Abstract
Pain is the leading symptom for most individuals with osteoarthritis (OA), a complex condition marked by joint discomfort. Recently, the dynamic interplay between the nervous and immune systems has become a focal point for understanding pain regulation. Despite this, there is still a substantial gap in our comprehensive understanding of the neuroimmune interactions and their effects on pain in OA. This review examines the bidirectional influences between immune cells and nerves in OA progression. It explores current approaches that target neuroimmune pathways, including promoting M2 macrophage polarization and specific neuronal receptor targeting, for effective pain reduction.
Translational potential statement
This review provides a comprehensive overview of the mechanisms underlying the interplay between the immune system and nervous system during the progression of OA, as well as their contributions to pain. Additionally, it compiles existing intervention strategies targeting neuroimmunity for the treatment of OA pain. This information offers valuable insights for researchers seeking to address the challenge of OA pain.
Keywords: Macrophage activation, Neuro-immune interaction, Osteoarthritis, Pain
Graphical abstract
1. Introduction
Osteoarthritis (OA) is a prevalent and disabling disease that imposes a significant burden on both patients and society. Globally, the prevalence of OA has increased by 132.2 % since 1990 and currently stands at 7.6 % as of 2020. This prevalence is projected to further rise by 2050 [1]. OA affects not only the articular cartilage but also the subchondral bone, intra-articular and periarticular soft tissues [2]. The main symptom of OA is pain, and pain relief is a primary concern for patients [2,3].
Currently, pain assessment in OA patients primarily relies on questionnaire-based methods, such as the Visual Analog Scale (VAS) [4], and the Knee injury and Osteoarthritis Outcome Score (KOOS) [5]. More objective methods, such as Quantitative Sensory Testing, including measurements of Pressure Pain Threshold, Conditioned Pain Modulation, and Temporal Summation, have also been employed to assess pain sensitization in OA [6]. Evaluation of pain intensity in experimental animals depends on behavioral experiments such as measuring mechanical and thermal pain thresholds, and gait analysis [7]. Additionally, some studies measure levels of inflammatory factors in the DRG of animals to assess pain severity [8].
In normal circumstances, the joint tissues, apart from cartilage, are richly innervated by sensory and sympathetic nerves. However, in OA, nerve endings proliferate in the synovium, subchondral bone, and osteochondral junction, heightening the joint's sensitivity [9]. Tissue injury in OA patients can activate nociceptive neurons, resulting in nociceptive pain. Interestingly, studies found that individuals with OA may also experience symptoms of neuropathic pain and neurosensitization [3,5,10]. These symptoms manifest as heightened sensitivity to stimulation within the joints or abnormal pain sensations in areas beyond the affected joints. The current consensus suggests that the mechanism driving chronic pain in OA is multifaceted, involving continuous stimulation and activation of nociceptive receptors within the joint, direct engagement of neurons in the dorsal root ganglion (DRG), and changes within the central nervous system. Furthermore, during the course of OA, there is a notable increase in markers indicative of nerve injury [11,12], suggesting that nerve injury may also contribute to OA pain.
OA, as a chronic inflammatory disease, involves a significant immune component throughout its course. In OA joints, inflammation primarily occurs in the synovium, where the number of immune cells significantly increases during OA progression [13]. Key immune cells involved include macrophages and T lymphocytes, whose infiltration levels correlate closely with OA progression and pain symptoms [14]. Inflammatory responses within the joint lead to upregulation of pro-inflammatory cytokines and pronociceptive substances, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, IL-17, prostaglandin E2 (PGE2), and nitric oxide (NO), which induce cartilage degradation and activation of nociceptors [15]. Apart from immune cells in the synovium, fibroblast-like synoviocytes (FLS), chondrocytes, osteoblasts, and osteoclasts also contribute to the secretion of these inflammatory factors, further driving the progression of joint inflammation and pain. However, regulating inflammation is not their primary function, and their capacity to secrete pro-inflammatory cytokines is lower compared to immune cells [15]. The mechanisms through which they regulate pain also partly differ from those of immune cells. For instance, FLS are able to stimulate nociceptors by influencing myofibroblast contraction to regulate synovial fibrosis [16], and osteoclasts can induce pain sensitization through the secretion of netrin-1 to promote nerve growth [17]. These cells’ modulation of OA pain extends beyond the realm of neuroimmune interactions; hence they are not extensively discussed in this review.
Recent advancements in understanding of OA pathogenesis have revealed the critical role played by interactions between the immune and nervous systems in mediating OA pain. This review is designed to provide a comprehensive overview of neuroimmune interactions in OA, and to summarize therapeutic approaches that target both the immune and nervous systems for the management of OA pain. It specifically delves into the role of immune cells and their secreted inflammatory mediators in the regulation of OA pain. Furthermore, the review examines the ways in which neurons modulate immune cell activity and inflammatory processes via neurotransmitters, chemokines, and microRNAs.
2. Regulation of nerves by immune cells in OA
Within the immune system's framework, there are two primary divisions: the innate and adaptive immunity mechanisms. Existing studies have primarily focused on the cells involved in innate immunity, particularly macrophages and the inflammatory mediators they release, as indispensable factors in the onset and progression of OA, closely linked to OA symptoms. Relatively few studies have explored the role of adaptive immunity in OA. However, given its strong association with chronic pain [18], further attention should be directed toward this aspect (Table 1).
Table 1.
The role of immune cells in different locations in mediating pain during OA progression.
| Types of Immune Cells | Sites |
||
|---|---|---|---|
| Joint | DRG | Spinal Cord Dorsal Horn | |
| Macrophages | Migration and M1 polarization in the synovium [19]. Releasing pain-promoting substances, such as IL-1β, IL-6, TNF-α, CCL2, PGE2, and NGF [20,21]. |
Infiltration and M1 polarization in the DRG [22]. The secretion of iNOS, IL-1β, TNF-α, IL-6, CCL2, and NGF induced pain [23]. |
The activation and proliferation of microglia occurred in the ipsilateral spinal dorsal horn [24]. Stimulating neurons through the release of IL-1β [25]. |
| Neutrophils | Leading to pain sensation by secreting IL-1β and NE [23,26]. | Neutrophil infiltration occurred during early-stage OA [27]. | |
| Mast Cells | The proportion of mast cells in synovium increased significantly [28]. The secretion of chymase, PGE2, PGD2, and NGF elicited pain [[29], [30], [31]]. |
||
| Dendritic Cells | The proportion of dendritic cells increased [32]. | ||
| T Cells | T-cell infiltration and Th1 polarization in the synovium resulting in pain [33,34]. | ||
OA, osteoarthritis; DRG, dorsal root ganglion; IL, Interleukin; TNF-α, tumor necrosis factor α; CCL2, C–C motif chemokine ligand 2; PGE2, prostaglandin E2; NGF, nerve growth factor; iNOS, inducible nitric oxide synthase; NE, norepinephrine; PAR, proteinase-activated receptor 2; MIA, monosodium iodoacetate; PGE2, prostaglandin E2; PGD2, prostaglandin D2; Th1, T-helper 1.
2.1. Regulation of nerves by innate immunity in OA
2.1.1. Macrophages
Macrophages are pivotal immune cells in OA pathology and have thus become a central focus in OA research. Throughout the progression of OA, macrophages accumulate not only in the joint, primarily within the synovium, leading to inflammation and cartilage degradation [35,19], but also in the DRG, where they provoke inflammatory responses [27] (Fig. 1). These macrophages can be categorized into pro-inflammatory macrophages (M1 macrophages) and anti-inflammatory macrophages (M2 macrophages). Considering the intimate link between pain and inflammation, the regulatory role of M1 and M2 macrophages on pain perception aligns with their respective regulatory roles in inflammatory processes [20].
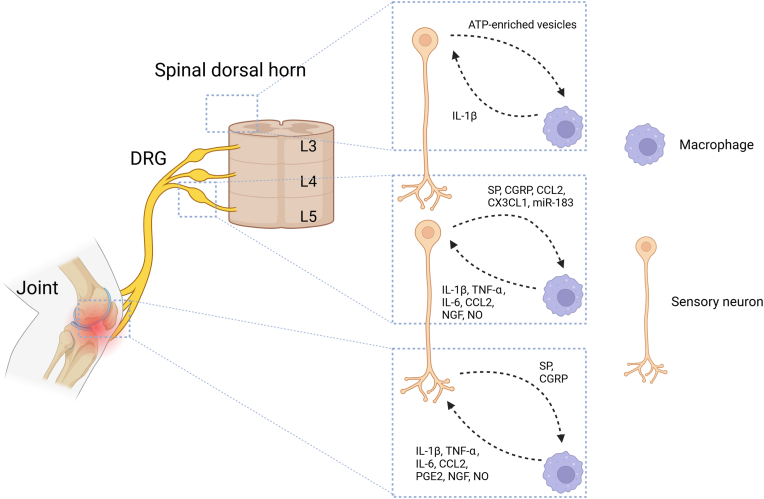
Figure 1.
The Interaction of Macrophages with Nerves at Multiple Sites During the Progression of Knee OA. Within the knee joint, macrophages are capable of directly stimulate nerve endings through various pro-inflammatory mediators. Concurrently, the release of nociceptive neurotransmitters from these nerve endings also plays a role in modulating macrophage activity. In the ipsilateral L3-L5 DRG, macrophages release pro-inflammatory mediators that have the potential to either stimulate or directly damage neuronal cell bodies. Meanwhile, neurons release nociceptive neurotransmitters, cytokines, and microRNAs, influencing macrophage function. Additionally, in the spinal dorsal horn ipsilateral to the joint affected by OA, activated microglia secreted IL-1β, which stimulates spinal neurons. Simultaneously, ATP-enriched vesicles released by neurons can promote microglial activation. CCL2, C–C motif chemokine ligand 2; CGRP, calcitonin gene-related peptide; CX3CL1, C-X3-C motif chemokine ligand 1; DRG, dorsal root ganglion; IL, interleukin; NGF, nerve growth factor; NO, nitric oxide; OA, osteoarthritis; PGE2, prostaglandin E2.
Within the joint, macrophages are predominantly located in the synovial membrane. During the progression of OA, macrophages become activated through the recognition of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) [36], or through the initiation of inflammasome activation [37]. The M1 polarization of macrophage is closely associated with OA development [19]. M1 macrophages release a variety of pro-inflammatory cytokines during the immune response, which have the ability to directly stimulate nociceptive sensory nerve endings [38]. For instance, in monosodium iodoacetate (MIA)-induced OA mice, M1 macrophages in the synovium exhibit a selective accumulation pattern around the terminations of neurons expressing transient receptor potential vanilloid 1 (TRPV1) [23]. TRPV1 is a non-selective cation ligand-gated channel mainly distributed on the surface of sensory neurons. Its activation can cause structural changes and trigger cations to flood the neuron, thereby playing a crucial role in the transmission of inflammatory pain [39]. These nociceptive TRPV1-positive neurons also co-express interleukin-1 receptor 1 (IL-1R1), and these two receptors exhibit functional coupling. As a result, IL-1β released by M1 macrophages can directly activate these neurons [23]. Additionally, other cytokines secreted by M1 macrophages, such as C–C motif chemokine ligand 2 (CCL2), can induce calcium influx by binding to the corresponding chemokine receptor C–C motif chemokine receptor (CCR) 2 on the surface of sensory neurons, directly activating these neurons [40]. Another crucial pro-inflammatory factor released by M1 macrophages, TNF-α, has been shown to increase sensitivity of sensory neurons to PGE2, thereby promoting hyperalgesia [41]. PGE2, a well-recognized pronociceptive substance and a metabolite of arachidonic acid, can bind to prostaglandin E2 receptor 4 (EP4R) on nociceptors’ surfaces, promoting pain sensitization [42]. In late-stage OA, synovial M1 macrophages are among its significant sources [43]. Other pronociceptive substances include nerve growth factor (NGF) and NO, which macrophages can promote expression of by modulating FLS and chondrocytes, or serve as direct sources themselves [21,44]. Interestingly, some studies suggested that M1 macrophages might also play a role in pain relief. For instance, M1 macrophages at nerve injury sites release opioid peptides in response to IL-4, thereby relieving pain [45]. In contrast, M2 macrophages exhibit anti-inflammatory and pain-relieving properties, suggesting that encouraging M2 polarization could be a key strategy in alleviating OA pain. This aspect will be further explored later in this review.
Recent studies have indicated that, macrophages in the DRG were indispensable for the maintenance of OA pain [22]. In mice with MIA-induced OA, the macrophages in the DRG exhibit a classical M1 phenotype, which is characterized by the expression of inducible nitric oxide synthase (iNOS) [22], resulting in direct neurotoxicity to neurons [46], which might explain the lack of strong correlation between pain and joint destruction in OA patients [5]. Same as in the joint, certain proinflammatory substances produced by M1 macrophages can stimulate neurons in the DRG. For example, IL-1β can induce pain by activating neuronal IL-1R1 [23]. CCR2 expressed by neurons in the DRG can also be activated and induce pain by CCL2 secreted by M1 macrophages [47,48]. Currently, the expression changes of molecules such as TNF-α, IL-1β, IL-6, and NGF in the DRG have become important indicators for evaluating pain in experimental animals [8,49].
In addition, acting as macrophages in the central nervous system, microglial cells also undergo changes during the progression of OA [24,25]. OA leads to the proliferation and activation of spinal microglia in mice, promoting the progression and sustaining of pain [24]. Research on the changes of cerebral microglial cells in OA is relatively lacking. Microglial cells can also be divided into M1/M2 phenotypes, with M1-polarized microglia capable of secreting various pro-inflammatory mediators, such as IL-1β, TNF-α, IL-6, and iNOS, thereby being a necessary condition for neuroinflammation and pain sensitization [50]. In MIA-induced OA mice, the upregulation of P2X7 receptors on spinal microglial cells promotes the release of IL-1β, affecting the excitability of dorsal horn neurons, leading to mechanical allodynia. Interestingly, in mice with unilateral OA, in addition to affecting the ipsilateral spinal dorsal horn, there is also proliferation in the contralateral spinal dorsal horn microglial cells [24,25], and the corresponding mechanisms require further clarification.
2.1.2. Neutrophils
Similar to macrophages, in the joint, neutrophils expressing IL-1β are observed to gather around sensory nerve terminals that express IL-1R1 [23], indicating that neutrophils in the joint play a role in neurology. Research on the effects of neutrophils on nerves in OA primarily focuses on elastase. Increased secretion of elastase by neutrophils in the joint can lead to cartilage damage [51,52], and is also strongly associated with synovial inflammation and pain of OA [[26], [53], [54]]. Neutrophil elastase (NE) could trigger protease-activated receptor 2 (PAR2) on nociceptors by cleaving its N-terminus [55], and the resultant pain might be attributed to the functional interaction between PAR2 and TRPV1 [56].
In MIA-induced OA mice, early-stage OA is characterized by the presence of neutrophil accumulation in the DRG. However, neutrophil levels return to baseline after 4 weeks, without a concomitant improvement in pain [27], suggesting that the presence of neutrophils in the DRG may be a reactive response to acute injury. Nonetheless, the early infiltration of neutrophils in the DRG may have a significant role in alleviating OA pain. This is supported by findings from mouse models using complete Freund's adjuvant (CFA) injection, where early administration of non-steroidal anti-inflammatory drugs (NSAIDs), which inhibited the aggregation of neutrophils, surprisingly led to a prolonged pain experience. A possible mechanism for this could be the secretion of calprotectin by neutrophils, which is thought to play a protective role against the development of chronic pain [57].
2.1.3. Mast cells
The level of mast cell infiltration in synovium of individuals with OA is significantly elevated, surpassing that seen in rheumatoid arthritis. This elevation correlates positively with OA severity as observed in imaging studies but does not directly correlate with patient-reported pain [28]. Upon degranulation, mast cells release proteases such as tryptase, which, similar to NE, can activate PAR2 [29], leading to similar effects. Mast cells may also serve as a source of NGF [30]. NGF can activate tropomyosin receptor kinase A (TrkA) on mast cells, inducing the release of PGE2 and prostaglandin D2 (PGD2). The activation of the prostaglandin D2 receptor 1 by PGD2 results in heightened sensitivity of pain receptors [31].
2.1.4. Dendritic cells
There is a lack of research investigating the interaction between dendritic cells (DCs) and neurons in OA. An elevated proportion of DCs in the DRG has been reported in elderly mice with OA [32]. Given that DCs are known to secrete IL-23, a factor implicated in inducing chemotherapy-induced pain in female mice [58], it is reasonable to speculate that DCs in the DRG might similarly influence OA pain.
2.2. Regulation of nerves by adaptive immunity in OA requires further confirmation
During the progression of OA, there is a noticeable increase in T cell migration into the synovium and synovial fluid. Importantly, the proportion of CD4+ T cells in the synovium exhibits a substantial correlation with the severity of pain and disability experienced in OA [59]. At the early stages of OA, a conspicuous accumulation of T cells occurs in the synovium, accompanied by a tendency towards T-helper 1 polarization [33]. This pattern persists even in the later stages of OA [34]. Although these T cells are known to exacerbate inflammation and cartilage deterioration in OA, thereby affecting pain perception [60], their precise role in neuroimmune crosstalk within this context remains largely unexplored.
Although the role of B cells in OA is not as prominent, specific phenotypic alterations are observed. In OA, synovial B cells gain the ability to secrete IL-10 [61]. Furthermore, Raffaeli et al. documented a decrease in opioid receptor expression on peripheral B cells in patients suffering from severe or chronic pain [62]. These findings suggest the potential engagement of B cells in the neuroimmune response of OA.
3. Regulation of immunity by nerves in OA
In the context of OA within the joint, activated sensory neurons subsequently release a diverse array of substances within the joint and in the DRG. This process simultaneously facilitates pain transmission and affects the immune system (Table 2).
Table 2.
Regulation of immune cells by sensory neuron secretions.
| Sensory Neuron Secretion | Pro-Inflammatory | Anti-Inflammatory |
|---|---|---|
| Nociceptive transmitter | SP induced activation of MAPK pathway in macrophages [63]. SP activated mast cells via Mrgprb2 [64]. CGRP promoted the release of CX3CL1 by endothelial cells, inducing macrophage chemotaxis and M1 polarization [65]. |
SP promoted M2 polarization of macrophages through inhibition of NF-kB/NLRP3 signaling pathway [66]. Intravenous injection of SP increased circulating IL-10 level, inducing macrophage M2 polarization [67]. CGRP promoted M2 polarization of macrophages via the PI3K/AKT signaling pathway [68]. |
| Cytokines | CCL2, CSF1, CX3CL1 promoted macrophage chemotaxis in the DRG [69,70,71]. CXCL1 facilitated the chemotaxis of neutrophils in the DRG [72]. |
|
| microRNA | miR-21 induced pro-inflammatory phenotype in macrophages [73]. | miR-183 suppressed macrophage chemotaxis [74]. |
| Vesicles | ATP-enriched vesicles released by spinal dorsal horn neurons induced activation of microglia [75]. |
SP, substance P; MAPK, mitogen-activated protein kinase; Mrgprb2, mas-related G protein-coupled receptor b2; CGRP, calcitonin gene-related peptide; NF-kB, Nuclear Factor kappa B; NLRP3, NOD-like receptor family pyrin domain containing 3; IL-10, Interleukin-10; PI3K, Phosphoinositide 3-kinase; AKT, Protein Kinase B; CX3CL1, C-X3-C motif chemokine ligand 1; CCL2, C–C motif chemokine ligand 2; DRG, dorsal root ganglion; CSF1, colony-stimulating factor 1; CCR2, C–C motif chemokine receptor 2.
Nociceptive transmitters released by sensory neurons have the capacity to modulate immunity. In the joint affected by OA, the activation of nociceptive receptors leads to an upregulation in the expression of nociceptive transmitters, primarily substance P (SP) and calcitonin gene-related peptide (CGRP) [76]. These nociceptive neurotransmitters are critical factors in pain perception. However, their regulatory roles in inflammation are complex and may involve dual pro-inflammatory and anti-inflammatory effects. For example, in vitro studies show that SP can stimulate macrophages and mast cells to secrete pro-inflammatory chemokines [63,64]. However, in the context of tissue injury, SP can promote the M2 polarization of macrophages, which is associated with anti-inflammatory effects [77]. More studies tend to support the anti-inflammatory role of SP during joint diseases. In RA mouse models, intravenous SP injection increases circulating IL-10 levels and induces macrophage M2 polarization, exerting anti-inflammatory effects and reducing arthritis inflammation [67]. Furthermore, Kim SJ et al. achieved sustained release of SP in OA mouse joints through intra-articular injection of self-assembled peptide-SP hydrogels, effectively reducing joint inflammation [78]. Conversely, while some reports suggest that CGRP can induce M2 polarization of macrophages in conditions such as keratitis and temporomandibular joint arthritis [68,79], current research on joint diseases tends to support a pro-inflammatory role for CGRP. In RA mice, CGRP released from DRG induces endothelial cells to secrete C-X3-C motif chemokine ligand 1 (CX3CL1), which recruits macrophages and promoting M1 polarization [65]. Additionally, in OA mice, intravenous administration of CGRP antagonists has been shown to alleviate joint inflammation [80].
Sensory neurons are capable of expressing various cytokines that influence immune cells. The heightened expression of CCL2 in sensory neurons within the DRG is associated with the activation of Toll-like receptor (TLR) 4 in neurons by DAMPs, which can attract macrophages originating from monocytes [32,76]. It is noteworthy that CCL2 can also activate CCR2 to promote pain through neuronal autocrine signaling [47]. Other research has demonstrated that TLR4 activation could also increase the expression of C-X-C Motif Chemokine Ligand 1 (CXCL1) in sensory neurons, promoting the chemotaxis of neutrophils and contributing to pain induction [72]. Similarly, in OA mice, neurons in the DRG release CXCL11, which attracts macrophages and induce M1 polarization to sustain pain [22]. Additionally, CX3CL1, present on the membrane of sensory neurons in the DRG, is released in response to nerve injury or other stimuli, promoting both the chemotaxis and proliferation of macrophages [69]. In the event of nerve damage, there is an upsurge in the expression of colony-stimulating factor 1 (CSF1) in DRG sensory neurons, leading to an expansion of macrophage within the DRG in male mice. Meanwhile, CSF1 levels also increase in the spinal dorsal horn, inducing the activation of microglial cells [70]. However, whether this phenomenon occurs during the course of OA requires further investigation.
Sensory neurons can also exert influence on the immune system through additional mediators. For example, neuron-derived microRNAs play a regulatory role in immunity. In mouse OA models, within the DRG, the inhibitory effect of miR-183 on transforming growth factor (TGF)-α is compromised, resulting in an increase in CCL2 and the exacerbation of inflammation [74]. In the DRG of peripheral nerve injury mouse model, there was a notable increase in miR-21 expression in neurons, accompanied with the neuron-mediated transfer of exosomes containing miR-21 to macrophages, resulting in the development of a pro-inflammatory macrophage phenotype [73]. Furthermore, ATP-enriched vesicles released by neurons in the posterior horn of the spinal cord could stimulate microglial activation, exacerbating nerve pain [75].
4. Treatment strategies for OA pain targeting the nervous system and immune system
As mentioned earlier in this paper, both the immune system and the nervous system contribute significantly to the pain experienced by patients with OA. Considering that pain is often the most troubling symptom for those with OA, numerous studies have been devoted to developing new treatment approaches to address OA-related pain. In this section, we categorize these approaches based on their targets - either the immune system or the nervous system - and explore the feasibility and effectiveness of these varied treatment methods (Table 3).
Table 3.
Strategies for the treatment of OA pain targeting neuroimmunity.
| Targets | Promising Therapeutic Measures | |
|---|---|---|
| Immune Cells | Macrophages | Inhibition of M1 polarization and induction of M2 polarization within the joint and DRG [35,27]. |
| Neutrophils | Neutralizing NE in the joint [81]. | |
| T cells | Inhibition of Th17 cell differentiation [82]. Induction of Treg cell differentiation [83]. |
|
| Neuronal Surface Receptors | TrkA | Blocking the binding of NGF to TrkA [84]. |
| TLRs | Inhibition of TLRs activation [71]. | |
| TRPV1 | Injection of high-purity synthetic trans-capsaicin into the joint [85]. | |
OA, osteoarthritis; TrkA, tropomyosin receptor kinase A; TLRs, toll-like receptors; TRPV1, transient receptor potential vanilloid 1; DRG, dorsal root ganglion; NE, neutrophil elastase; Th17, T-helper 17; Treg cell, regulatory T cell; NGF, nerve growth factor.
4.1. Strategies for targeting immune cells
As previously mentioned, macrophages play a crucial role in OA pain through their involvement in neural immunity. While local or systemic depletion of macrophages in mouse models of OA can alleviate pain, it significantly disrupts immune homeostasis in the entire body and joint [86,87], making it unsuitable for clinical treatment. Modulating polarization of macrophages appears to be a promising avenue for therapeutic intervention of OA pain. Numerous studies have demonstrated that promoting the anti-inflammatory phenotype of macrophages within the joint effectively alleviates OA lesions and reduces pain in mice [35,88]. Likewise, the direct application of in vitro-induced M2 macrophages to the DRG successfully relieved OA pain in mice [27].
The role of M2 macrophages in suppressing pain can be achieved through multiple mechanisms. Firstly, they release anti-inflammatory factors like IL-10 and TGF-β, which have been shown to reduce neuroinflammation [89,90]. Secondly, M2 macrophages secrete opioid peptides to mitigate pain hypersensitivity [91]. Thirdly, M2 macrophages secrete specialized pro-resolving mediators including lipoxins, protectins, and resolvin E1, which modulate various immune cells to decrease the release of inflammatory mediators [92]. Additionally, the activation of resolvin E1 receptors on DRG neurons has been shown to block TRPV1 function, thus alleviating pain [93]. Finally, M2 macrophages release exosomes containing miRNA, which can be internalized by neurons and glial cells, leading to the downregulation of target genes associated with pro-neuroinflammatory responses [94,95]. Therefore, inhibiting M1 polarization and inducing M2 polarization of macrophages is an effective approach to alleviate OA pain.
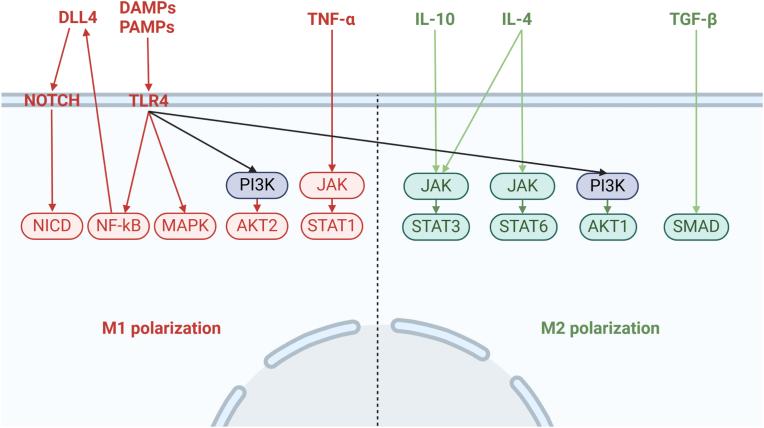
Macrophage polarization is a complex process involving multiple signaling pathways. Representative pathways include Janus kinase (JAK)/signal transducer and activator of transcription (JAK/STAT), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PI3K/AKT), Nuclear Factor kappa B (NF-kB), mitogen-activated protein kinase (MAPK), Notch, and TGF-β dependent pathways [[96], [97], [98], [99], [100]] (Fig. 2). Modulating these signaling pathways is crucial for regulating macrophage polarization. Numerous biologically active materials have been utilized in current research, including inhibitors, specially treated cells, and extracellular vesicles, all proven to regulate the polarization of macrophages within joints and DRGs, thereby relieving OA pain [49,[101], [102], [103], [104], [105], [106], [107]] (Table 4). Currently, there is insufficient evidence regarding the induction of M2 polarization of macrophages in the central nervous system to alleviate OA pain; however, research by Ban D et al. has shown that intrathecal injection of cerium oxide nanoparticles induces M2 polarization of macrophages, mitigating chronic neuropathic pain following spinal cord injury [108].
Figure 2.
Representative signaling pathways regulating macrophage polarization. Red arrows indicate pathways promoting M1 polarization of macrophages, green arrows indicate pathways inducing M2 polarization, and black arrows represent pathways contributing to both M1 and M2 polarization, without a clear polarization preference. AKT, Protein kinase B; DAMPs, Damage-associated molecular patterns; DLL4, Delta-like ligand 4; IL, Interleukin; JAK, Janus kinase; MAPK, Mitogen-activated protein kinase; NF-kB, Nuclear Factor kappa B; NICD, Notch intracellular domain; PAMPs, Pathogen-associated molecular patterns; PI3K, Phosphoinositide 3-kinase; TGF-β, Transforming growth factor-β; TLR4, Toll-like receptor 4; TNF-α, Tumor necrosis factor-α.
Table 4.
Biological materials improving OA pain through promotion of macrophage M2 polarization.
| Biological Materials | Mechanism of Action | Route of Administration | Target Site |
|---|---|---|---|
| MJN110 (a kind of MAGL inhibitor) [101] | Promoting increased expression of PINK1 and Parkin in M1 macrophages to induce mitochondrial autophagy. | Intraperitoneal injection | Joint |
| BTZ@PTK (co-assembly of bortezomib and an amphiphilic copolymer with ROS-cleaved thioketal linkages) [102] | Clearing ROS within the joints, and inhibiting the JAK/STAT signaling pathway in M1 macrophages, thus suppressing M1 polarization of macrophages. | Intra-articular injection | Joint |
| TissueGene-C (a combination of human allogeneic chondrocytes and irradiated GP2-293 cells overexpressing TGF-β1) [103] | Inducing an increase in intra-articular levels of IL-10 and TGF-β1 to promote M2 polarization of macrophages. | Intra-articular injection | Joint |
| Neonatal umbilical cord blood mesenchymal stem cells [104] | The secretion of PTX-3 acts on macrophages to induce M2 phenotype. | Intra-articular injection | Joint |
| Tyrosine hydroxylase-positive cells derived from bone marrow stem cells [105] | The secretion of IL-4 induces M2 polarization of macrophages and catecholamines to exert anti-inflammatory effects. | Intravenous injection | DRG |
| M2 macrophage-derived exosomes miR-26 b-5p [106] | Suppressing the expression of TLR3 on macrophages to inhibit M1 polarization, while promoting the expression of CD206 on their surface to facilitate the transition to M2 type. | Intra-articular injection | Joint |
| Membrane vesicles from Lactobacillus johnsonii [107] | The Membrane vesicles highly enriched with GS can inhibit the mTOR pathway within macrophages, hindering macrophage migration and M1 polarization. | Intraperitoneal injection | Joint |
| Dexamethasone liposomes [49] | Binding to glucocorticoid receptors on the surface of synovial macrophages promotes their polarization to the M2 phenotype. | Intra-articular injection | Joint |
OA, osteoarthritis; MAGL, Monoacylglycerol lipase; PINK1, Phosphatase and Tensin Homolog-Induced Putative Kinase 1; TGF-β1, transforming growth factor-β1; IL-10, Interleukin-10; PTX-3, Pentraxin-3; IL-4, Interleukin-4; TLR3, Toll-like receptor 3; GS, glutamine synthetase; mTOR, mammalian target of rapamycin.
Significant research is focused on targeting cytokines secreted by macrophages for the treatment of OA pain. As mentioned earlier, certain pro-inflammatory cytokines, including TNF-α and IL-1β, which are secreted by M1 macrophages, can directly stimulate nerve cells. However, clinical trials targeting a single cytokine alone have showed limited efficacy in alleviating OA pain [109,110]. Conversely, the direct application of M2 macrophage-related cytokines has achieved gratifying results. Animal studies have shown that direct application of the fusion protein of IL-4 and IL-10 in the joints or DRGs significantly alleviates OA pain, underscoring the significant potential of these cytokines in OA therapy [27,111].
Significant strides have also been made in targeting other immune cells in the context of OA. For instance, prophylactic inhibition of NE has been successful in preventing chronic OA pain in animal models [81]. In the management of OA, therapeutic approaches that focus on modifying T cell behavior are showing promise. These methods are proving to be effective in both mitigating pain and protecting joints, representing a key avenue in OA treatment [82,83]. However, most current studies focus on safeguarding joint tissues like cartilage and synovium, with less emphasis on the involvement of these immune cells in neuroimmunity. This oversight highlights the need for further research to understand how these immune cells contribute to neuroimmune interactions and to explore their potential implications in the development of new OA therapies.
4.2. Strategies for targeting nerves
Strategies targeting nerves include inhibiting neurogenic pain mediators and pro-inflammatory factors to reduce neurogenic inflammation, as well as blocking relevant receptors on nerves to alleviate inflammatory stimulation. Rimegepant, a CGRP receptor antagonist, has been shown to inhibit synovitis and pain in destabilization of the medial meniscus (DMM) mouse models when administered intraperitoneally [80]. Another competitive antagonist, CGRP8-37, demonstrates similar effects [112]. Additionally, intra-articular injection of a combination of magnesium ions and vitamin C has been shown to reduce SP and CGRP levels in OA mouse joints, alleviating hyperalgesia and suppressing joint inflammation [113]. Furthermore, intrathecal injection of antibodies against CXCL11, which is released by neurons, improves pain-related behaviors in OA mice by inhibiting M1 polarization of macrophages in the DRG [22].
Significant progress has been made in research focused on blocking the actions of pain-promoting substances on nerves. NGF can activate the TrkA and p75 neurotrophic factor receptor (p75NTR) on sensory neurons, leading to hyperalgesia [114]. Although anti-NGF treatment can improve pain, it also carries the risk of causing structural damage to the joint [115], which has led to the suspension of related clinical trials. Recent studies on animal models suggested that blocking TrkA might offer a more promising avenue for treating OA pain compared to anti-NGF therapies [116,84]. Regarding another pro-inflammatory substance PGE2, the competitive EP4R antagonist Grapiprant has been used to treat osteoarthritic pain in dogs [117], but its applicability to humans requires further investigation and confirmation through research. Moreover, sensory neurons express various TLRs in the DRG, and the activation of these TLRs contributes to OA pain [71]. The specific mechanisms of this process remain to be further elucidated. It is hypothesized that this phenomenon is related to the interaction between neutrophils and neurons. As previously mentioned, activation of TLRs on the surface of neurons leads to the release of CXCL1, recruiting neutrophils, and ultimately inducing nociceptive sensitization [72]. Additionally, it is proposed that DAMPs generated in OA can activate TLRs on damage neurons co-expressing TLRs and TRPV1, subsequently activating associated TRPV1 channels, leading to cation influx and neuronal activation [71]. Experimental studies have shown that targeted knockout of these TLRs or the downstream myeloid differentiation primary reactive protein 88, the central connector protein of TLRs, can inhibit hyperalgesia [72,118]. Therefore, exploring the blocking of TLRs as a potential therapeutic approach for OA pain is worthy of further investigation.
TRPV1 is at the forefront of current research, particularly due to its expression in sensory neurons and its association with inflammation and transmission of nociceptive signals [119]. The impact of TRPV1 activation, however, is multifaceted, owing to its widespread presence in various cell types. Notably, several studies have illustrated that activating TRPV1 can actually reduce inflammation and pain. For instance, capsaicin, a well-known TRPV1 activator, has been shown to inhibit M1 macrophage polarization by activating TRPV1 receptors present on their surface [120]. A recent clinical study revealed that high-purity synthetic trans-capsaicin (CNTX-4975) significantly reduced pain in patients with moderate to severe OA knee pain, particularly during walking [85]. Nonetheless, further research and practical investigations are necessary to fully comprehend the role of TRPV1 in modulating nerve function and immunity in OA patients.
5. Discussion
This review offers a detailed summary of the mechanisms underlying pain associated with OA, with a particular focus on the interaction between immune cells (especially macrophages) and nerves. It also analyzes potential methods and their feasibility of targeting immune cells and nerves in the treatment of OA pain. Throughout the various stages of OA, immune cells located in the joint, DRG, and spinal cord can modulate pain by affecting sensory neurons through the production of cytokines. Inversely, nerves can also influence immune responses by releasing chemokines and neurotransmitters. Consequently, strategies that regulate the infiltration of immune cells and cytokines in the joint and DRG, as well as those modulating neuronal surface receptors, might be effective in pain inhibition. The insights provided in this review offer a fresh perspective for researchers investigating the mechanisms and treatment of OA-associated pain.
It is noteworthy that pain symptoms in some OA patients appear before X-ray evidence of cartilage damage. This phenomenon occurs because synovitis and bone marrow edema beneath the cartilage can sometimes precede structural cartilage damage during the progression of OA, and both are highly correlated with pain [17,121]. Furthermore, mice undergoing DMM surgery showed changes in gene expression in the DRG and pain behavior as early as three days post-operation, even in the absence of significant cartilage structural damage [122,123]. This suggests that targeting neuroimmune regulation in the early stages of OA progression for pain management should be considered, and treatment sites should include joints and DRGs. Additionally, the contribution of subchondral bone to OA pain should be emphasized. While synovitis is a typical feature of OA, some patients exhibit milder synovial inflammation, with pathology primarily characterized by cartilage degeneration and subchondral bone remodeling [124]. In these cases, specific mechanisms of pain include increased intraosseous pressure stimulating nerve endings [125], netrin-1 secretion by osteoclasts [17], acidification of the subchondral bone microenvironment sensitizing nerve endings [126], and spontaneous firing of nerve endings due to subchondral bone overgrowth [127]. Although immune cell populations in OA subchondral bone have been identified [128], the interaction of neuroimmune factors within subchondral bone has not been definitively validated.
Exploration into the crosstalk of immune cells in OA remains limited, potentially obscuring some underlying neuroimmune mechanisms. Insights from studies on other diseases can be informative. For example, Luo et al. discovered that in cases of chemotherapy-induced nerve injury in female mice, T cells were the primary inducers of neuropathic pain in standard strains. In contrast, in female nude mice lacking adaptive immunity, macrophages assumed this pain-inducing role [129]. This suggests that employing immune-deficient animal models may offer substantial insights into understanding neuroimmune interactions in OA.
Research into the role of the sympathetic nervous system in neuroimmune responses throughout OA progression remains underexplored. Neurotransmitters secreted by the sympathetic nervous system, present within the joint, could have varying effects on different cell types, potentially leading to both pro-inflammatory and anti-inflammatory responses [130]. Notably, several studies have documented the impacts of sympathetic nerve activation and ablation in mouse models of OA [131,132]. The involvement of sympathetic nerves in neuroimmune interactions in OA thus presents an intriguing area for more in-depth research in future studies.
In animal models of OA, variances in pain-induced behaviors and pain-related signaling pathways were observed across different sexes [32]. This pattern is mirrored in other pain research, where immune responses and subsequent pain responses varied between experimental animals of different genders [58,129]. Taking into account the disparities in OA prevalence and symptoms between men and women in the general population [2], the inclusion of both genders in neuroimmunity studies related to OA would be beneficial. Such an inclusive approach is likely to shed light on gender-specific differences in the mechanisms of OA pain and aid in creating more targeted and effective treatment methods.
Author contributions
YZ and CYL provided conception and design of study, YZ drafted the manuscript, ZGW provided writing and illustration support, GHL and JX revised the manuscript critically for important intellectual content.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of generative AI in scientific writing
ChatGPT 4 has provided valuable suggestions for improvements in vocabulary, grammar, and sentence structure, aimed at enhancing the linguistic fluency and readability of the paper. Furthermore, we ensure high-quality writing standards in all aspects of the paper through a combination of manual revisions, professional proofreading, and domain knowledge. We place great importance on the scientific accuracy and reliability of the paper. Utilizing ChatGPT 4 for English polishing does not affect our commitment to quality assurance in the scientific content and empirical results of the paper.
Contributor Information
Guanghui Li, Email: liguanghui@tjh.tjmu.edu.cn.
Jun Xiao, Email: jun_xiao@hust.edu.cn.
References
- 1.Collaborators G.B.D.O. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508–e522. doi: 10.1016/S2665-9913(23)00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Duong V., Oo W.M., Ding C., Culvenor A.G., Hunter D.J. Evaluation and treatment of knee pain: a review. JAMA. 2023;330(16):1568–1580. doi: 10.1001/jama.2023.19675. [DOI] [PubMed] [Google Scholar]
- 4.Averbuch M., Katzper M. Assessment of visual analog versus categorical scale for measurement of osteoarthritis pain. J Clin Pharmacol. 2004;44(4):368–372. doi: 10.1177/0091270004263995. [DOI] [PubMed] [Google Scholar]
- 5.van Helvoort E.M., Welsing P.M.J., Jansen M.P., Gielis W.P., Loef M., Kloppenburg M., et al. Neuropathic pain in the IMI-APPROACH knee osteoarthritis cohort: prevalence and phenotyping. RMD Open. 2021;7(3) doi: 10.1136/rmdopen-2021-002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arant K.R., Katz J.N., Neogi T. Quantitative sensory testing: identifying pain characteristics in patients with osteoarthritis. Osteoarthritis Cartilage. 2022;30(1):17–31. doi: 10.1016/j.joca.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piel M.J., Kroin J.S., van Wijnen A.J., Kc R., Im H.J. Pain assessment in animal models of osteoarthritis. Gene. 2014;537(2):184–188. doi: 10.1016/j.gene.2013.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh G., I O.S., Natarajan Anbazhagan A., Ranjan K.C., Farooqui Z., Ma K., et al. Loss of PKCdelta/Prkcd prevents cartilage degeneration in joints but exacerbates hyperalgesia in an experimental osteoarthritis mouse model. Gene. 2024;893 doi: 10.1016/j.gene.2023.147920. [DOI] [PubMed] [Google Scholar]
- 9.Aso K., Walsh D.A., Wada H., Izumi M., Tomitori H., Fujii K., et al. Time course and localization of nerve growth factor expression and sensory nerve growth during progression of knee osteoarthritis in rats. Osteoarthritis Cartilage. 2022;30(10):1344–1355. doi: 10.1016/j.joca.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Zolio L., Lim K.Y., McKenzie J.E., Yan M.K., Estee M., Hussain S.M., et al. Systematic review and meta-analysis of the prevalence of neuropathic-like pain and/or pain sensitization in people with knee and hip osteoarthritis. Osteoarthritis Cartilage. 2021;29(8):1096–1116. doi: 10.1016/j.joca.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira-Gomes J., Garcia M.M., Nascimento D., Almeida L., Quesada E., Castro-Lopes J.M., et al. TLR4 antagonism reduces movement-induced nociception and ATF-3 expression in experimental osteoarthritis. J Pain Res. 2021;14:2615–2627. doi: 10.2147/JPR.S317877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira-Gomes J., Adães S., Sousa R.M., Mendonça M., Castro-Lopes J.M. Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat. Mol Pain. 2012;8:50. doi: 10.1186/1744-8069-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Lopez E., Coras R., Torres A., Lane N.E., Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–275. doi: 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein-Wieringa I.R., de Lange-Brokaar B.J., Yusuf E., Andersen S.N., Kwekkeboom J.C., Kroon H.M., et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol. 2016;43(4):771–778. doi: 10.3899/jrheum.151068. [DOI] [PubMed] [Google Scholar]
- 15.De Roover A., Escribano-Nunez A., Monteagudo S., Lories R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthritis Cartilage. 2023;31(10):1303–1311. doi: 10.1016/j.joca.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Maglaviceanu A., Wu B., Kapoor M. Fibroblast-like synoviocytes: role in synovial fibrosis associated with osteoarthritis. Wound Repair Regen. 2021;29(4):642–649. doi: 10.1111/wrr.12939. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S., Zhu J., Zhen G., Hu Y., An S., Li Y., et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129(3):1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiore N.T., Debs S.R., Hayes J.P., Duffy S.S., Moalem-Taylor G. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol. 2023;19(4):199–220. doi: 10.1038/s41582-023-00777-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H., Lin C., Zeng C., Wang Z., Wang H., Lu J., et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77(10):1524–1534. doi: 10.1136/annrheumdis-2018-213450. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Cai D., Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28(5):555–561. doi: 10.1016/j.joca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Takano S., Uchida K., Inoue G., Miyagi M., Aikawa J., Iwase D., et al. Nerve growth factor regulation and production by macrophages in osteoarthritic synovium. Clin Exp Immunol. 2017;190(2):235–243. doi: 10.1111/cei.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin Gil C., Raoof R., Versteeg S., Willemen H., Lafeber F., Mastbergen S.C., et al. Myostatin and CXCL11 promote nervous tissue macrophages to maintain osteoarthritis pain. Brain Behav Immun. 2023;116:203–215. doi: 10.1016/j.bbi.2023.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Mailhot B., Christin M., Tessandier N., Sotoudeh C., Bretheau F., Turmel R., et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med. 2020;217(9) doi: 10.1084/jem.20191430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran P.B., Miller R.E., Ishihara S., Miller R.J., Malfait A.M. Spinal microglial activation in a murine surgical model of knee osteoarthritis. Osteoarthritis Cartilage. 2017;25(5):718–726. doi: 10.1016/j.joca.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousseau M., Burma N.E., Lee K.Y., Leduc-Pessah H., Kwok C.H.T., Reid A.R., et al. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv. 2018;4(8) doi: 10.1126/sciadv.aas9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muley M.M., Reid A.R., Botz B., Bolcskei K., Helyes Z., McDougall J.J. Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2. Br J Pharmacol. 2016;173(4):766–777. doi: 10.1111/bph.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raoof R., Martin Gil C., Lafeber F., de Visser H., Prado J., Versteeg S., et al. Dorsal root ganglia macrophages maintain osteoarthritis pain. J Neurosci. 2021;41(39):8249–8261. doi: 10.1523/JNEUROSCI.1787-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lange-Brokaar B.J., Kloppenburg M., Andersen S.N., Dorjee A.L., Yusuf E., Herb-van Toorn L., et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage. 2016;24(4):664–671. doi: 10.1016/j.joca.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Habuchi H., Izumi M., Dan J., Ushida T., Ikeuchi M., Takeuchi K., et al. Bone marrow derived mast cells injected into the osteoarthritic knee joints of mice induced by sodium monoiodoacetate enhanced spontaneous pain through activation of PAR2 and action of extracellular ATP. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Cheng J., Zhang Y., Chen J.D.Z., Seralu F.M. Electroacupuncture at ST36 relieves visceral hypersensitivity via the NGF/TrkA/TRPV1 peripheral afferent pathway in a rodent model of post-inflammation rectal hypersensitivity. J Inflamm Res. 2021;14:325–339. doi: 10.2147/JIR.S285146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sousa-Valente J., Calvo L., Vacca V., Simeoli R., Arevalo J.C., Malcangio M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthritis Cartilage. 2018;26(1):84–94. doi: 10.1016/j.joca.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Geraghty T., Obeidat A.M., Ishihara S., Wood M.J., Li J., Lopes E.B.P., et al. Age-associated changes in knee osteoarthritis, pain-related behaviors, and dorsal root ganglia immunophenotyping of male and female mice. Arthritis Rheumatol. 2023;75(10):1770–1780. doi: 10.1002/art.42530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosshirt N., Trauth R., Platzer H., Tripel E., Nees T.A., Lorenz H.M., et al. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res Ther. 2021;23(1):37. doi: 10.1186/s13075-020-02410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosshirt N., Hagmann S., Tripel E., Gotterbarm T., Kirsch J., Zeifang F., et al. A predominant Th1 polarization is present in synovial fluid of end-stage osteoarthritic knee joints: analysis of peripheral blood, synovial fluid and synovial membrane. Clin Exp Immunol. 2019;195(3):395–406. doi: 10.1111/cei.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou K., Yang C., Shi K., Liu Y., Hu D., He X., et al. Activated macrophage membrane-coated nanoparticles relieve osteoarthritis-induced synovitis and joint damage. Biomaterials. 2023;295 doi: 10.1016/j.biomaterials.2023.122036. [DOI] [PubMed] [Google Scholar]
- 36.Sun Z., Liu Q., Lv Z., Li J., Xu X., Sun H., et al. Targeting macrophagic SHP2 for ameliorating osteoarthritis via TLR signaling. Acta Pharm Sin B. 2022;12(7):3073–3084. doi: 10.1016/j.apsb.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z., Xie S., Qian J., Gao F., Jin W., Wang L., et al. Targeting macrophagic PIM-1 alleviates osteoarthritis by inhibiting NLRP3 inflammasome activation via suppressing mitochondrial ROS/Cl(-) efflux signaling pathway. J Transl Med. 2023;21(1):452. doi: 10.1186/s12967-023-04313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook A.D., Christensen A.D., Tewari D., McMahon S.B., Hamilton J.A. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39(3):240–255. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Maximiano T.K.E., Carneiro J.A., Fattori V., Verri W.A. TRPV1: receptor structure, activation, modulation and role in neuro-immune interactions and pain. Cell Calcium. 2024;119 doi: 10.1016/j.ceca.2024.102870. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H., Cui H., Wang T., Shimada S.G., Sun R., Tan Z., et al. CCL2/CCR2 signaling elicits itch- and pain-like behavior in a murine model of allergic contact dermatitis. Brain Behav Immun. 2019;80:464–473. doi: 10.1016/j.bbi.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 41.de Magalhaes S.F., Manzo L.P., de Faria F.M., de Oliveira-Fusaro M.C., Nishijima C.M., Vieira W.F., et al. Inflammatory pain in peripheral tissue depends on the activation of the TNF-alpha type 1 receptor in the primary afferent neuron. Eur J Neurosci. 2021;53(2):376–389. doi: 10.1111/ejn.14985. [DOI] [PubMed] [Google Scholar]
- 42.Gahbauer S., DeLeon C., Braz J.M., Craik V., Kang H.J., Wan X., et al. Docking for EP4R antagonists active against inflammatory pain. Nat Commun. 2023;14(1):8067. doi: 10.1038/s41467-023-43506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai Y., Fujita M., Kawasaki S., Sanaki T., Yoshioka T., Higashino K., et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain. 2019;160(4):895–907. doi: 10.1097/j.pain.0000000000001466. [DOI] [PubMed] [Google Scholar]
- 44.Miyasaka N., Hirata Y. Nitric oxide and inflammatory arthritides. Life Sci. 1997;61(21):2073–2081. doi: 10.1016/s0024-3205(97)00585-7. [DOI] [PubMed] [Google Scholar]
- 45.Labuz D., Celik M.O., Seitz V., Machelska H. Interleukin-4 induces the release of opioid peptides from M1 macrophages in pathological pain. J Neurosci. 2021;41(13):2870–2882. doi: 10.1523/JNEUROSCI.3040-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li T., Xu T., Zhao J., Gao H., Xie W. Depletion of iNOS-positive inflammatory cells decelerates neuronal degeneration and alleviates cerebral ischemic damage by suppressing the inflammatory response. Free Radic Biol Med. 2022;181:209–220. doi: 10.1016/j.freeradbiomed.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Du S., Wu S., Feng X., Wang B., Xia S., Liang L., et al. A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. J Clin Invest. 2022;132(13) doi: 10.1172/JCI153563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L., Liu Y., Hu W., Yang J., Ma S., Tian Z., et al. Peripheral CCL2 induces inflammatory pain via regulation of I(h) currents in small diameter DRG neurons. Front Mol Neurosci. 2023;16 doi: 10.3389/fnmol.2023.1144614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teng H., Chen S., Fan K., Wang Q., Xu B., Chen D., et al. Dexamethasone liposomes alleviate osteoarthritis in miR-204/-211-deficient mice by repolarizing synovial macrophages to M2 phenotypes. Mol Pharm. 2023;20(8):3843–3853. doi: 10.1021/acs.molpharmaceut.2c00979. [DOI] [PubMed] [Google Scholar]
- 50.Atta A.A., Ibrahim W.W., Mohamed A.F., Abdelkader N.F. Microglia polarization in nociplastic pain: mechanisms and perspectives. Inflammopharmacology. 2023;31(3):1053–1067. doi: 10.1007/s10787-023-01216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson D.J., Falconer A.M.D., Wright H.L., Lin H., Yamamoto K., Cheung K., et al. Matrix metalloproteinase-13 is fully activated by neutrophil elastase and inactivates its serpin inhibitor, alpha-1 antitrypsin: implications for osteoarthritis. FEBS J. 2022;289(1):121–139. doi: 10.1111/febs.16127. [DOI] [PubMed] [Google Scholar]
- 52.Wang G., Jing W., Bi Y., Li Y., Ma L., Yang H., et al. Neutrophil elastase induces chondrocyte apoptosis and facilitates the occurrence of osteoarthritis via caspase signaling pathway. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.666162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haraden C.A., Huebner J.L., Hsueh M.F., Li Y.J., Kraus V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. 2019;21(1):146. doi: 10.1186/s13075-019-1923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsueh M.F., Zhang X., Wellman S.S., Bolognesi M.P., Kraus V.B. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol. 2021;73(1):89–99. doi: 10.1002/art.41486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassler S.N., Kume M., Mwirigi J.M., Ahmad A., Shiers S., Wangzhou A., et al. The cellular basis of protease-activated receptor 2-evoked mechanical and affective pain. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.137393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helyes Z., Sandor K., Borbely E., Tekus V., Pinter E., Elekes K., et al. Involvement of transient receptor potential vanilloid 1 receptors in protease-activated receptor-2-induced joint inflammation and nociception. Eur J Pain. 2010;14(4):351–358. doi: 10.1016/j.ejpain.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Parisien M., Lima L.V., Dagostino C., El-Hachem N., Drury G.L., Grant A.V., et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med. 2022;14(644):eabj9954. doi: 10.1126/scitranslmed.abj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo X., Chen O., Wang Z., Bang S., Ji J., Lee S.H., et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109(17):2691–26706 e5. doi: 10.1016/j.neuron.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nees T.A., Rosshirt N., Zhang J.A., Platzer H., Sorbi R., Tripel E., et al. T helper cell infiltration in osteoarthritis-related knee pain and disability. J Clin Med. 2020;9(8):2423. doi: 10.3390/jcm9082423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Na H.S., Park J.S., Cho K.H., Kwon J.Y., Choi J., Jhun J., et al. Interleukin-1-Interleukin-17 signaling Axis induces cartilage destruction and promotes experimental osteoarthritis. Front Immunol. 2020;11:730. doi: 10.3389/fimmu.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun H., Zhang Y., Song W., Yin L., Wang G., Yu D., et al. IgM(+)CD27(+) B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum Immunol. 2019;80(4):263–269. doi: 10.1016/j.humimm.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Raffaeli W., Malafoglia V., Bonci A., Tenti M., Ilari S., Gremigni P., et al. Identification of MOR-positive B cell as possible innovative biomarker (mu lympho-marker) for chronic pain diagnosis in patients with fibromyalgia and osteoarthritis diseases. Int J Mol Sci. 2020;21(4):1499. doi: 10.3390/ijms21041499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun J., Ramnath R.D., Zhi L., Tamizhselvi R., Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294(6):C1586–C1596. doi: 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- 64.Green D.P., Limjunyawong N., Gour N., Pundir P., Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 2019;101(3):412–420 e3. doi: 10.1016/j.neuron.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oggero S., Cecconello C., Silva R., Zeboudj L., Sideris-Lampretsas G., Perretti M., et al. Dorsal root ganglia CX3CR1 expressing monocytes/macrophages contribute to arthritis pain. Brain Behav Immun. 2022;106:289–306. doi: 10.1016/j.bbi.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Z., Sui X., Cao Z., Li X., Qing L., Tang J. Substance P promote macrophage M2 polarization to attenuate secondary lymphedema by regulating NF-kB/NLRP3 signaling pathway. Peptides. 2023;168 doi: 10.1016/j.peptides.2023.171045. [DOI] [PubMed] [Google Scholar]
- 67.Hong H.S., Son Y. Substance P ameliorates collagen II-induced arthritis in mice via suppression of the inflammatory response. Biochem Biophys Res Commun. 2014;453(1):179–184. doi: 10.1016/j.bbrc.2014.09.090. [DOI] [PubMed] [Google Scholar]
- 68.Yuan K., Zheng J., Shen X., Wu Y., Han Y., Jin X., et al. Sensory nerves promote corneal inflammation resolution via CGRP mediated transformation of macrophages to the M2 phenotype through the PI3K/AKT signaling pathway. Int Immunopharm. 2022;102 doi: 10.1016/j.intimp.2021.108426. [DOI] [PubMed] [Google Scholar]
- 69.Aníbal-Silva C.E., Guimarães R.M., Davoli-Ferreira M., Gomes F.I.F., Mendes A., Cavallini M.C.M., et al. Neuron-associated macrophage proliferation in the sensory ganglia is associated with peripheral nerve injury-induced neuropathic pain involving CX3CR1 signaling. Elife. 2023;12 doi: 10.7554/eLife.78515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu X., Liu H., Hamel K.A., Morvan M.G., Yu S., Leff J., et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11(1):264. doi: 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller R.E., Belmadani A., Ishihara S., Tran P.B., Ren D., Miller R.J., et al. Damage‐associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through toll‐like receptor 4. Arthritis Rheumatol. 2015;67(11):2933–2943. doi: 10.1002/art.39291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J., Harada Y., Hayashi Y. A TLR-CXCL1 pathway in DRG neurons induces neutrophil accumulation in the DRG and mechanical allodynia in EAE mice. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-48558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeboudj L., Sideris-Lampretsas G., Silva R., Al-Mudaris S., Picco F., Fox S., et al. Silencing miR-21-5p in sensory neurons reverses neuropathic allodynia via activation of TGF-beta-related pathway in macrophages. J Clin Invest. 2023;133(11) doi: 10.1172/JCI164472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao Z., Zhou Y., Zeng B., Yang X., Su M. MicroRNA-183 attenuates osteoarthritic pain by inhibiting the TGFalpha-mediated CCL2/CCR2 signalling axis. Bone Joint Res. 2021;10(8):548–557. doi: 10.1302/2046-3758.108.BJR-2019-0308.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin Y., Hong J., Pham T.L., Shin J., Gwon D.H., Kwon H.H., et al. Evans blue reduces neuropathic pain behavior by inhibiting spinal ATP release. Int J Mol Sci. 2019;20(18):4443. doi: 10.3390/ijms20184443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller R.E., Tran P.B., Das R., Ghoreishi-Haack N., Ren D., Miller R.J., et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109(50):20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim J.E., Chung E., Son Y. A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNgamma. Sci Rep. 2017;7(1):9417. doi: 10.1038/s41598-017-09639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S.J., Kim J.E., Choe G., Song D.H., Kim S.J., Kim T.H., et al. Self-assembled peptide-substance P hydrogels alleviate inflammation and ameliorate the cartilage regeneration in knee osteoarthritis. Biomater Res. 2023;27(1):40. doi: 10.1186/s40824-023-00387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tao J., Wang X., Xu J. Expression of CGRP in the trigeminal ganglion and its effect on the polarization of macrophages in rats with temporomandibular arthritis. Cell Mol Neurobiol. 2024;44(1):22. doi: 10.1007/s10571-024-01456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nekomoto A., Nakasa T., Ikuta Y., Ding C., Miyaki S., Adachi N. Feasibility of administration of calcitonin gene-related peptide receptor antagonist on attenuation of pain and progression in osteoarthritis. Sci Rep. 2023;13(1) doi: 10.1038/s41598-023-42673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muley M.M., Krustev E., Reid A.R., McDougall J.J. Prophylactic inhibition of neutrophil elastase prevents the development of chronic neuropathic pain in osteoarthritic mice. J Neuroinflammation. 2017;14(1):168. doi: 10.1186/s12974-017-0944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faust H.J., Zhang H., Han J., Wolf M.T., Jeon O.H., Sadtler K., et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest. 2020;130(10):5493–5507. doi: 10.1172/JCI134091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sohn H.S., Choi J.W., Jhun J., Kwon S.P., Jung M., Yong S., et al. Tolerogenic nanoparticles induce type II collagen-specific regulatory T cells and ameliorate osteoarthritis. Sci Adv. 2022;8(47) doi: 10.1126/sciadv.abo5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nwosu L.N., Mapp P.I., Chapman V., Walsh D.A. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis. 2016;75(6):1246–1254. doi: 10.1136/annrheumdis-2014-207203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stevens R.M., Ervin J., Nezzer J., Nieves Y., Guedes K., Burges R., et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019;71(9):1524–1533. doi: 10.1002/art.40894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey K.N., Furman B.D., Zeitlin J., Kimmerling K.A., Wu C.L., Guilak F., et al. Intra-articular depletion of macrophages increases acute synovitis and alters macrophage polarity in the injured mouse knee. Osteoarthritis Cartilage. 2020;28(5):626–638. doi: 10.1016/j.joca.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu C.L., McNeill J., Goon K., Little D., Kimmerling K., Huebner J., et al. Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage fas-induced apoptosis-transgenic mice. Arthritis Rheumatol. 2017;69(9):1772–1783. doi: 10.1002/art.40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ko C.Y., Lin Y.Y., Achudhan D., Chang J.W., Liu S.C., Lai C.Y., et al. Omentin-1 ameliorates the progress of osteoarthritis by promoting IL-4-dependent anti-inflammatory responses and M2 macrophage polarization. Int J Biol Sci. 2023;19(16):5275–5289. doi: 10.7150/ijbs.86701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun Q., Hu T., Zhang Y., Wang X., Liu J., Chen W., et al. IRG1/itaconate increases IL-10 release to alleviate mechanical and thermal hypersensitivity in mice after nerve injury. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1012442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xin W., Pan Y., Wei W., Gerner S.T., Huber S., Juenemann M., et al. TGF-β1 decreases microglia-mediated neuroinflammation and lipid droplet accumulation in an in vitro stroke model. Int J Mol Sci. 2023;24(24) doi: 10.3390/ijms242417329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Celik M.O., Labuz D., Keye J., Glauben R., Machelska H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight. 2020;5(4) doi: 10.1172/jci.insight.133093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dalli J., Serhan C. Macrophage proresolving mediators-the when and where. Microbiol Spectr. 2016;4(3) doi: 10.1128/microbiolspec.MCHD-0001-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16(5):592–597. doi: 10.1038/nm.2123. 1pp. following 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jean-Toussaint R., Lin Z., Tian Y., Gupta R., Pande R., Luo X., et al. Therapeutic and prophylactic effects of macrophage-derived small extracellular vesicles in the attenuation of inflammatory pain. Brain Behav Immun. 2021;94:210–224. doi: 10.1016/j.bbi.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qu Y., Xu Y., Jiang Y., Yu D., Jiang X., Zhao L. Macrophage-derived extracellular vesicles regulates USP5-mediated HDAC2/NRF2 axis to ameliorate inflammatory pain. Faseb J. 2021;35(9) doi: 10.1096/fj.202001185RR. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y., Wang L., Li S., Zhang T., Chen C., Hu J., et al. Mechanical stimulation improves rotator cuff tendon-bone healing via activating IL-4/JAK/STAT signaling pathway mediated macrophage M2 polarization. J Orthop Translat. 2022;37:78–88. doi: 10.1016/j.jot.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3):1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 98.Hu J., Wang H., Li X., Liu Y., Mi Y., Kong H., et al. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. 2020;10(21):9702–9720. doi: 10.7150/thno.44297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fung E., Tang S.-M.T., Canner J.P., Morishige K., Arboleda-Velasquez J.F., Cardoso A.A., et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115(23):2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 100.Geng K., Ma X., Jiang Z., Gu J., Huang W., Wang W., et al. WDR74 facilitates TGF-β/Smad pathway activation to promote M2 macrophage polarization and diabetic foot ulcer wound healing in mice. Cell Biol Toxicol. 2023;39(4):1577–1591. doi: 10.1007/s10565-022-09748-8. [DOI] [PubMed] [Google Scholar]
- 101.Gu C., Chen M., Li X., Geng D., Wang C. MAGL regulates synovial macrophage polarization vis inhibition of mitophagy in osteoarthritic pain. Mol Med Rep. 2023;27(6) doi: 10.3892/mmr.2023.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xue C., Tian J., Cui Z., Liu Y., Sun D., Xiong M., et al. Reactive oxygen species (ROS)-mediated M1 macrophage-dependent nanomedicine remodels inflammatory microenvironment for osteoarthritis recession. Bioact Mater. 2024;33:545–561. doi: 10.1016/j.bioactmat.2023.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee H., Kim H., Seo J., Choi K., Lee Y., Park K., et al. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology. 2020;28(5):1237–1252. doi: 10.1007/s10787-020-00738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee M., Kim G.H., Kim M., Seo J.M., Kim Y.M., Seon M.R., et al. PTX-3 secreted by intra-articular-injected SMUP-cells reduces pain in an osteoarthritis rat model. Cells. 2021;10(9):2420. doi: 10.3390/cells10092420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ebbinghaus M., Jenei-Lanzl Z., Segond von Banchet G., Stangl H., Gajda M., Straub R.H., et al. A promising new approach for the treatment of inflammatory pain: transfer of stem cell-derived tyrosine hydroxylase-positive cells. Neuroimmunomodulation. 2018;25(4):225–237. doi: 10.1159/000495349. [DOI] [PubMed] [Google Scholar]
- 106.Qian Y., Chu G., Zhang L., Wu Z., Wang Q., Guo J.J., et al. M2 macrophage-derived exosomal miR-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting TLR3 and COL10A1 to alleviate osteoarthritis. J Nanobiotechnol. 2024;22(1):72. doi: 10.1186/s12951-024-02336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu R., Zhou Y., Chen H., Xu H., Zuo M., Chen B., et al. Membrane vesicles from Lactobacillus johnsonii delay osteoarthritis progression via modulating macrophage glutamine synthetase/mTORC1 axis. Biomed Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115204. [DOI] [PubMed] [Google Scholar]
- 108.Ban D., Yu H., Xiang Z., Li C., Yu P., Wang J., et al. Cerium oxide nanoparticles alleviate neuropathic pain by modulating macrophage polarization in a rat SCI model. J Pain Res. 2022;15:3369–3380. doi: 10.2147/JPR.S371789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kloppenburg M., Peterfy C., Haugen I.K., Kroon F., Chen S., Wang L., et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1alpha and anti-interleukin-1beta dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann Rheum Dis. 2019;78(3):413–420. doi: 10.1136/annrheumdis-2018-213336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kloppenburg M., Ramonda R., Bobacz K., Kwok W.Y., Elewaut D., Huizinga T.W.J., et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(12):1757–1764. doi: 10.1136/annrheumdis-2018-213202. [DOI] [PubMed] [Google Scholar]
- 111.van Helvoort E.M., de Visser H.M., Lafeber F., Coeleveld K., Versteeg S., Weinans H.H., et al. IL4-10 fusion protein shows DMOAD activity in a rat osteoarthritis model. Cartilage. 2021;13(2_suppl):1155S. doi: 10.1177/19476035211026736. 64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liebmann K., Castillo M.A., Jergova S., Best T.M., Sagen J., Kouroupis D. Modification of mesenchymal stem/stromal cell-derived small extracellular vesicles by calcitonin gene related peptide (CGRP) antagonist: potential implications for inflammation and pain reversal. Cells. 2024;13(6):484. doi: 10.3390/cells13060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao H., Xu J., Wang J., Zhang Y., Zheng N., Yue J., et al. Combination of magnesium ions and vitamin C alleviates synovitis and osteophyte formation in osteoarthritis of mice. Bioact Mater. 2021;6(5):1341–1352. doi: 10.1016/j.bioactmat.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sung K., Ferrari L.F., Yang W., Chung C., Zhao X., Gu Y., et al. Swedish nerve growth factor mutation (NGFR100W) defines a role for TrkA and p75NTR in nociception. J Neurosci. 2018;38(14):3394–3413. doi: 10.1523/JNEUROSCI.1686-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Menges S., Michaelis M., Kleinschmidt-Dorr K. Anti-NGF treatment worsens subchondral bone and cartilage measures while improving symptoms in floor-housed rabbits with osteoarthritis. Front Physiol. 2023;14 doi: 10.3389/fphys.2023.1201328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.I O.S., Kc R., Singh G., Das V., Ma K., Li X., et al. Sensory neuron-specific deletion of tropomyosin receptor kinase A (TrkA) in mice abolishes osteoarthritis (OA) pain via NGF/TrkA intervention of peripheral sensitization. Int J Mol Sci. 2022;23(20) doi: 10.3390/ijms232012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kirkby Shaw K., Rausch-Derra L.C., Rhodes L. Grapiprant: an EP4 prostaglandin receptor antagonist and novel therapy for pain and inflammation. Vet Med Sci. 2016;2(1):3–9. doi: 10.1002/vms3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu X.J., Liu T., Chen G., Wang B., Yu X.L., Yin C., et al. TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep. 2016;6 doi: 10.1038/srep28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Logashina Y.A., Palikova Y.A., Palikov V.A., Kazakov V.A., Smolskaya S.V., Dyachenko I.A., et al. Anti-inflammatory and analgesic effects of TRPV1 polypeptide modulator APHC3 in models of osteo- and rheumatoid arthritis. Mar Drugs. 2021;19(1):39. doi: 10.3390/md19010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lv Z., Xu X., Sun Z., Yang Y.X., Guo H., Li J., et al. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca(2+)/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021;12(6):504. doi: 10.1038/s41419-021-03792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Atukorala I., Kwoh C.K., Guermazi A., Roemer F.W., Boudreau R.M., Hannon M.J., et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390–395. doi: 10.1136/annrheumdis-2014-205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaki S., Smith M.M., Smith S.M., Little C.B. Differential patterns of pathology in and interaction between joint tissues in long-term osteoarthritis with different initiating causes: phenotype matters. Osteoarthritis Cartilage. 2020;28(7):953–965. doi: 10.1016/j.joca.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 123.Zaki S., Smith M.M., Little C.B. Pathology-pain relationships in different osteoarthritis animal model phenotypes: it matters what you measure, when you measure, and how you got there. Osteoarthritis Cartilage. 2021;29(10):1448–1461. doi: 10.1016/j.joca.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 124.Wang X., Chen T., Liang W., Fan T., Zhu Z., Cao P., et al. Synovitis mediates the association between bone marrow lesions and knee pain in osteoarthritis: data from the Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium. Osteoarthritis Cartilage. 2022;30(9):1270–1277. doi: 10.1016/j.joca.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 125.Simkin P.A. Bone pain and pressure in osteoarthritic joints. Novartis Found Symp. 2004;260:179–186. [PubMed] [Google Scholar]
- 126.Yoneda T., Hiasa M., Nagata Y., Okui T., White F.A. Acidic microenvironment and bone pain in cancer-colonized bone. BoneKEy Rep. 2015;4:690. doi: 10.1038/bonekey.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morgan M., Thai J., Nazemian V., Song R., Ivanusic J.J. Changes to the activity and sensitivity of nerves innervating subchondral bone contribute to pain in late-stage osteoarthritis. Pain. 2022;163(2):390–402. doi: 10.1097/j.pain.0000000000002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu Y., Cui J., Liu H., Wang S., Zhou Q., Zhang H., et al. Single-cell RNA-sequencing analysis reveals the molecular mechanism of subchondral bone cell heterogeneity in the development of osteoarthritis. RMD Open. 2022;8(2) doi: 10.1136/rmdopen-2022-002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luo X., Huh Y., Bang S., He Q., Zhang L., Matsuda M., et al. Macrophage toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J Neurosci. 2019;39(35):6848–6864. doi: 10.1523/JNEUROSCI.3257-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sohn R., Jenei-Lanzl Z. Role of the sympathetic nervous system in mild chronic inflammatory diseases: focus on osteoarthritis. Neuroimmunomodulation. 2023;30(1):143–166. doi: 10.1159/000531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen W., Zhang X.-N., Su Y.-S., Wang X.-Y., Li H.-C., Liu Y.-H., et al. Electroacupuncture activated local sympathetic noradrenergic signaling to relieve synovitis and referred pain behaviors in knee osteoarthritis rats. Front Mol Neurosci. 2023;16 doi: 10.3389/fnmol.2023.1069965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rösch G., El Bagdadi K., Muschter D., Taheri S., Dorn C., Meurer A., et al. Sympathectomy aggravates subchondral bone changes during osteoarthritis progression in mice without affecting cartilage degeneration or synovial inflammation. Osteoarthritis Cartilage. 2022;30(3):461–474. doi: 10.1016/j.joca.2021.11.016. [DOI] [PubMed] [Google Scholar]