Abstract
Background/Aim
The overexpression of matrix metalloproteinase-9 (MMP9) has been observed in asthmatic patients, yet the role of MMP9 genotype in determining asthma susceptibility remains unresolved. This study aimed to elucidate the contribution of MMP9 promoter rs3918242 genotype to asthma risk in Taiwan.
Materials and Methods
A cohort comprising 453 non-asthmatic healthy controls and 198 asthmatic cases was assembled, and the MMP9 rs3918242 genotypes were determined using polymerase chain reaction-restriction fragment length polymorphism methodology.
Results
Our findings indicated that people carrying the variant CT or TT genotype of MMP9 rs3918242 did not demonstrate an elevated risk of asthma compared to wild-type CC carriers (odds ratio=1.28 and 1.72, 95% confidence interval=0.87-1.87 and 0.72-4.13; p=0.2417 and 0.3201, respectively). Furthermore, individuals carrying the T allele at MMP9 rs3918242 did not exhibit a higher risk of asthma than those carrying the C allele (odds ratio=1.31, 95% confidence interval=0.96-1.79, p=0.0869). Interestingly, a positive association was observed between MMP9 rs3918242 CT or TT genotypes and the severity of asthma symptoms among asthmatic patients (p=0.0035).
Conclusion
Although the T allele at MMP9 rs3918242 was not associated with asthma risk, it may serve as a predictor for asthma symptom severity. These findings warrant validation in larger and more diverse populations to further elucidate the significance of MMP9 in asthma etiology.
Keywords: Asthma, genotype, MMP9, polymorphism, Taiwanese
Asthma is a chronic respiratory inflammatory disease characterized by airway hyper-responsiveness, reversible airway obstruction, and airway wall remodeling, which involves significant thickening of the reticular basement membrane and deposition of extracellular matrix (ECM) components (1-3). In this context, matrix metalloproteinases (MMPs) have been identified as key players in ECM regulation and metabolism (4,5). MMPs constitute a family of endoproteinases comprising more than 26 members (6,7).
MMP9, also known as 92 kDa type IV collagenase, 92 kDa gelatinase, or gelatinase B, has been shown to play a crucial role in the development and progression of various human malignancies, including lung cancer (8), breast cancer (9,10), osteosarcoma (11), pancreatic cancer (12), hepatocellular carcinoma (13), cervical cancer (14,15), and ovarian cancer (16). The ratio of MMP9 to its natural inhibitor (TIMP1) in bronchoalveolar lavage fluid was also found to be higher in children with symptomatic asthma compared to healthy controls (17-19). Additionally, MMP9 deficiency in mice leads to enhanced allergen-induced airway inflammation and increased numbers of eosinophils and levels of cytokines such as interleukins 4 and 13 (20-22). These accumulated data support the notion that MMP9 plays an important role in asthma pathogenesis.
Among the plethora of genetic variations linked to MMP9, extensive attention has been directed towards the MMP9 rs3918242 polymorphism situated within the promoter region. The MMP9 rs3918242 polymorphic genotypes have undergone thorough scrutiny for their potential correlation with diverse cancer forms, including breast cancer (23), gastric cancer (24), colorectal cancer (25,26), prostate cancer (27), renal cell carcinoma (28), and childhood leukemia (29). Regarding asthma, several reports have examined the significant role of MMP9 rs3918242 polymorphic genotypes (18,30-34).
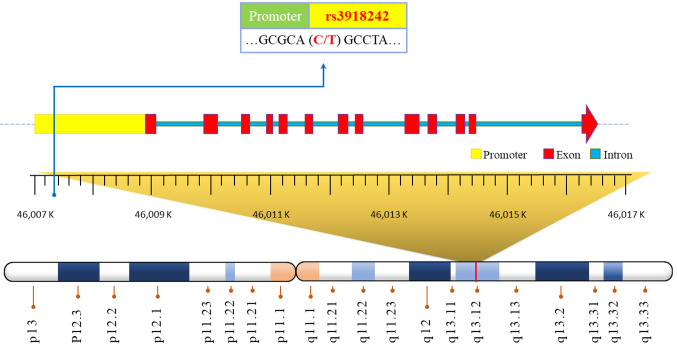
Based on the preceding information, the objective of this study was to assess the correlation between MMP9 rs3918242 (depicted in Figure 1) and the susceptibility to asthma in Taiwan. Additionally, we also endeavor to provide an updated summary and discuss the highlighted findings and their limitations.
Figure 1. The physical map for the matrix metalloproteinase-9 rs3918242 polymorphic site.
Materials and Methods
Enrollments of asthmatic cases and non-asthmatic controls. The research cohort consisted of 198 individuals diagnosed with asthma who were meticulously selected from China Medical University Hospital. The diagnosis of asthma was established based on the following criteria: (i) experiencing more than two or three episodes of wheezing and shortness of breath within the previous year; (ii) a confirmed diagnosis of asthma by pulmonologists, accompanied by spirometry evidence of reversible and variable airflow obstruction; (iii) the presence of relevant symptoms; and (iv) the prescription of asthma medications. Participants were all adults, with the youngest being 25 years old, and there were no restrictions based on sex. Furthermore, a control cohort comprising 453 non-asthmatic individuals, meticulously matched for sex and age (within ±5 years), was included, following the methodologies outlined in our previous publications (35,36). Our study was approved and supervised by the Research Ethics Committee of China Medical University Hospital (CMUH106-REC1-004). To ensure the precision of evaluation, each asthmatic participant underwent thorough validation of the severity of their symptoms by a panel consisting of a minimum of two experienced pulmonary physicians, overseen by Dr. Hsia. This validation procedure adhered to the guidelines stipulated by the Global Initiative for Asthma (37). The clinical characteristics displayed by the asthmatic patients facilitated their classification into four distinct severity stages, meticulously defined, recorded, and closely monitored throughout the study duration.
DNA extraction and storage. DNA extraction was performed on buffy coats obtained from peripheral blood samples of all participants within 24 h of collection using QIAamp Blood Mini Kit (38-40). The extracted DNA samples were stored at −80˚C for long-term preservation. Furthermore, DNA samples from both asthmatic patients and non-asthmatic controls were diluted, aliquoted, and prepared as a working stock collection, following previously established protocols (41,42). The working stock DNA samples from asthmatic patients and non-asthmatic controls in 96-well plates were stored at −20˚C until further analysis.
Processes for the determination of MMP9 rs3918242 genotype. The MMP9 rs3918242 genotype was determined following the methodology outlined in our previous publication (24,43). Briefly, amplification of MMP9 rs3918242 utilized the appropriate forward and reverse primer sequences. Then the DNA adducts were digested with Sph I (New England Biolabs, Taipei, Taiwan) before undergoing 3% DNA gel electrophoresis. The DNA fragments for MMP9 rs3918242 CC, CT, and TT genotypes were determined to be 386 bp, 386+320+66 bp, and 320+66 bp, respectively.
Methodology of statistical analysis. The age distributions in the asthmatic and non-asthmatic groups were compared using unpaired Student’s t-test. Genotype comparisons among subgroups were conducted using Pearson’s chi-square test with Yates’ correction. The assessment of associations between MMP9 genotype and asthma risk involved the utilization of odds ratios (ORs) alongside their corresponding 95% confidence intervals (CIs) for each genotyping comparison. Statistical significance was identified as any statistical p-value less than 0.05.
Results
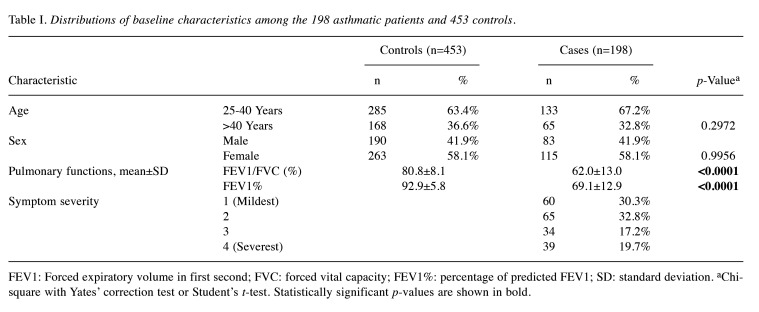
Analysis of demographic characteristics between the asthmatic and non-asthmatic groups. Table I provides a comprehensive summary of age, sex, and specific clinical attributes, including pulmonary function and symptom severity, for both the 198 asthmatics and the 453 non-asthmatic subjects. Due to the careful matching of age and sex between the two cohorts no statistically significant differences were observed regarding age and sex distributions (p=0.2972 and 0.9956, respectively). Regarding pulmonary functions, notable disparities were observed. Within the asthmatic group, both the mean ratio of forced expiratory volume in the first second to forced vital capacity (FEV1/FVC, %) and the percentage of predicted FEV1 (FEV1%) were observed to be lower when compared to the non-asthmatic group (both p<0.0001). Regarding symptom severity, among asthmatic patients, the distribution was as follows: 30.3% for stage 1, 32.8% for stage 2, 17.2% for stage 3, and 19.7% for stage 4 (Table I).
Table I. Distributions of baseline characteristics among the 198 asthmatic patients and 453 controls.
FEV1: Forced expiratory volume in first second; FVC: forced vital capacity; FEV1%: percentage of predicted FEV1; SD: standard deviation. aChi-square with Yates’ correction test or Student’s t-test. Statistically significant p-values are shown in bold.
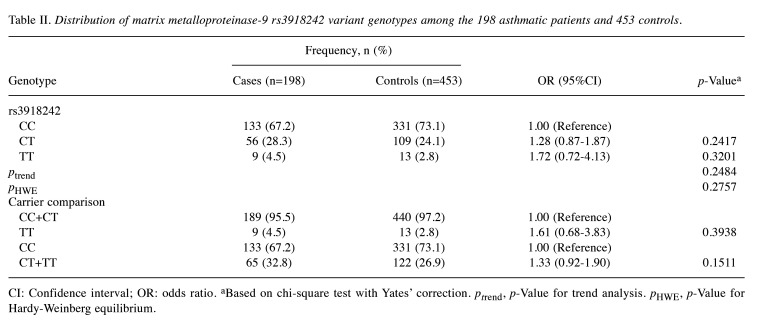
Association of MMP9 rs3918242 genotypes with asthma risk in Taiwanese. Table II presents the genotypic distributions of MMP9 rs3918242 among the 453 non-asthmatic controls and the 198 asthmatic patients. Firstly, the genotypic frequencies of MMP9 rs3918242 among non-asthmatic controls were in accordance with the Hardy-Weinberg equilibrium (p=0.2757). Secondly, the genotypic frequencies of MMP9 rs3918242 did not display any statistically significant distinction between the asthmatic case and non-asthmatic groups (p for trend=0.2484). Specifically, neither the heterozygous CT nor the homozygous variant TT genotypes of MMP9 rs3918242 were associated with asthma susceptibility (OR=1.28 and 1.72, 95% CI=0.87-1.87 and 0.72-4.13, p=0.2417 and 0.3201, respectively). Thirdly, in a recessive model contrasting individuals bearing the TT genotype with those bearing the CC or CT genotype, the OR for asthma was 1.61 (95% CI=0.68-3.83, p=0.3938). Lastly, in a dominant model comparing individuals carrying the CT or TT genotype with those carrying the CC genotype, there was a non-significantly increased asthma risk in the former (OR=1.33, 95% CI=0.92-1.90; p=0.1511, Table II).
Table II. Distribution of matrix metalloproteinase-9 rs3918242 variant genotypes among the 198 asthmatic patients and 453 controls.
CI: Confidence interval; OR: odds ratio. aBased on chi-square test with Yates’ correction. ptrend, p-Value for trend analysis. pHWE, p-Value for Hardy-Weinberg equilibrium.
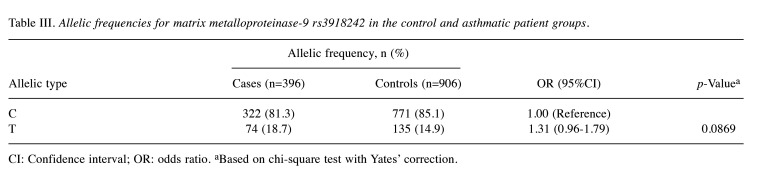
Association of MMP9 rs3918242 allelic frequencies with asthma risk in Taiwanese. To further corroborate the findings in Table II, an analysis for allelic frequency was conducted to assess the involvement of MMP9 rs3918242 in asthma risk in Taiwanese, as illustrated in Table III. The data indicated that the presence of variant T alleles at MMP9 rs3918242 did not significantly differ between the asthmatic case and non-asthmatic control groups (p=0.0869). Individuals harboring the variant T allele at MMP9 rs3918242 demonstrated a 1.31-fold odds ratio (95% CI=0.96-1.79) for asthma susceptibility compared to those carrying the wild-type C allele (Table III). Thus, the findings from the allelic frequency analysis suggest that the MMP9 rs3918242 variant CT or TT genotype may not be a major determinant for elevated risk of asthma in Taiwanese.
Table III. Allelic frequencies for matrix metalloproteinase-9 rs3918242 in the control and asthmatic patient groups.
CI: Confidence interval; OR: odds ratio. aBased on chi-square test with Yates’ correction.
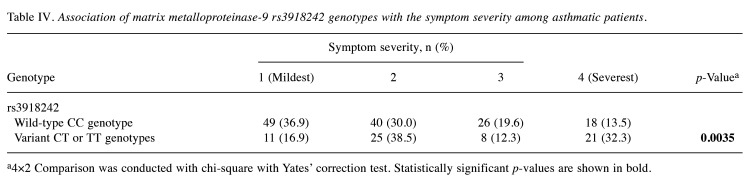
Stratified analysis of MMP9 rs3918242 genotypes according to severity of asthmatic symptoms. While MMP9 rs3918242 genotypes may not serve as practical predictors for asthma risk, we are intrigued by the intricate interplay between MMP9 genotypes and the severity of asthmatic symptoms within the clinical context. To explore these further, asthmatic patients were stratified based on their MMP9 rs3918242 genotypes and corresponding symptom severity profiles. The results of this analysis are detailed in Table IV. We combined the variant genotypes (CT and TT) due to their minor proportions compared to the wild-type CC genotype at MMP9 rs3918242. The results presented in Table IV emphasize that individuals carrying variant genotypes (CT or TT) at MMP9 rs3918242 were more prone to experiencing severe symptomatology compared to their wild-type (CC) counterparts (p=0.0035) (Table IV). Upon detailed examination, the distribution of wild-type carriers across the first (mildest), second, third, and fourth (most severe) stages among asthmatic patients was 36.9%, 30.0%, 19.6%, and 13.5%, respectively. Remarkably, a notable discrepancy was observed: the percentage of wild-type CC carriers was significantly higher among patients in the mildest stage (36.9% versus 16.9%), while conversely it was lower among those in the most severe stage (13.5% versus 32.3%) (p=0.0035). Conversely, the opposite pattern was evident for carriers of the variant genotypes CT or TT at MMP9 rs3918242, with distributions diverging in an opposite direction (Table IV).
Table IV. Association of matrix metalloproteinase-9 rs3918242 genotypes with the symptom severity among asthmatic patients.
a4×2 Comparison was conducted with chi-square with Yates’ correction test. Statistically significant p-values are shown in bold.
Discussion
Chronic asthma is characterized by abnormal remodeling and altered airway function, partly attributed to changes in ECM deposition, potentially influenced by MMP9 imbalance (44,45). Elevated levels of MMP9 in airways or alveolar macrophages may indicate chronic airway inflammation in patients with asthma (46). Studies have reported associations between a higher MMP9 level and reduced lung function (47), as well as an elevated serum MMP9 level in children with asthma compared to healthy controls (48). The current study aimed to investigate the association between MMP9 rs3918242 genotypes and asthma in a Taiwanese population consisting of individuals distributed across different stages of asthma and in non-asthmatic controls (Table I). The results indicate that CC, CT, or TT genotypes at MMP9 rs3918242 are not determinants of asthma susceptibility (Table II and Table III). However, variant CT or TT genotypes at MMP9 rs3918242 may predict severe asthma severity (Table IV). Although previous research has shown that the MMP9 rs3918242 genotype can lead to increased MMP9 expression (48,49), we did not provide direct evidence of this in this study.
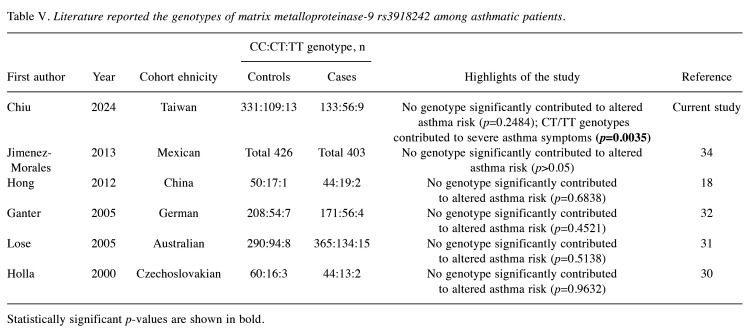
In 2000, Holla and colleagues conducted the first investigation into the association of MMP9 rs3918242 polymorphism with asthma risk in a Czechoslovakian population, but found no significant association (30). In 2005, Lose and colleagues similarly found no association between MMP9 rs3918242 and asthma or asthma severity in an Australian population (31). In the same year, Ganter and colleagues reported another negative association among German children (32). In 2006, Nakashima and colleagues reported similar results but revealed a significant association between MMP9 rs2274755, which was in complete linkage disequilibrium with MMP9 rs3918242, and childhood asthma risk (33). In 2010, Pinto and colleagues found that another MMP9 polymorphism, rs2664538 at exon 6, significantly increased the risk of non-atopic asthma (50). In 2012, Hong and colleagues examined the contribution of MMP9 rs3918242 genotype to childhood asthma and found no association in a small Chinese pediatric population (18). In 2013, Jimenez-Morales and colleagues investigated the role of other polymorphisms at MMP9 in childhood asthma in a Mexican population. They proposed a haplotype of rs2274755, rs17577, and rs3918249 of MMP9 as being associated with asthma risk, but found no association with MMP9 rs3918242 genotype (34). We have summarized the authors, ethnicities, sample sizes, and key findings of all literature focused on MMP9 rs3918242 and asthma risk for a comprehensive understanding of the role of MMP9 in asthma (Table V).
Table V. Literature reported the genotypes of matrix metalloproteinase-9 rs3918242 among asthmatic patients.
Statistically significant p-values are shown in bold.
The genotypic distribution for MMP9 rs3918242 in our control group adheres well to the Hardy-Weinberg equilibrium (p=0.2757). Information obtained from the results of 3,120 East Asian samples indicates that C and T alleles constitute 86.54% and 13.43%, respectively. Among the non-asthmatic controls in our study, C and T alleles were represented at frequencies of 85.10% and 14.90%, respectively. This concordance suggests that our cohort’s genotyping accurately represents the entire Taiwanese population, with allelic frequencies aligning closely with those of East Asians (51). The current study reveals that the MMP9 rs3918242 polymorphism was not associated with asthma risk in this Taiwanese population. Additionally, we found no significant associations among subgroups stratified by age or sex (data not shown). Several plausible explanations exist for this lack of functional association. Firstly, elevated MMP9 levels are observed not only in asthma but also in other inflammatory diseases, such as acute respiratory tract diseases and chronic obstructive pulmonary disease (52). Thus, elevated MMP9 levels may be attributed to other factors rather than the MMP9 rs3918242 polymorphism. Secondly, the T allele at MMP9 rs3918242 may have minor effects but is closely linked to other potentially functional polymorphisms within MMP9 or other genes involved in the inflammatory response (53), which may play more fundamental roles in asthma.
An important finding of this current study is the correlation detected between MMP9 rs3918242 genotypes and the severity of symptoms among the asthmatic patient cohort (Table IV). Particularly noteworthy is the trend suggesting that individuals with the variant CT or TT genotypes of MMP9 rs3918242 may be more prone to experiencing heightened symptom severity compared to those with the wild-type CC genotype (Table IV). Our findings may underscore the potential of the MMP9 rs3918242 T allele as a practical predictor of severe asthma symptoms in Taiwanese. This finding contrasts with the report by Lose and colleagues, which indicated no association between MMP9 rs3918242 genotype and asthma severity in an Australian population (31). This discrepancy might be attributed to differences in investigated populations, sampling criteria, sample sizes, and definitions of symptom severity.
In summary, our study provides evidence indicating that the T allele of the MMP9 promoter rs3918242 polymorphism may have a minor role in predicting adult asthma susceptibility in Taiwanese. Conversely, our results indicate that the MMP9 rs3918242 T allele has an exacerbating effect on the severity of symptoms among asthmatic patients, potentially serving as a predictor for asthma symptom severity. Overall, these preliminary findings warrant validation with larger sample sizes and/or diverse ethnicities. Further studies are urgently needed to deepen our understanding of the involvement of MMP9 genotype/phenotype in asthma etiology.
Conflicts of Interest
The Authors declare no conflicts of interest regarding this study.
Authors’ Contributions
Research design: Chiu KL, Chen GL, Bau DT, and Hsia TC; patient and questionnaire summaries: Chiu KL, Chen GL, Shen TC, Chen LH and Hsia TC; experimental work: He JL, Chang WS and Tsai CW; data clearance and identification: He JL, Chen JC and Hsia TC; statistical analysis: Chen JC, Chang WS and Tsai CW; article writing: Chiu KL, Hsia TC and Bau DT; review and revision: Tsai CW, Chang WS, Hsia TC and Bau DT.
Acknowledgements
The Authors are grateful to Yun-Chi Wang, Yu-Ting Chin, and Hou-Yu Shih for their excellent technical assistance. The contribution of all the participants in this study is highly appreciated. This study was supported by China Medical University and Hospital (DMR-111-036), Taichung Tzu Chi Hospital (grant number: TTCRD113-14), Taichung Armed Forces General Hospital (TCAFGH-D-111016), Asia and China Medical University (grant number: CMU112-ASIA-01 and ASIA-112-CMUH-17). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the article.
References
- 1.Wijsman PC, Goorsenberg AWM, Keijzer N, d’Hooghe JNS, Ten Hacken NHT, Shah PL, Weersink EJM, de Brito JM, de Souza Xavier Costa N, Mauad T, Nawijn MC, Vonk JM, Annema JT, Burgess JK, Bonta PI. Airway wall extracellular matrix changes induced by bronchial thermoplasty in severe asthma. J Allergy Clin Immunol. 2024;153(2):435–446.e4. doi: 10.1016/j.jaci.2023.09.035. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Cooley MA, Nair PM, Donovan C, Hsu AC, Jarnicki AG, Haw TJ, Hansbro NG, Ge Q, Brown AC, Tay H, Foster PS, Wark PA, Horvat JC, Bourke JE, Grainge CL, Argraves WS, Oliver BG, Knight DA, Burgess JK, Hansbro PM. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c. J Pathol. 2017;243(4):510–523. doi: 10.1002/path.4979. [DOI] [PubMed] [Google Scholar]
- 3.Rogers NK, Clements D, Dongre A, Harrison TW, Shaw D, Johnson SR. Extra-cellular matrix proteins induce matrix metalloproteinase-1 (MMP-1) activity and increase airway smooth muscle contraction in asthma. PLoS One. 2014;9(2):e90565. doi: 10.1371/journal.pone.0090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurya S, Prasad D, Mukherjee S. Matrix metalloproteinases in oral cancer pathogenesis and their use in therapy. Anticancer Agents Med Chem. 2024;24(1):3–17. doi: 10.2174/0118715206270002231108071917. [DOI] [PubMed] [Google Scholar]
- 5.Mustafa S, Koran S, AlOmair L. Insights into the role of matrix metalloproteinases in cancer and its various therapeutic aspects: a review. Front Mol Biosci. 2022;9:896099. doi: 10.3389/fmolb.2022.896099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei C. The multifaceted roles of matrix metalloproteinases in lung cancer. Front Oncol. 2023;13:1195426. doi: 10.3389/fonc.2023.1195426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almutairi S, Kalloush HM, Manoon NA, Bardaweel SK. Matrix metalloproteinases inhibitors in cancer treatment: an updated review (2013-2023) Molecules. 2023;28(14):5567. doi: 10.3390/molecules28145567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco-Prieto S, Barcia-Castro L, Páez de la Cadena M, Rodríguez-Berrocal FJ, Vázquez-Iglesias L, Botana-Rial MI, Fernández-Villar A, De Chiara L. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer. 2017;17(1):823. doi: 10.1186/s12885-017-3842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousef EM, Tahir MR, St-Pierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer. 2014;14:609. doi: 10.1186/1471-2407-14-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Qiu Z, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol Lett. 2017;14(5):5865–5870. doi: 10.3892/ol.2017.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Shi Q, Yuan TX, Song QL, Zhang Y, Wei Q, Zhou L, Luo J, Zuo G, Tang M, He TC, Weng Y. Matrix metalloproteinase 9 (MMP-9) in osteosarcoma: Review and meta-analysis. Clin Chim Acta. 2014;433:225–231. doi: 10.1016/j.cca.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Tian M, Cui YZ, Song GH, Zong MJ, Zhou XY, Chen Y, Han JX. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:241. doi: 10.1186/1471-2407-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh HC, Lin SM, Chen MF, Pan TL, Wang PW, Yeh CT. Evaluation of serum matrix metalloproteinase (MMP)-9 to MMP-2 ratio as a biomarker in hepatocellular carcinoma. Hepatogastroenterology. 2010;57(97):98–102. [PubMed] [Google Scholar]
- 14.Li Y, Wu T, Zhang B, Yao Y, Yin G. Matrix metalloproteinase-9 is a prognostic marker for patients with cervical cancer. Med Oncol. 2012;29(5):3394–3399. doi: 10.1007/s12032-012-0283-z. [DOI] [PubMed] [Google Scholar]
- 15.Zajkowska M, Zbucka-Krętowska M, Sidorkiewicz I, Lubowicka E, Będkowska GE, Gacuta E, Szmitkowski M, Ławicki S. Human plasma levels of vascular endothelial growth factor, matrix metalloproteinase 9, and tissue inhibitor of matrix metalloproteinase 1 and their applicability as tumor markers in diagnoses of cervical cancer based on ROC analysis. Cancer Control. 2018;25(1):1073274818789357. doi: 10.1177/1073274818789357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiner AT, Tan S, Agreiter C, Auer K, Bachmayr-Heyda A, Aust S, Pecha N, Mandorfer M, Pils D, Brisson AR, Zeillinger R, Lim SK. EV-associated MMP9 in high-grade serous ovarian cancer is preferentially localized to annexin V-binding EVs. Dis Markers. 2017;2017:9653194. doi: 10.1155/2017/9653194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlewyn-Lajeunesse MD, Hunt LP, Pohunek P, Dobson SJ, Kochhar P, Warner JA, Warner JO. Bronchoalveolar lavage MMP-9 and TIMP-1 in preschool wheezers and their relationship to persistent wheeze. Pediatr Res. 2008;64(2):194–199. doi: 10.1203/PDR.0b013e318175dd2d. [DOI] [PubMed] [Google Scholar]
- 18.Hong Z, Lin YM, Qin X, Peng JL. Serum MMP-9 is elevated in children with asthma. Mol Med Rep. 2011;5(2):462–4. doi: 10.3892/mmr.2011.656. [DOI] [PubMed] [Google Scholar]
- 19.Grzela K, Zagórska W, Krejner A, Litwiniuk M, Zawadzka-Krajewska A, Kulus M, Grzela T. Polymorphic variants 279R and 668Q augment activity of matrix metalloproteinase-9 in breath condensates of children with asthma. Arch Immunol Ther Exp. 2017;65(2):183–187. doi: 10.1007/s00005-016-0412-z. [DOI] [PubMed] [Google Scholar]
- 20.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol. 2004;172(4):2586–2594. doi: 10.4049/jimmunol.172.4.2586. [DOI] [PubMed] [Google Scholar]
- 21.Vermaelen KY, Cataldo D, Tournoy K, Maes T, Dhulst A, Louis R, Foidart J, Noël A, Pauwels R. Matrix metalloproteinase-9-mediated dendritic cell recruitment into the airways is a critical step in a mouse model of asthma. J Immunol. 2003;171(2):1016–1022. doi: 10.4049/jimmunol.171.2.1016. [DOI] [PubMed] [Google Scholar]
- 22.Cataldo DD, Tournoy KG, Vermaelen K, Munaut C, Foidart JM, Louis R, Noël A, Pauwels RA. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am J Pathol. 2002;161(2):491–498. doi: 10.1016/S0002-9440(10)64205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felizi RT, Veiga MG, Carelli Filho I, Souto RPD, Fernandes CE, Oliveira E. Association between matrix metallopeptidase 9 polymorphism and breast cancer risk. Rev Bras Ginecol Obstet. 2018;40(10):620–624. doi: 10.1055/s-0038-1673366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu CK, Chang WS, Tsai CW, Wang YC, Yang MD, Hsu HS, Chao CY, Yu CC, Chen JC, Pei JS, Bau DT. The association of MMP9 promoter Rs3918242 genotype with gastric cancer. Anticancer Res. 2021;41(7):3309–3315. doi: 10.21873/anticanres.15118. [DOI] [PubMed] [Google Scholar]
- 25.Hoelzle CR, Magalhães KC, Carvalho SS, Santos GA, Maia IM, Sousa MC, Andrade-Filho JS, Cruz GM, Simões RT. Matrix metalloproteinase 9 -1562C/T polymorphism increased protein levels in patients with colorectal cancer in a sample from southeastern Brazil. Genet Mol Res. 2016;15(1):gmr7478. doi: 10.4238/gmr.15017478. [DOI] [PubMed] [Google Scholar]
- 26.Wu MH, Tzeng HE, Wu CN, Yueh TC, Peng YC, Tsai CH, Wang YC, Ke TW, Pei JS, Chang WS, Tsai CW, Bau DT. Association of matrix metalloproteinase-9 rs3918242 promoter genotypes with colorectal cancer risk. Anticancer Res. 2019;39(12):6523–6529. doi: 10.21873/anticanres.13867. [DOI] [PubMed] [Google Scholar]
- 27.Schveigert D, Valuckas KP, Kovalcis V, Ulys A, Chvatovic G, Didziapetriene J. Significance of MMP-9 expression and MMP-9 polymorphism in prostate cancer. Tumori. 2013;99(4):523–529. doi: 10.1177/030089161309900414. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, An T, Zhou H, Wan Z, Huang Z, Chong T. MMP9 and TYROBP affect the survival of circulating tumor cells in clear cell renal cell carcinoma by adapting to tumor immune microenvironment. Sci Rep. 2023;13(1):6982. doi: 10.1038/s41598-023-34317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo CC, Tsai CW, Chang WS, Shen TC, Tzeng HE, Li CH, Wang YC, Tsai FJ, Bau DT. Contribution of matrix metalloproteinase-9 rs3918242 genotypes to childhood leukemia risk. Anticancer Res. 2020;40(10):5751–5756. doi: 10.21873/anticanres.14591. [DOI] [PubMed] [Google Scholar]
- 30.Hollá LI, Vašků A, Stejskalová A, Znojil V. Functional polymorphism in the gelatinase B gene and asthma. Allergy. 2000;55(9):900–901. doi: 10.1034/j.1398-9995.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 31.Lose F, Thompson PJ, Duffy D, Stewart GA, Kedda MA. A novel tissue inhibitor of metalloproteinase-1 (TIMP-1) polymorphism associated with asthma in Australian women. Thorax. 2005;60(8):623–628. doi: 10.1136/thx.2004.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganter K, Deichmann KA, Heinzmann A. Association study of polymorphisms within matrix metalloproteinase 9 with bronchial asthma. Int J Immunogenet. 2005;32(4):233–236. doi: 10.1111/j.1744-313X.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima K, Hirota T, Obara K, Shimizu M, Doi S, Fujita K, Shirakawa T, Enomoto T, Yoshihara S, Ebisawa M, Matsumoto K, Saito H, Suzuki Y, Nakamura Y, Tamari M. A functional polymorphism in MMP-9 is associated with childhood atopic asthma. Biochem Biophys Res Commun. 2006;344(1):300–307. doi: 10.1016/j.bbrc.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 34.Jiménez-Morales S, Martínez-Aguilar N, Gamboa-Becerra R, Jiménez-Ruíz JL, López-Ley D, Lou H, Saldaña-Alvarez Y, Dean M, Orozco L. Polymorphisms in metalloproteinase-9 are associated with the risk for asthma in Mexican pediatric patients. Hum Immunol. 2013;74(8):998–1002. doi: 10.1016/j.humimm.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 35.Chen LH, Li CH, Wang SC, Chiu KL, Wu MF, Yang JS, Tsai CW, Chang WS, Hsia TC, Bau DT. Association of matrix metalloproteinase-1 promoter polymorphisms with asthma risk. In Vivo. 2024;38(1):365–371. doi: 10.21873/invivo.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu KL, Chang WS, Tsai CW, Mong MC, Hsia TC, Bau DT. Novel genetic variants in long non-coding RNA MEG3 are associated with the risk of asthma. PeerJ. 2023;11:e14760. doi: 10.7717/peerj.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2023. Available at: https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf. [Last accessed on April 22, 2024]
- 38.Chiu KL, Wang SC, Li CH, Shen TC, Chen LH, Shen YC, Chang WS, Tsai CW, Hsia TC, Bau DT. The contribution of double-strand break repair radiation sensitive protein 51 genotypes to lung cancer in Taiwan. Anticancer Res. 2024;44(4):1409–1416. doi: 10.21873/anticanres.16937. [DOI] [PubMed] [Google Scholar]
- 39.Chen CC, Chang WS, Pei JS, Kuo CC, Wang CH, Wang YC, Hsu PC, He JL, Gu J, Bau DT, Tsai CW. Non-homologous end-joining genotype, mRNA expression, and DNA repair capacity in childhood acute lymphocytic leukemia. Cancer Genomics Proteomics. 2024;21(2):144–157. doi: 10.21873/cgp.20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang MD, Lin KC, Lu MC, Jeng LB, Hsiao CL, Yueh TC, Fu CK, Li HT, Yen ST, Lin CW, Wu CW, Pang SY, Bau DT, Tsai FJ. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine (Taipei) 2017;7(2):10. doi: 10.1051/bmdcn/2017070203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu PS, Hsia NY, Wang ZH, Chen HC, Hsia TC, Lin ML, Wang YC, Chang WS, Bau DT, Tsai CW. Contribution of matrix metalloproteinase-2 genotypes to Taiwan pterygium risk. In Vivo. 2024;38(2):539–545. doi: 10.21873/invivo.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu CK, Mong MC, Tzeng HE, Yang MD, Chen JC, Hsia TC, Hsia NY, Tsai CW, Chang WS, Chen CP, Bau DT. The significant contribution of interleukin-16 genotypes, smoking, alcohol drinking, and helicobacter pylori infection to gastric cancer. In Vivo. 2024;38(1):90–97. doi: 10.21873/invivo.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao CH, Tsai CL, Chang SY, Lin YH, Wang YC, Huang WC, Mong MC, Yang YC, Wu WT, Chen JC, Tsai CW, Bau DT, Chang WS. Impacts of matrix metalloproteinase 9 genotypes on renal cell carcinoma. In Vivo. 2023;37(6):2452–2458. doi: 10.21873/invivo.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang LF, Du LZ, Zou CC, Gu WZ. Levels of matrix metalloproteinase-9 and its inhibitor in guinea pig asthma model following ovalbumin challenge. Fetal Pediatr Pathol. 2005;24(2):81–87. doi: 10.1080/15227950591008277. [DOI] [PubMed] [Google Scholar]
- 45.Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005;4(2):177–181. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- 46.Chung FT, Huang HY, Lo CY, Huang YC, Lin CW, He CC, He JR, Sheng TF, Wang CH. Increased ratio of matrix metalloproteinase-9 (MMP-9)/tissue inhibitor metalloproteinase-1 from alveolar macrophages in chronic asthma with a fast decline in FEV(1) at 5-year follow-up. J Clin Med. 2019;8(9):1451. doi: 10.3390/jcm8091451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naik SP, P A M, B S J, Madhunapantula SV, Jahromi SR, Yadav MK. Evaluation of inflammatory markers interleukin-6 (IL-6) and matrix metalloproteinase-9 (MMP-9) in asthma. J Asthma. 2017;54(6):584–593. doi: 10.1080/02770903.2016.1244828. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney AM. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999;99(14):1788–1794. doi: 10.1161/01.cir.99.14.1788. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Fang S, Wei L, Wang R, Jin X, Wen D, Li Y, Guo W, Wang N, Zhang J. No association between the C-1562T polymorphism in the promoter of matrix metalloproteinase-9 gene and non-small cell lung carcinoma. Lung Cancer. 2005;49(2):155–161. doi: 10.1016/j.lungcan.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Pinto LA, Depner M, Klopp N, Illig T, Vogelberg C, von Mutius E, Kabesch M. MMP-9 gene variants increase the risk for non-atopic asthma in children. Respir Res. 2010;11(1):23. doi: 10.1186/1465-9921-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reference SNP (rs) Report. rs3918242. Available at: https://www.ncbi.nlm.nih.gov/snp/rs3918242. [Last accessed on April 22, 2024]
- 52.Brajer B, Batura-Gabryel H, Nowicka A, Kuznar-Kaminska B, Szczepanik A. Concentration of matrix metalloproteinase-9 in serum of patients with chronic obstructive pulmonary disease and a degree of airway obstruction and disease progression. J Physiol Pharmacol. 2008;59 Suppl 6:145–152. [PubMed] [Google Scholar]
- 53.Slezakova S, Borilova Linhartova P, Bartova J, Petanova J, Kuklinek P, Fassmann A, Dusek L, Izakovicova Holla L. Gene variability in matrix metalloproteinases in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2020;49(3):271–277. doi: 10.1111/jop.12993. [DOI] [PubMed] [Google Scholar]