Abstract
Trabectedin is an antineoplastic drug used to treat soft tissue sarcomas. Trabectedin is mainly infused from the central venous port (CVP) because trabectedin leakage causes serious skin and soft tissue complications. Characteristic sterile inflammation has recently been reported after infusion of trabectedin from the CVP. Here, we report a case of sterile inflammation along a tunneled catheter pathway after trabectedin infusion from the CVP, with residual postinflammatory changes even after CVP removal.
A 57-year-old man with myxoid liposarcoma developed skin erythema, swelling, and induration along a tunneled catheter pathway of the CVP after 16 cycles of trabectedin infusion through the CVP. The patient was diagnosed with sterile inflammation because various tests were negative for infection. The CVP was removed because the increasing injection resistance made trabectedin infusion difficult. The catheter firmly adhered to the surrounding tissue during removal. The induration and pigmentation along the catheter persisted for 4 months after CVP removal.
Keywords: Central venous port, Trabectedin, Sterile inflammation
Introduction
Trabectedin is an antineoplastic drug used to treat soft tissue sarcomas [1]. Trabectedin is a vesicant drug that can cause serious skin and soft tissue complications, such as skin necrosis, when leaked [2]; therefore, the central venous port (CVP) is the primary choice of infusion route. In addition, a few retrospective studies have reported that the infusion of trabectedin from the CVP causes a characteristic sterile inflammation along the tunneled catheter, distinct from anticancer drug extravasation and infections [[3], [4], [5]]. However, no reports have described the subsequent changes after sterile inflammation. Here, we report a case of sterile inflammation along a tunneled catheter after trabectedin infusion from the CVP with residual post-inflammatory changes even after CVP removal.
Case report
A 57-year-old man with myxoid liposarcoma presented with skin erythema, swelling, and induration along the tunneled catheter pathway of a CVP (PowerPort; Bard Access Systems, Medicon Inc., Osaka, Japan) implanted through the right internal jugular vein after sixteen cycles of trabectedin infusion (Fig. 1). Various blood tests were negative for infection; therefore, the patient was diagnosed with a characteristic sterile inflammation caused by trabectedin infusion from the CVP. After 3 additional cycles of trabectedin infusion, induration and swelling worsened and injection resistance increased. Ultrasonography revealed a structure with a low-intensity rim around the catheter and acoustic shadows (Fig. 2). Ultrasonography and contrast-enhanced computed tomography revealed no thrombus around the catheter tip. CVP removal was planned because the increased injection resistance made it difficult to infuse trabectedin.
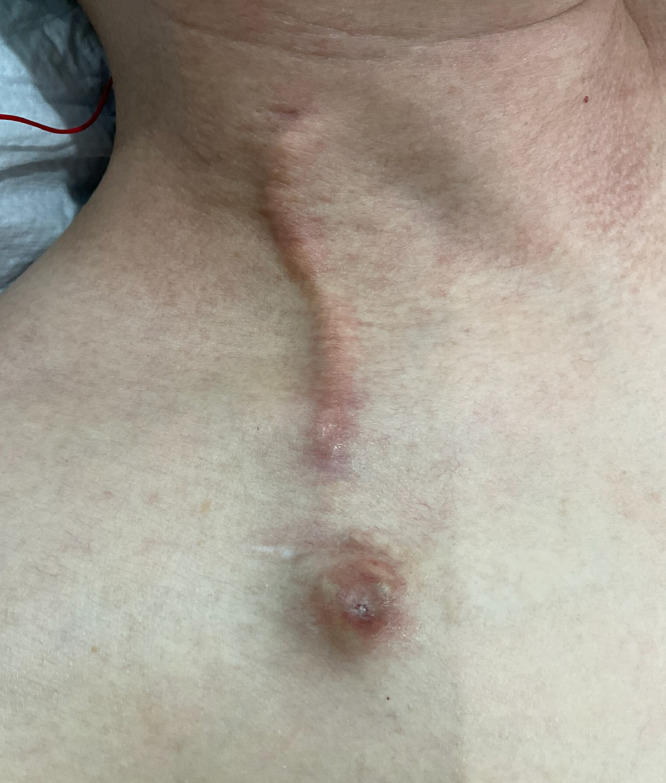
Fig. 1.
Skin changes after 16 cycles of trabectedin infusion through the central venous port (CVP). Skin erythema, swelling, and induration presented along the tunneled catheter of the CVP implanted through the right internal jugular vein.
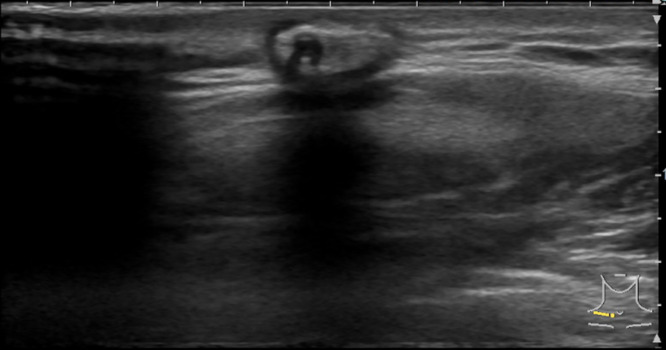
Fig. 2.
Ultrasound before central venous port removal. After 19 cycles of trabectedin infusion, an ultrasound showed a structure with a low-intensity rim around the catheter with acoustic shadows.
A small incision was made near the port site under local anesthesia, and the subcutaneous tissue was detached to expose the port body. Manual port removal was attempted; however, the subcutaneous catheter was firmly adhered to the surrounding tissue, making removal difficult. Therefore, the adherent surrounding tissue around the subcutaneous catheter was incised and detached before successful removal. The removed catheter showed no noticeable damage.
The skin erythema spontaneously faded after CVP removal. However, a radiograph taken 1 month after CVP removal showed residual calcified edges along the preexisting catheter pathway (Fig. 3A). In addition, skin induration and pigmentation remained 4 months after CVP removal (Fig. 3B).
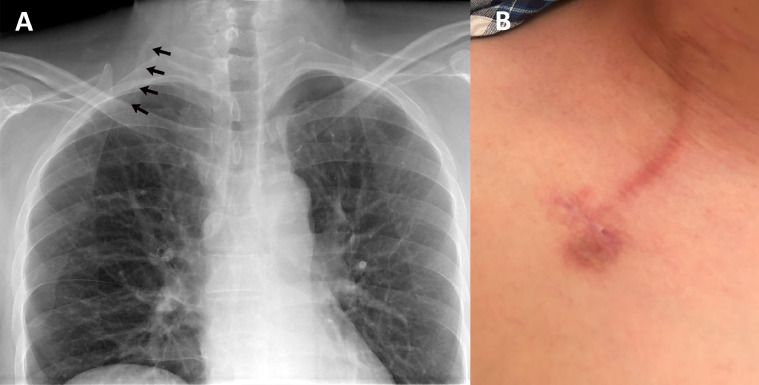
Fig. 3.
(A) chest radiograph and (B) skin changes after central venous port (CVP) removal. (A) A radiograph 1 month after CVP removal showed residual calcified edges along the preexistent catheter pathway (arrows). (B) Skin induration and pigmentation remained 4 months after CVP removal.
Discussion
In a retrospective study of trabectedin in metastatic sarcoma, Hoiczyk et al. first reported a unique noninfectious irritation along the subcutaneous catheter after trabectedin infusion [5]. Two retrospective studies were subsequently reported, focusing on this characteristic sterile inflammation along the tunneled catheter after trabectedin infusion from a CVP [3,4]. In these studies, sterile inflammation typically caused skin erythema, swelling, pain, and induration along the tunneled catheter, without detecting the causative microorganisms [[3], [4], [5]]. These symptoms were found to abate between cycles of trabectedin infusion but often flared up a few days after trabectedin infusion [3,4]. Compared to the severe skin and soft tissue complications caused by trabectedin leakage [2], sterile inflammation along the catheter is considered a different phenomenon, as it differs in localization and has relatively mild symptoms. In addition, sterile inflammation may be a characteristic complication of trabectedin infusion via CVPs, as no complications similar to those of other drugs have been reported. The mechanism of sterile inflammation is thought to be mild inflammation due to microleakage of trabectedin from the porous catheter at the tunneled catheter site [3] or spillback of the drug caused by small thrombi at the tip of the catheter [5]; however, this has not been proven. In the present study, sterile inflammation along the tunneled catheter occurred after 16 cycles of trabectedin infusion from the CVP. We considered this characteristic sterile inflammation of trabectedin infusion to be similar to that observed in previous reports.
Few detailed reports are available on the course of sterile inflammation. Erythema has been reported to persist for several weeks before spontaneously fade [5]. In this case, the erythema faded, but induration and pigmentation remained along the catheter trajectory even after CVP removal, causing a burden on the patient's cosmetic appearance. In addition, Kubo et al. found that CVP removal was necessary in 4 of 5 patients with sterile inflammation because of worsening pain [4]. Hoiczyk et al. also found that several consecutive port replacements were required in some cases [5]. In the current study, increased infusion resistance made it difficult to infuse trabectedin and the CVP had to be removed. Therefore, it is important to know that sterile inflammation can create a cosmetic burden for patients and to necessitate CVP removal is important.
The incidence of sterile inflammation has been reported to be approximately 30% [3,4], which is not low. Several methods have been proposed to reduce the incidence of this characteristic complication caused by trabectedin infusion through CVPs. Verboom et al. did not detect any cases of sterile inflammation when tunneled catheters were placed deeply, and therefore proposed placing the catheter deeply into the subcutaneous tissue [3]. In contrast, Kubo et al. found that the incidence of sterile inflammation differed significantly between the CVP systems, and suggested using systems with a low incidence rate [4]. Although the PowerPort used in this study is a CVP system with no previous reports of sterile inflammation, it may cause sterile inflammation, and may be avoided. Furthermore, selecting a route with as few subcutaneous catheter pathways as possible may be effective when implanting a CVP for trabectedin infusion.
This case report suggests that the characteristic sterile inflammation caused by trabectedin infusion from CVP may lead to the removal of CVP and patient cosmetic burden.
Patient consent
Written informed consent was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: We would like to thank Editage (www.editage.com) for their writing support.
References
- 1.Nakamura T, Sudo A. The role of trabectedin in soft tissue sarcoma. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.777872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theman TA, Hartzell TL, Sinha I, Polson K, Morgan J, Demetri GD, et al. Recognition of a new chemotherapeutic vesicant: trabectedin (ecteinascidin-743) extravasation with skin and soft tissue damage. J Clin Oncol. 2009;27:e198–e200. doi: 10.1200/JCO.2008.21.6473. [DOI] [PubMed] [Google Scholar]
- 3.Verboom MC, Ouwerkerk J, Steeghs N, Lutjeboer J, Martijn Kerst J, van der Graaf WTA, et al. H. Central venous access related adverse events after trabectedin infusions in soft tissue sarcoma patients; experience and management in a nationwide multi-center study. Clin Sarcoma Res. 2017;7:2. doi: 10.1186/s13569-017-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubo T, Yasaka K, Kobayashi H. Differences in the sterile inflammation incidence after trabectedin infusion with two central venous port systems: a retrospective study. Cureus. 2024;16:e57507. doi: 10.7759/cureus.57507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoiczyk M, Grabellus F, Podleska L, Ahrens M, Schwindenhammer B, Taeger G, et al. Trabectedin in metastatic soft tissue sarcomas: role of pretreatment and age. Int J Oncol. 2013;43:23–28. doi: 10.3892/ijo.2013.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]