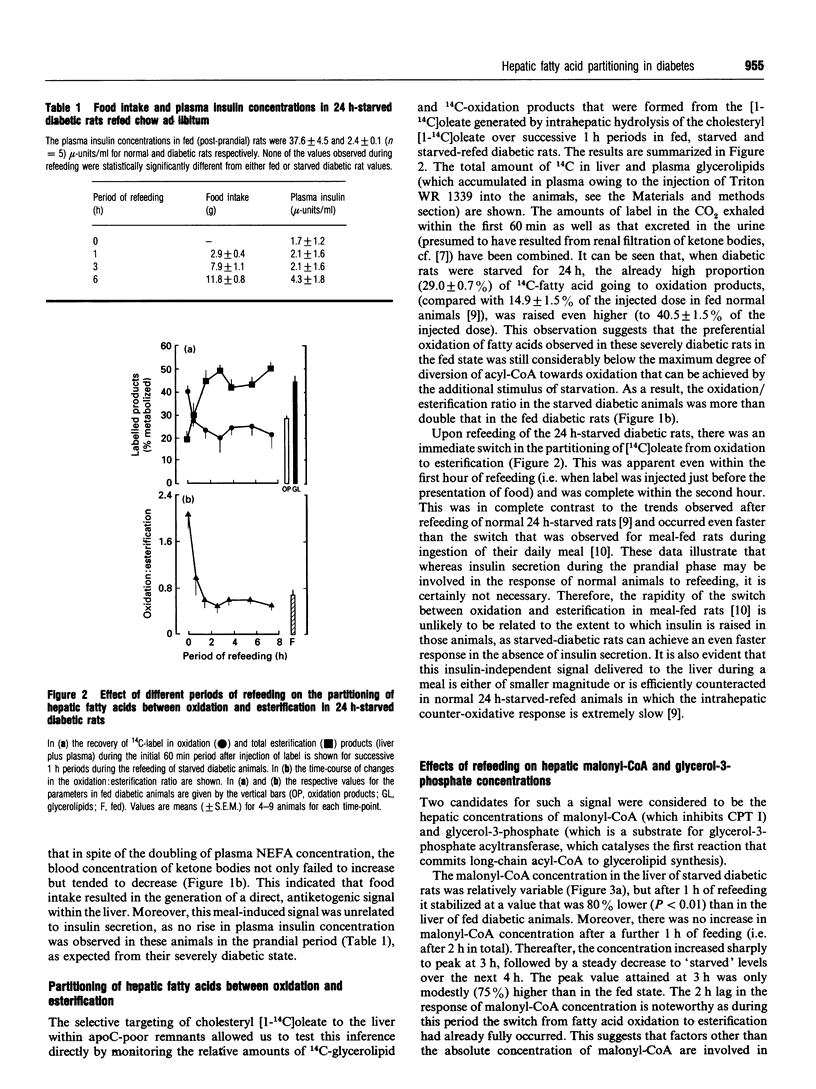
- Roberts D. C., Miller N. E., Price S. G., Crook D., Cortese C., La Ville A., Masana L., Lewis B. An alternative procedure for incorporating radiolabelled cholesteryl ester into human plasma lipoproteins in vitro. Biochem J. 1985 Feb 15;226(1):319–322. doi: 10.1042/bj2260319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson D. Carnitine palmitoyltransferase in extrahepatic tissues. Biochem Soc Trans. 1986 Aug;14(4):679–681. doi: 10.1042/bst0140679. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Bowen N. L., Hems D. A. Synthesis of fatty acids in the perused mouse liver. Biochem J. 1974 Sep;142(3):611–618. doi: 10.1042/bj1420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Stakkestad J. A., Bremer J., Borrebaek B. Determination of malonyl-coenzyme A in rat heart, kidney, and liver: a comparison between acetyl-coenzyme A and butyryl-coenzyme A as fatty acid synthase primers in the assay procedure. Anal Biochem. 1984 Apr;138(1):107–111. doi: 10.1016/0003-2697(84)90776-0. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
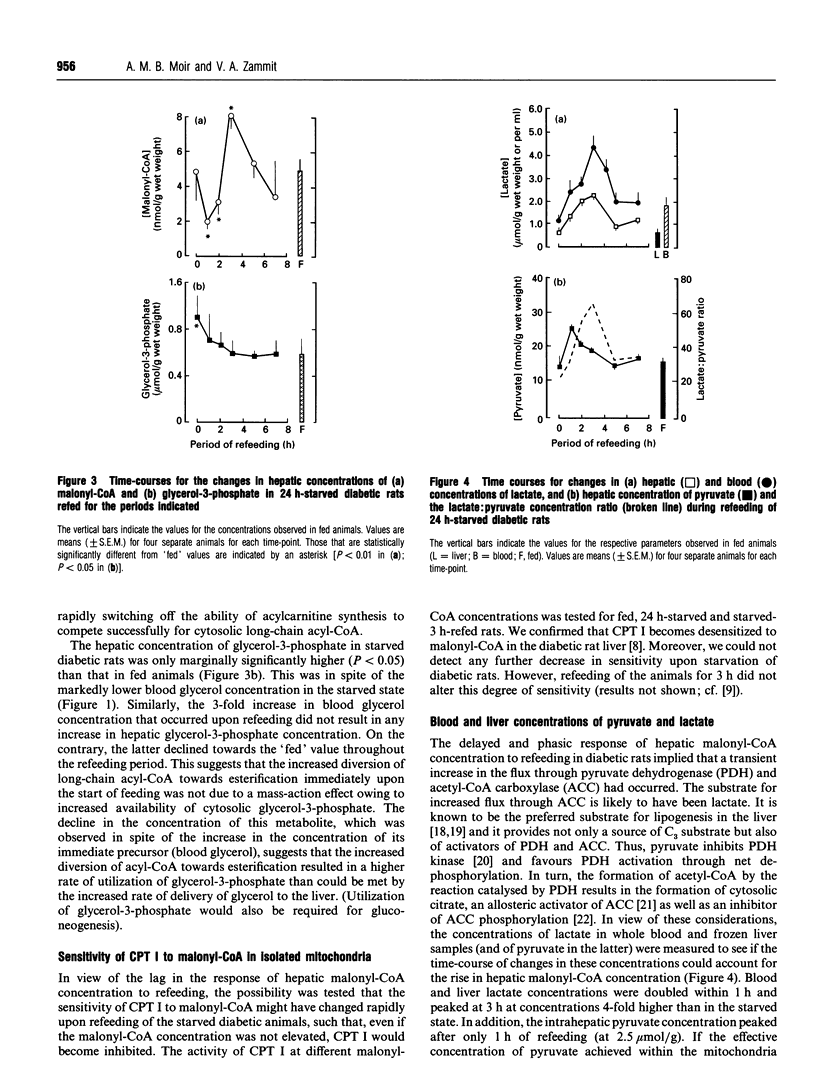
- Stephens T. W., Cook G. A., Harris R. A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 May 15;212(2):521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. H., Leveille G. A. Significance of insulin in the metabolic adaptation of rats to meal ingestion. J Nutr. 1970 Sep;100(9):1073–1080. doi: 10.1093/jn/100.9.1073. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]


