Highlights
-
•
Carbapenem-resistant Enterobacteriaceae (CRE) are routinely cultured from clinical specimens in Nigerian hospitals.
-
•
Empiric antibiotics are often inappropriate for the treatment of CRE.
-
•
Inappropriate treatment for CRE is continued or initiated despite culture data.
Keywords: Carbapenem-resistant Enterobacteriaceae, Antimicrobial resistance, Antimicrobial stewardship, Infection prevention and control, Bacteria
Abstract
Objectives
This study aims to provide lacking data on antibiotics and treatment strategies used in the management of carbapenem-resistant Enterobacteriaceae (CRE) infections in Nigeria.
Methods
A cross-sectional study was carried out at the University College Hospital in Ibadan. CRE isolated from routine culture of specimens from hospitalized patients from December 2021 to September 2022 was identified. Treatment information and other data were collected from the patients’ medical records.
Results
The hospital laboratory isolated CRE from 55 patients during the study period and 27 (49.1%) of them had data available for the study. The most frequently isolated CRE was Klebsiella spp. (13 of 27, 48.1%). Of the 24 patients who received empiric antibiotics, only two (8.3%) of their CRE isolates were susceptible. After receiving culture results, 18 (66.7%) patients were treated with at least one antibiotic, to which resistance was documented. Only three (11.1%) patients overall commenced or remained on an antibiotic, to which their CRE isolate was susceptible.
Conclusions
Despite culture data, we found a high prevalence of drug-pathogen mismatch in CRE treatment, including new or persistent use of antibiotics, to which resistance was documented. Antimicrobial stewardship efforts need to be strengthened to specifically address CRE treatment and effective antibiotics need to be made accessible.
Introduction
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) are a growing concern in developing countries and often associated with poor clinical outcomes [1]. CREs are designated as the highest level of World Health Organization (WHO) priority pathogens [2]. The optimal choice of drugs to treat CRE depends on various factors such as the mechanism of resistance to carbapenems, the minimum inhibitory concentration (MIC) and the site and severity of infection [3]. Antibiotic use guidelines, which take all these factors into consideration, as well as the local molecular and clinical epidemiology of pathogens, provide a good guide for treatment of CRE and other bacterial infections. The Africa Center for Disease Control developed treatment guidelines for bacterial infections; notably absent are recommendations for treatment of CRE infections [4]. The WHO AWaRe antibiotic book’s section on “Reserve” antibiotics, however, does include recommendations for treatment of carbapenem-resistant bacteria [5]. Notwithstanding, it is not clear what treatment strategies are used for managing CRE infections in most sub-Saharan African countries. We set out to describe antibiotic use in treatment of CRE infections at a tertiary hospital in Ibadan, Nigeria.
Methods
A retrospective cross-sectional study was carried out at the University College Hospital in Ibadan, a 1000-bed tertiary hospital affiliated with the University of Ibadan. Clinical specimens obtained for culture during routine care of patients and processed at the hospital's microbiology laboratory at University College Hospital from December 2021 to September 2022 were reviewed. All microbiology records of culture reports of urine, blood, sputum, pleural fluid, cerebrospinal fluid, wound swab or biopsy, and intra-abdominal infections were included in our study and reviewed during the study period to identify CRE. No routine clinical specimens processed in the laboratory during the study period were excluded. Pathogens were isolated through bacteria culture and phenotypic identification methods. Briefly, the modified Kirby–Bauer disk diffusion method was used and it involves inoculating an agar plate with a standardized bacterial suspension, placing antibiotic-impregnated disks on the surface, incubating the plate, and measuring the inhibition zones to assess antibiotic susceptibility. The Clinical and Laboratory Standards Institute 2021 criteria were used for MIC evaluation. After the identification of CRE, a research nurse reviewed the medical records of the patient to obtain data including biodata and treatment information. Ethical approval was obtained from the University of Ibadan/University College Hospital, Ibadan, research ethics committee approval number: UI/EC/20/0009. Informed consent was waived because the study involved the use of de-identified secondary data.
Results
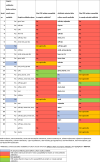
Over the 9-month study period, 923 isolates of Enterobacteriaceae were isolated from clinical specimens. A total of 55 (6%) were CRE, of which 27 (2.9%) of them had available records and were included in this report. Each of the 27 isolates were obtained from different patients. Of the 27 isolates, 19 (70.5%) were obtained from male patients and the median (interquartile range) age of the patients was 42 (25-58) years (Table 1). The most frequently cultured clinical specimen reported was urine (12, 44.4%), whereas Klebsiella spp. (13, 48.1%) were the most frequently isolated pathogens (Table 1). A total of 24 of the 27 patients with CRE infection had received empiric antibiotics before culture results were available and only two of these turned out to be effective against the CRE later isolated from their specimens. In both cases, there was a history of previously treated CRE infection (Table 2). Of the 24 patients on empiric antibiotics, seven (29.2%) of them discontinued antibiotics entirely after receiving culture results, including one of the patients who had empirically received an antibiotic effective against CRE. After receiving culture results, 18 (66.7%) patients added at least one antibiotic (including carbapenems), to which resistance was documented. None of these 18 patients received an antibiotic, to which susceptibility was documented after cultures were made available. Only three (11.1%) patients commenced or remained on an effective antibiotic after culture results became available. In these patients, these antibiotics were a polymyxin for two and amikacin for the third. Regarding antimicrobial susceptibility, all the CRE isolates were resistant to gentamicin and quinolones. In vitro susceptibility was documented to polymyxins, tigecycline, and amikacin in 21 of 27 (80.8%), 21 of 26 (77.8%), and six of 22 (27.3%) of CRE isolates, respectively.
Table 1.
Characteristics of patients, culture specimens, and CRE isolates.
| Characteristics | Number (%) |
|---|---|
| Gender | |
| Male | 19 (70.4) |
| Female | 8 (29.6) |
| Age group | |
| Child (<15 years) | 6 (22.8) |
| Adult (≥15 years) | 21 (77.8) |
| Specimen type | |
| Urine | 12 (44.4) |
| Wound swab/biopsy | 7 (25.9) |
| Blood | 4 (14.8) |
| Sputum/Pleural fluid | 2 (7.4) |
| Others | 2 (7.4) |
| Received antibiotics before culture results available | |
| Yes | 24 (88.9) |
| No | 3 (11.1) |
| CRE isolated | |
| Klebsiella spp | 13 (48.1) |
| Escherichia coli | 5 (18.5) |
| Enterobacter spp | 3 (11.1) |
| Serratia spp | 3 (11.1) |
| Raoultella spp | 2 (7.4) |
| Cronobacter spp | 1 (3.7) |
CRE, carbapenem-resistant Enterobacteriaceae.
Table 2.
Antibiotic choices before and after culture results were available.
 |
Discussion
Our brief report describes the use of antibiotics for treatment of CRE infections in hospitalized patients in Nigeria, which, to the best of our knowledge, is the first of its kind. The number of available antibiotics for treatment of CREs worldwide has gradually increased in recent years and they are variably effective on different species and strains of pathogens [6]. Although newer agents for treating CRE are not available in Nigeria, polymyxins and tigecycline, to which CRE susceptibility has often been demonstrated in vitro locally, are available but are costly and scarce. Therefore, local experience with the available agents is limited. The few patients who received the appropriate empiric antibiotics (polymyxins) for CRE infection in our study had a history of confirmed CRE infections during the same admission, hence the decision to place them on a polymyxin empirically. This indicates an appropriate indication for the use of polymyxins, which are WHO Reserve antibiotics [5]. Unfortunately, several patients with confirmed CRE infection remained on antibiotics, to which resistance was documented. The reasons for this are unclear and may include poor provider knowledge of therapeutic options or unavailability or unaffordability of polymyxins and tigecycline [7]. Nevertheless, a smaller number of patients on ineffective empiric antibiotics had the antibiotics discontinued. The optimal drugs for treating CRE in this and similar settings have yet to be identified; however, using drugs without documented in vitro susceptibility may not be a good strategy [8]. The genes responsible for carbapenem resistance are also not well-described locally, which is important for identifying the appropriate antibiotics [9]. There are instances in which CREs with MIC values at the lower end of carbapenem resistance may still benefit from carbapenem therapy. In our study, this does not appear to be the rationale behind continued carbapenem use for CRE treatment because MIC data are not usually provided with culture results [10]. Meropenem and quinolone dual therapy was used in a few instances in our study, even though there was documented in vitro resistance to levofloxacin in all CRE isolates tested. Although there is evidence to suggest that dual therapy might be superior to monotherapy in certain circumstances, it does not appear to have been a guiding principle in the selection of this antibiotic combination [6]. In the cases where effective antibiotics were used after culture results became available, two of them received polymyxins, whereas the third received amikacin. Our descriptive study has some limitations. Our study was small and performed in a single center; medical records for about half of the patients with CRE infections were not available and thus not included in this report. We also do not have information on diagnosis, dosing, duration of treatment, and treatment outcomes, thus clinical information was lacking to interpret some of the treatment decisions made by the clinicians.
Conclusion
The treatment of CRE infections in Nigeria is sub-optimal and existing treatment guidelines need to be adapted for institutions urgently in this and other resource-limited settings. Health care workers need to be trained as part of an antimicrobial stewardship intervention for CRE treatment. Research needs to be targeted toward understanding the local molecular epidemiology of CRE and the most effective antibiotics for treatment. Infection prevention and control practices need to be strengthened to prevent the spread of these difficult-to-treat pathogens in health care settings. Lastly, there is a need for equitable access to the most appropriate treatment agents in settings where this is not the case.
Declarations of competing interest
Olukemi Adekanmbi reports financial support from Pfizer. The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by an independent medical grant from Pfizer [grant number 54775481] as part of the Carbapenem-Resistant Enterobacteriaceae in WeST Africa project.
Ethical approval
Ethical approval was obtained from the University of Ibadan/University College Hospital, Ibadan, research ethics committee approval number: UI/EC/20/0009. Informed consent was waived because the study only entailed the use of secondary data from microbiology laboratory records and clinical records.
Author contributions
OA participated in conceptualization of the research, study design, study coordination and drafted the manuscript. OP participated in the study design, statistical analysis and helped to draft the manuscript. AF participated in conceptualization of the research study, laboratory work and helped to draft the manuscript. OI participated in study design and data collection. BO participated in conceptualization of the research, data collection and helped with study coordination. SL participated in conceptualization of the research, study design and helped to draft the manuscript. IA carried out laboratory work and data collection. All authors read and approved the final manuscript.
References
- 1.Lowe M, Shuping L, Perovic O. Carbapenem-resistant Enterobacterales in patients with bacteraemia at tertiary academic hospitals in South Africa, 2019–2020: an update. S Afr Med J. 2022;112:542–552. doi: 10.7196/SAMJ.2022.v112i8.16351. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO priority pathogens list for R&D of new antibiotics, http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/; 2017 [accessed 13 March 2024].
- 3.Trecarichi EM, Tumbarello M. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence. 2017;8:470–484. doi: 10.1080/21505594.2017.1292196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Africa Centres for Disease Control and Prevention, Center for Disease Dynamics EP, editor. African antibiotic treatment guidelines for common bacterial infections and syndromes. 1st ed. Addis Ababa: Africa Centres for Disease Control and Prevention Center for Disease Dynamics, Economics & Policy, 2021.
- 5.World Health Organization . World Health Organization; Geneva: 2022. The WHO AWaRe (access, watch, reserve) antibiotic book. [Google Scholar]
- 6.Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25:943–950. doi: 10.1016/j.cmi.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Ogoina D, Iliyasu G, Kwaghe V, Otu A, Akase IE, Adekanmbi O, et al. Predictors of antibiotic prescriptions: a knowledge, attitude and practice survey among physicians in tertiary hospitals in Nigeria. Antimicrob Resist Infect Control. 2021;10:1. doi: 10.1186/s13756-020-00855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise MG, Karlowsky JA, Mohamed N, Kamat S, Sahm DF. In vitro activity of aztreonam–avibactam against Enterobacterales isolates collected in Latin America, Africa/Middle East, Asia, and Eurasia for the ATLAS Global Surveillance Program in 2019–2021. Eur J Clin Microbiol Infect Dis. 2023;42:1135–1143. doi: 10.1007/s10096-023-04645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedišaletše M, Phumuzile D, Angela D, Andrew W, Mae N-F. Epidemiology, risk factors, and clinical outcomes of carbapenem-resistant Enterobacterales in Africa: a systematic review. J Glob Antimicrob Resist. 2023;35:297–306. doi: 10.1016/j.jgar.2023.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Chiotos K, Hayes M, Gerber JS, Tamma PD. Treatment of carbapenem-resistant Enterobacteriaceae infections in children. J Pediatric Infect Dis Soc. 2020;9:56–66. doi: 10.1093/jpids/piz085. [DOI] [PMC free article] [PubMed] [Google Scholar]


