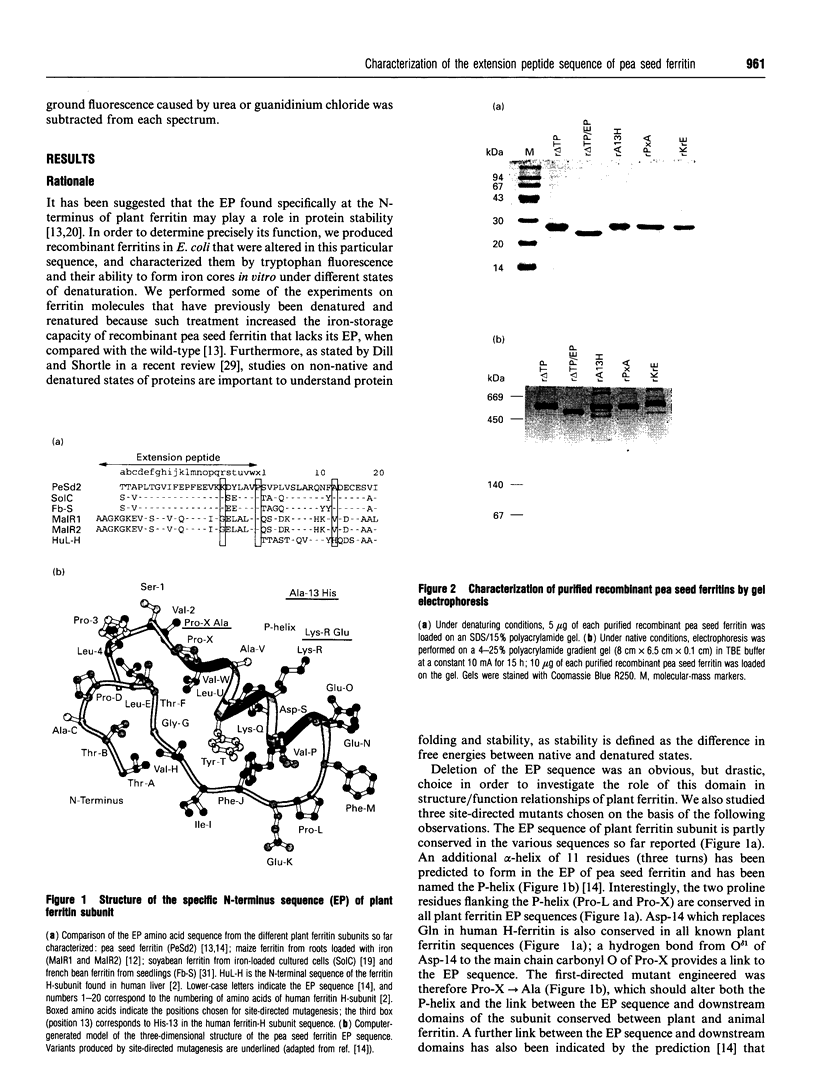
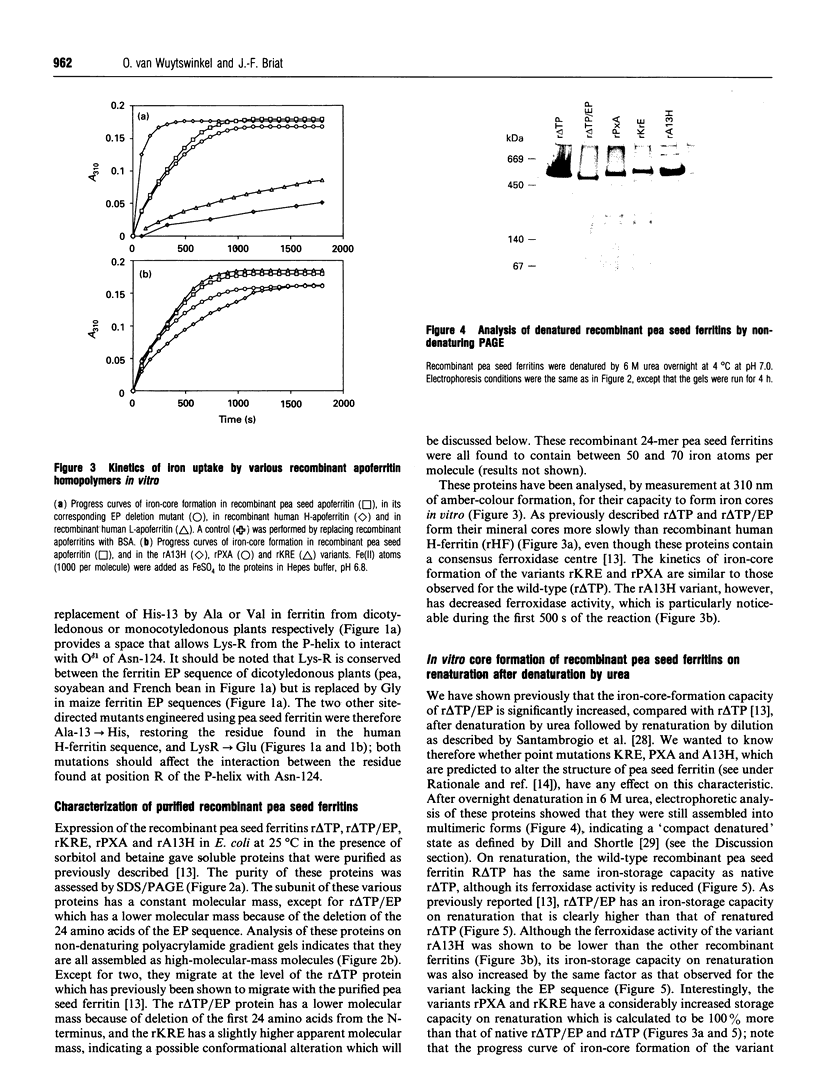
Abstract
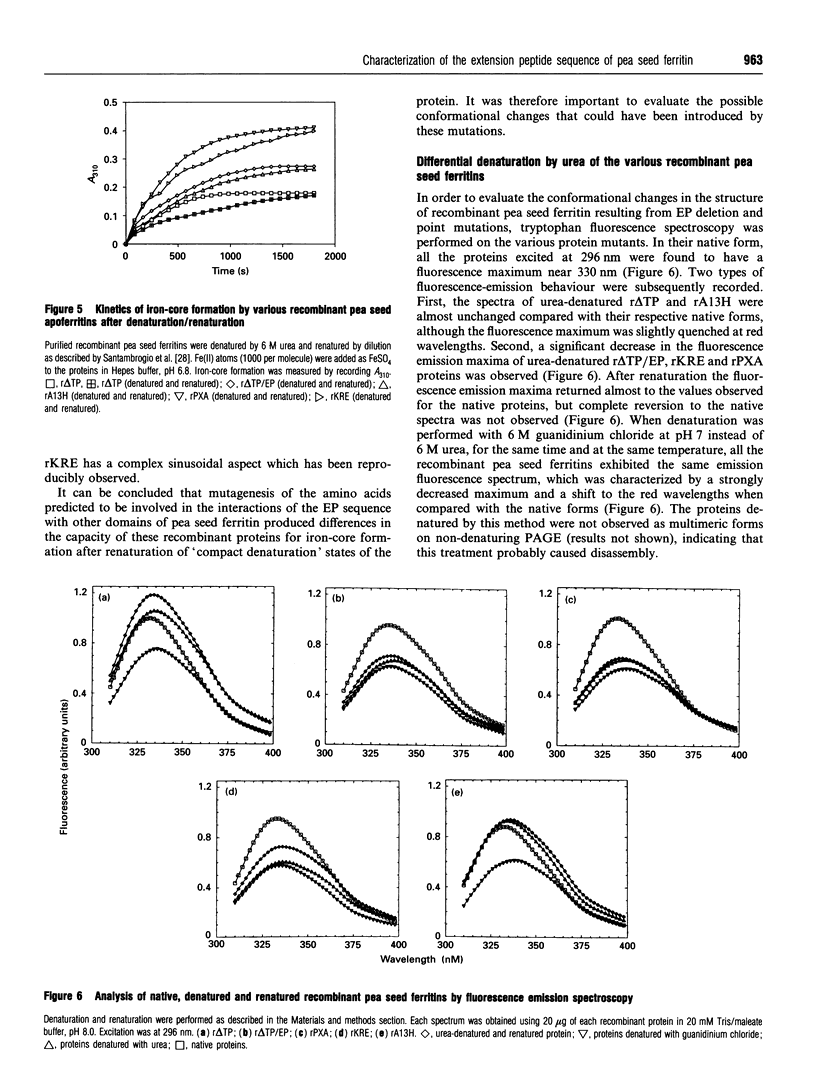
Plant ferritin has a three-dimensional structure predicted to be very similar to that of animal ferritin. It has, however, an additional specific sequence of 24 amino acids at its N-terminus named extension peptide (EP). In order to determine precisely the interactions between EP and other domains of the pea seed ferritin subunit, three point mutations were performed. The mutated residues were chosen by three-dimensional computer modelling of the pea seed ferritin subunit structure [Lobréaux, Yewdall, Briat and Harrison (1992) Biochem. J. 228, 931-939]. The mutant recombinant proteins were expressed in Escherichia coli and purified to homogeneity; all the mutants were found to be assembled as 24-mers. When Ala-13 was replaced by His, as in mammalian ferritins, ferroxidase activity was significantly reduced. Moreover, in vitro iron-core formation in Pro-X-->Ala, Lys-R-->Glu and Ala-13-->His mutants was increased after denaturation by urea followed by renaturation; this was also observed with the EP deletion mutant (r delta TP/EP). The recombinant ferritins were also analysed using tryptophan fluorescence spectra. The r delta TP/EP, Pro-X-->Ala and Lys-R-->Glu mutants were found to be more susceptible to denaturation by urea than the native r delta TP pea seed ferritin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Arosio P., Bottke W., Briat J. F., von Darl M., Harrison P. M., Laulhère J. P., Levi S., Lobreaux S., Yewdall S. J. Structure, function, and evolution of ferritins. J Inorg Biochem. 1992 Aug 15;47(3-4):161–174. doi: 10.1016/0162-0134(92)84062-r. [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Harrison P. M., Guest J. R. Cloning, sequencing, and mapping of the bacterioferritin gene (bfr) of Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3940–3947. doi: 10.1128/jb.171.7.3940-3947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Bauminger E. R., Harrison P. M., Hechel D., Hodson N. W., Nowik I., Treffry A., Yewdall S. J. Iron (II) oxidation and early intermediates of iron-core formation in recombinant human H-chain ferritin. Biochem J. 1993 Dec 15;296(Pt 3):709–719. doi: 10.1042/bj2960709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. R., Horgan R. A novel strategy for production of a highly expressed recombinant protein in an active form. FEBS Lett. 1991 Dec 16;295(1-3):10–12. doi: 10.1016/0014-5793(91)81372-f. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cozzi A., Santambrogio P., Levi S., Arosio P. Iron detoxifying activity of ferritin. Effects of H and L human apoferritins on lipid peroxidation in vitro. FEBS Lett. 1990 Dec 17;277(1-2):119–122. doi: 10.1016/0014-5793(90)80823-2. [DOI] [PubMed] [Google Scholar]
- Dill K. A., Shortle D. Denatured states of proteins. Annu Rev Biochem. 1991;60:795–825. doi: 10.1146/annurev.bi.60.070191.004051. [DOI] [PubMed] [Google Scholar]
- Izuhara M., Takamune K., Takata R. Cloning and sequencing of an Escherichia coli K12 gene which encodes a polypeptide having similarity to the human ferritin H subunit. Mol Gen Genet. 1991 Mar;225(3):510–513. doi: 10.1007/BF00261694. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laulhere J. P., Laboure A. M., Briat J. F. Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem. 1989 Feb 25;264(6):3629–3635. [PubMed] [Google Scholar]
- Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991 Feb 7;349(6309):541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. M., Treffry A., Artymiuk P. J., Harrison P. M., Yewdall S. J., Luzzago A., Cesareni G., Levi S., Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989 Aug 28;254(1-2):207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. Partial specific volumes and interactions with solvent components of proteins in guanidine hydrochloride. Biochemistry. 1974 Jan 15;13(2):257–265. doi: 10.1021/bi00699a005. [DOI] [PubMed] [Google Scholar]
- Lescure A. M., Proudhon D., Pesey H., Ragland M., Theil E. C., Briat J. F. Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8222–8226. doi: 10.1073/pnas.88.18.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S., Santambrogio P., Albertini A., Arosio P. Human ferritin H-chains can be obtained in non-assembled stable forms which have ferroxidase activity. FEBS Lett. 1993 Dec 27;336(2):309–312. doi: 10.1016/0014-5793(93)80826-g. [DOI] [PubMed] [Google Scholar]
- Levi S., Yewdall S. J., Harrison P. M., Santambrogio P., Cozzi A., Rovida E., Albertini A., Arosio P. Evidence of H- and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem J. 1992 Dec 1;288(Pt 2):591–596. doi: 10.1042/bj2880591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S., Briat J. F. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J. 1991 Mar 1;274(Pt 2):601–606. doi: 10.1042/bj2740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S., Massenet O., Briat J. F. Iron induces ferritin synthesis in maize plantlets. Plant Mol Biol. 1992 Jul;19(4):563–575. doi: 10.1007/BF00026783. [DOI] [PubMed] [Google Scholar]
- Lobreaux S., Yewdall S. J., Briat J. F., Harrison P. M. Amino-acid sequence and predicted three-dimensional structure of pea seed (Pisum sativum) ferritin. Biochem J. 1992 Dec 15;288(Pt 3):931–939. doi: 10.1042/bj2880931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudhon D., Briat J. F., Lescure A. M. Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol. 1989 Jun;90(2):586–590. doi: 10.1104/pp.90.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland M., Briat J. F., Gagnon J., Laulhere J. P., Massenet O., Theil E. C. Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem. 1990 Oct 25;265(30):18339–18344. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio P., Levi S., Cozzi A., Rovida E., Albertini A., Arosio P. Production and characterization of recombinant heteropolymers of human ferritin H and L chains. J Biol Chem. 1993 Jun 15;268(17):12744–12748. [PubMed] [Google Scholar]
- Spence M. J., Henzl M. T., Lammers P. J. The structure of a Phaseolus vulgaris cDNA encoding the iron storage protein ferritin. Plant Mol Biol. 1991 Sep;17(3):499–504. doi: 10.1007/BF00040644. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Van Wuytswinkel O., Savino G., Briat J. F. Purification and characterization of recombinant pea-seed ferritins expressed in Escherichia coli: influence of N-terminus deletions on protein solubility and core formation in vitro. Biochem J. 1995 Jan 1;305(Pt 1):253–261. doi: 10.1042/bj3050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade V. J., Levi S., Arosio P., Treffry A., Harrison P. M., Mann S. Influence of site-directed modifications on the formation of iron cores in ferritin. J Mol Biol. 1991 Oct 20;221(4):1443–1452. doi: 10.1016/0022-2836(91)90944-2. [DOI] [PubMed] [Google Scholar]
- Wade V. J., Treffry A., Laulhère J. P., Bauminger E. R., Cleton M. I., Mann S., Briat J. F., Harrison P. M. Structure and composition of ferritin cores from pea seed (Pisum sativum). Biochim Biophys Acta. 1993 Jan 15;1161(1):91–96. doi: 10.1016/0167-4838(93)90201-2. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]