Abstract
Background/Aim
Angiotensinogen (AGT), a precursor of angiotensin II (AngII), contributes to regulating (patho)physiological conditions, including blood pressure changes, inflammation, and kidney fibrosis. However, the precise role of tissue-specific AGT in kidney fibrosis independent of blood pressure remains to be fully understood. This study investigated the source of intrarenal AGT and its role in kidney injury and fibrosis during obstructive nephropathy.
Materials and Methods
Proximal tubule- (PT, major source secreting AGT in the kidney; PKO) or liver- (major source of circulating AGT; LKO) AGT knockout (KO) mice were subjected to unilateral ureteral obstruction (UUO), a blood pressure-independent fibrosis model.
Results
UUO increased AGT mRNA and protein levels in the kidneys. PKO decreased AGT mRNA, but LKO enhanced it in UUO kidneys compared with the control. In contrast, the intrarenal protein levels of AGT increased in PKO, but not in LKO in UUO kidneys, indicating that the liver is a major source of intrarenal AGT protein. Expression of megalin, a PT receptor involved in the uptake of circulating AGT, was down-regulated in UUO kidneys and was independent of PKO or LKO. However, none of these changes prevented UUO-induced tubular injury and kidney fibrosis.
Conclusion
Hepatic and proximal tubule AGT play distinct roles in contributing to intrarenal AGT levels during UUO, and their genetic inhibitions fail to prevent kidney injury and fibrosis, suggesting a highly complicated signaling pathway of the renin-angiotensin system and an associated compensatory mechanism in obstructive nephropathy.
Keywords: Obstructive nephropathy, ureteral obstruction, kidney fibrosis, kidney injury, angiotensinogen
Chronic kidney disease (CKD) is a life-threatening disease with no effective treatable option except renal replacement therapy, such as kidney transplant and dialysis (1-3). CKD accompanied by fibrosis progression is a common feature along with tubular injury and inflammation, which causes chronic loss of kidney function (4-6). Several drugs targeting the renin-angiotensin system (RAS), such as angiotensin (Ang)-converting enzyme inhibitor (ACEi) and Ang II type 1 receptor inhibitor (ARB), are clinically used not only for controlling blood pressure, but also for halting CKD progression. However, this approach may, at best, reduce proteinuria, a surrogate marker of renal disease, but it only partially reduces progression of CKD and can have harmful side effects (7-9). Considering the incomplete efficacy of RAS blockade, it is necessary to find new drugs that could either exert a complementary action to ACEi and ARB or better define the cellular and molecular mechanism by which RAS signaling exerts the pathophysiological processes of CKD progression.
Angiotensinogen (AGT) is a precursor of AngII, a well-established molecule driving diverse signaling pathways, including tissue fibrosis. A growing body of evidence reveals that both systemically and locally generated AGTs contribute to intrarenal AGT levels and disease progression, primarily through blood pressure (BP) effects (10-12). We and others have reported that liver-derived AGT is a major source of circulating AGT and plays a major role in BP regulation, whereas proximal tubule (PT)-derived AGT acts locally in the kidney with minor or no contribution to the levels of circulating AGT and the regulation of BP (13-15). However, despite considerable efforts, the mechanism by which intrarenal AGT contributes to CKD pathogenesis, particularly in a BP-independent manner, remains undefined.
The present study aimed to investigate the precise role of liver- and PT-derived AGT in obstructive nephropathy, a BP-independent kidney fibrosis model.
Materials and Methods
Animal and surgical procedure. Mice were cared for according to the principles and guidelines of the Institutional Animal Care and Use Committee (IACUC), University of Nebraska Medical Center (UNMC), and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All protocols were approved by the UNMC-IACUC prior to the experiments (#14-023). Angiotensinogen (AGT) floxed mice (Jackson Lab, Bar Harbor, ME) were crossed with Pepck Cre mice, a kind gift from Dr. Volker Haase at Vanderbilt University (16), or Albumin Cre mice (Jackson Lab, Bar Harbor, ME, USA) to create PT- and liver-specific knockouts of AGT, respectively. A cocktail containing ketamine (200 mg/kg body weight) and xylazine (16 mg/kg body weight) was intraperitoneally administered to anesthetize the AGT knockouts and their controls. As described previously (17-19), to generate unilateral ureteral obstruction, the right ureter near the renal pelvis was ligated completely using a 5-0 silk. The same surgical procedure except for the ureter ligation was performed to generate sham controls. Seven days post-surgery, the mice were sacrificed, and kidney samples were collected for analysis as described previously (17).
Genomic PCR and electrophoresis. Tail biopsies were collected to determine the genotype of mice using electrophoresis, as previously described (20).
Collagen deposition. Sirius red staining was used for evaluating collagen deposition, as previously described (21). In five randomly chosen fields per kidney, Sirius red-positive areas were calculated by a ratio of Sirius red-positive area to total area. ImageJ software (NIH, Bethesda, MD, USA) was used to isolate Sirius red-positive areas and quantify their area.
Histology and evaluation of tubular injury. Tubular injury was calculated using PAS-stained sections (20). Tubular atrophy and dilatation were evaluated as injury markers in five randomly chosen fields per kidney. The ratio of the number of atrophied or dilated tubules to the total number of tubules was calculated for analysis.
Immunohistochemistry. Paraffin-embedded kidney sections were used for immunohistochemistry to detect AGT (22). Briefly, the kidney sections were rehydrated, and incubated with antibodies against AGT (IBL, Minneapolis, MN, USA) overnight at 4˚C. Meyer’s hematoxylin (Electron Microscopy Sciences, Hatfield, PA, USA) was used for counterstaining. The representative images were chosen from five randomly selected fields per kidney. ImageJ software (NIH) was used for evaluating AGT-positive areas.
Western blot analysis. Western blot analysis was performed as we recently described (22). Antibodies were used for evaluating kidney fibrosis-related factors, including α-SMA (Sigma, St. Louis, MO, USA) and p-Smad3 (Abcam, Cambridge, MA, USA). For loading control, GAPDH immunoblotting was used on stripped membranes. ImageJ software (NIH, Bethesda, MD, USA) was used for analyzing band intensities.
RNA extraction. Total RNA from kidneys was isolated using RNeasy mini kit (Qiagen, Hilden, Germany) as we recently described (22). Complementary DNA (cDNA) was synthesized from 1 μg total RNA using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA).
Quantitative reverse transcription-PCR. Quantitative real-time PCR was performed with 1 μl cDNA using SYBR Premix Ex Taq (Applied Biosystems, Foster City, CA, USA) and specific primers (Table I) as we have previously described (22). mRNA level as a relative expression (R) to an internal control gene (GAPDH) was calculated using the 2–ΔΔCt method.
Table I. Primer sequences used for quantitative PCR.
Statistical analyses. Data are presented as the mean±SD. Differences were assessed by 2-tailed unpaired Student’s t-test between two groups and by ordinary one-way analysis of variance followed by Bonferroni analysis for multiple group comparison (GraphPrism 10 software). p-Values less than 0.05 were considered statistically significant.
Results
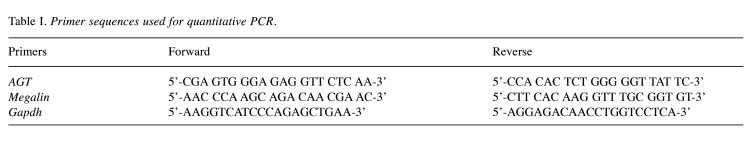
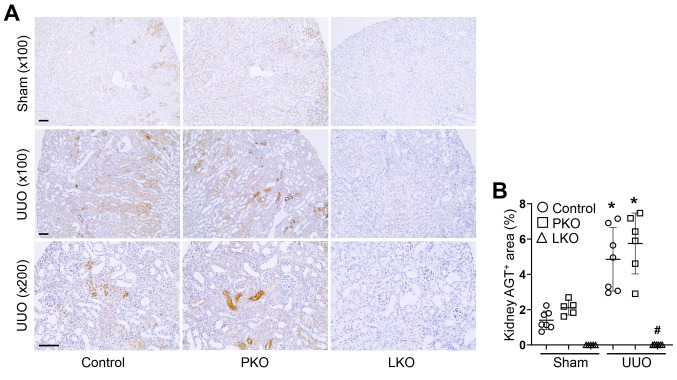
Distinct role of PT- and liver-derived AGT in kidney expression levels of AGT and megalin. We generated tissue-specific AGT KO in PT (PKO) or liver (LKO) by crossing AGT floxed mice with Pepck- or albumin-cre recombinase and the genotypes were confirmed using RT-PCR (data not shown). To determine how PT- or liver-derived AGT contributes to AGT mRNA and protein levels in sham and UUO kidneys, we carried out immunohistochemistry and RT-PCR for AGT in the kidneys. UUO increased both AGT mRNA and protein levels (Figure 1 and Figure 2A and B). PKO, not LKO, suppressed AGT mRNA in sham kidneys, compared with those of WT control (Figure 2A and B). In UUO kidneys, PKO inhibited UUO-induced up-regulation of AGT mRNA. Intriguingly, LKO enhanced the UUO-induced increase in AGT mRNA (Figure 2A and B). These data indicate that PT is the main source of AGT mRNA in sham and UUO kidneys. The level of AGT protein was completely absent in LKO kidneys, but increased in PKO (Figure 1A and B), suggesting that liver-derived circulating AGT primarily contributes to kidney AGT protein level in UUO.
Figure 1. Expression of angiotensinogen (AGT) in unilateral ureteral obstruction (UUO) kidneys of WT, proximal tubule-specific AGT KO (PKO) and liver-specific AGT KO (LKO) mice. WT control, PKO, and LKO mice were subjected to UUO or sham operation for seven days. (A) Paraffin-embedded kidney sections were used to carry out immunohistochemistry for evaluating AGT expression. Brown color indicates AGT localization. (B) Expression levels were evaluated using Image J software (n=5-7). Scale bar, 50 μm. Data are expressed as means±SD. *p<0.05 vs. WT-Sham; #p<0.05 vs. WT-UUO.
Figure 2. Changes in angiotensinogen (AGT) and megalin mRNA expression in unilateral ureteral obstruction (UUO) kidneys of WT, proximal tubule-specific AGT KO (PKO), and liver-specific AGT KO (LKO) mice. WT control, PKO, and LKO mice were subjected to UUO or sham operation for seven days. (A-B) Kidney AGT mRNA levels were measured using quantitative real time RT-PCR. The levels are expressed as a fold increase to Sham (n=5-9). (C-D) Levels of megalin mRNA were measured using quantitative real time RT-PCR. The levels are expressed as a fold increase to Sham in WT control (n=5-7). Data are expressed as means±SD. *p<0.05 vs. WT-Sham; #p<0.05 vs. WT-UUO.
Next, we examined the expression level of megalin, a major receptor involved in the absorption of circulating AGT, since it can affect the intrarenal protein levels of AGT through PT absorption (23,24). The expression of megalin was similar among sham kidneys of WT, PKO, and LKO mice (Figure 2C and D). However, UUO significantly down-regulated megalin expression in the kidneys, with no significant difference observed among the groups (Figure 2C and D). These data demonstrate that liver-derived AGT plays a major role in the intrarenal expression of AGT protein, in both sham and UUO mice.
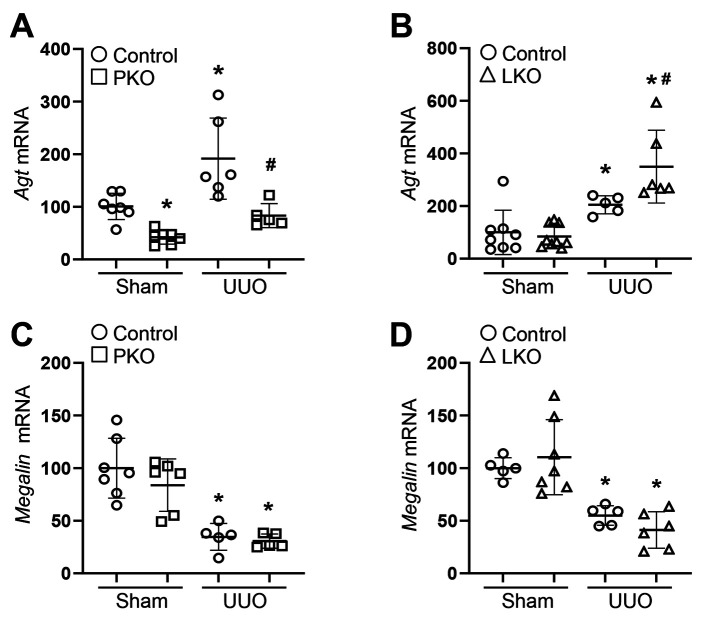
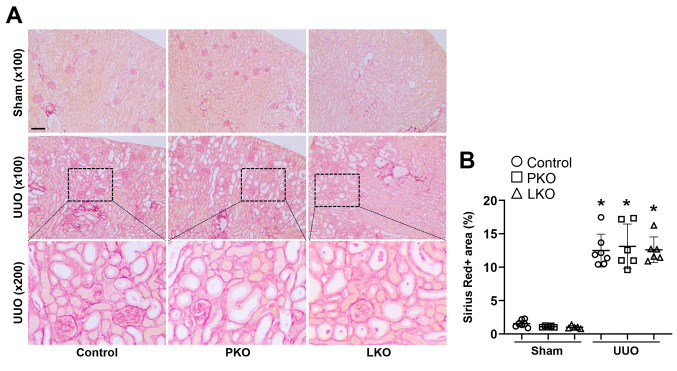
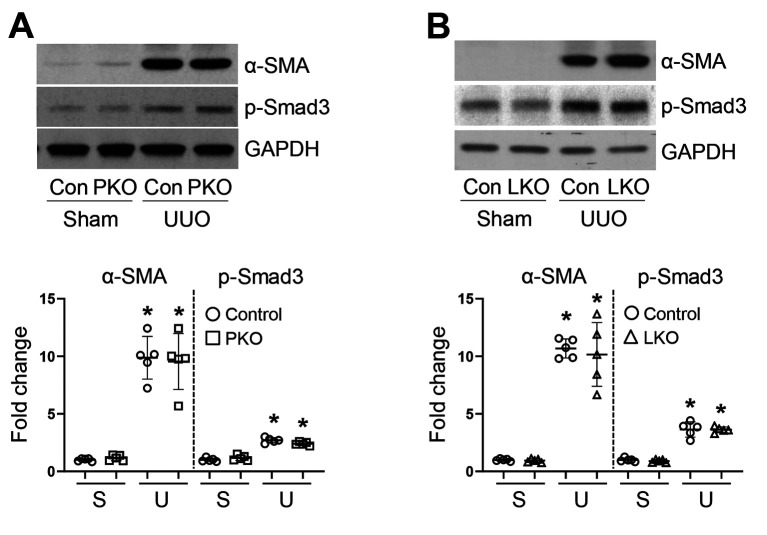
Tubular injury and tubulointerstitial fibrosis in PT- and liver-specific AGT KO mice during UUO. To evaluate whether tissue-derived AGTs affect obstructive nephropathy, we confirmed tubular injury and tubulointerstitial fibrosis in UUO kidneys. UUO resulted in a significant increase in kidney injury, tubular atrophy, and dilation (Figure 3). In parallel, kidney fibrosis was markedly up-regulated in the kidneys of WT UUO mice (Figure 4). This was accompanied by an increase in fibroblast activation, as indicated by elevated expression levels of alpha-SMA, a marker of myofibroblasts, and p-Smad3, a well-established pro-fibrogenic molecule (Figure 4 and Figure 5). The UUO-induced tubular injury and fibrosis progression was not changed in the kidneys of PKO and LKO mice, compared with those of WT control (Figure 4 and Figure 5). These findings suggest that in the absence of PT- or liver-derived AGT, the obstructed kidney could develop tubular injury and fibrosis through complementary mechanisms or other source(s) of AGT and components of the RAS such as Ang II.
Figure 3. Effect of proximal tubule-specific angiotensinogen (AGT) KO (PKO) and liver-specific AGT KO (LKO) in unilateral ureteral obstruction (UUO)-induced kidney tubular injury and fibrosis. WT control, PKO, and LKO mice were subjected to UUO or sham operation for seven days. (A) Paraffin-embedded kidney sections were used for PAS staining (n=6-7). (B) Ratios of atrophied and dilated tubule to total tubules were evaluated as described in the Methods section. Insets indicate atrophied tubule. Scale bar, 50 μm. Data are expressed as means±SD. *p<0.05 vs. WT-Sham.
Figure 4. Effect of proximal tubule-specific angiotensinogen (AGT) KO (PKO) and liver-specific AGT KO (LKO) in unilateral ureteral obstruction (UUO)-induced kidney fibrosis. WT control, PKO, and LKO mice were subjected to UUO or sham operation for seven days. (A) Paraffin-embedded kidney section was used to carry out Sirius Red stain (n=6-7). (B) Sirius Red-positive collagen deposition was quantified from randomly chosen five fields per kidney. Scale bar, 50 μm. Data are expressed as means±SD. *p<0.05 vs. WT-Sham.
Figure 5. Expression of α-SMA and p-Smad3 in unilateral ureteral obstruction (UUO) kidneys of WT, proximal tubule-specific angiotensinogen (AGT) KO (PKO), and liver-specific AGT KO (LKO) mice. (A-C) Levels of α-SMA and p-Smad3 were examined by western blot analysis using specific antibodies. Representative western blots are shown (n=5). Anti-GAPDH antibody was used as a loading control. Expression levels were evaluated using Image J software. S: Sham; Con: control. Data are expressed as means±SD. *p<0.05 vs. WT-Sham.
Discussion
In the present study, we showed the distinct role of liver- and PT-derived AGT in intrarenal AGT expression during obstructive nephropathy. PT-specific AGT increased intrarenal AGT mRNA expression during UUO and that was enhanced when liver-derived AGT is blocked. However, intrarenal AGT protein, which was derived from liver, was not compensated by inhibition of PT-derived AGT during UUO. Interestingly, in PT-specific AGT-KO, intrarenal AGT protein level was increased due to megalin-mediated uptake of circulating liver-derived AGT during UUO. Nevertheless, UUO-induced tubular injury and fibrosis progression was comparable among WT, PT-, and liver-AGT KO, suggesting a compensatory mechanism by systemic AGT or RAS components from other sources. These findings suggest that during obstructive nephropathy, intrarenal levels of AGT and AngII can be compensated by diverse sources, other than the liver and kidney. This compensation contributes to kidney injury and fibrosis progression.
RAS components, including AGT and Ang II, serve as regulators in a number of cellular and molecular signaling pathways as well as in BP regulation. Our research and others have demonstrated that blockage of AGT or Ang II can halt CKD progression and reduce BP in diverse clinical and experimental models (12,20,25-28). Systemic inhibition of AGT or Ang II type 1 receptor modestly suppresses the pathogenesis of CKD, but also has unwanted side effects, such as hypotension-related issues and fatigue, or limited efficacy in the treatment of CKD (7-9), suggesting the need for elucidating the precise mechanism of intrarenal RAS. Recently, significant efforts have been committed to define the respective role of systemic and local AGT in kidney diseases, but our understanding remains incomplete due to their complexity (29). Studies have demonstrated that liver- or adipocyte-derived AGT affects BP regulation, whereas kidney-derived AGT does not (14,30,31). However, over-expression of AGT or Ang II in the kidneys is likely to overcome its limited effect on BP control (13,32), suggesting a potential renal mechanism involving AGT/AngII that can affect BP regulation.
However, BP-independent mechanisms of intrarenal AGT have rarely been studied in kidney injury and fibrosis. In the present study, we sought to delineate the precise role of tissue-specific AGT in obstructive nephropathy using genetic deletion of AGT in the PT or liver, which are major sites generating systemic or intrarenal AGT in mice. The mouse models with tissue-specific AGT deletion were useful in defining the role of AGT during UUO, a BP-independent established model of kidney tubular injury and fibrosis. We demonstrated that PT-derived AGT is required for maintaining intrarenal levels of AGT mRNA under normal conditions and that its expression increases in the kidney during UUO. In contrast, liver-derived AGT is necessary for maintaining the intrarenal levels of AGT protein in both normal and UUO kidneys. In addition, when liver-derived AGT was absent during UUO, it was compensated by PT-derived AGT, as evidenced by the increased intrarenal AGT mRNA levels. However, unlike in BP-dependent CKD models (20), these changes did not prevent kidney injury and fibrosis, which may be associated to the dynamics of renal AGT observed in UUO kidneys. Further studies are required to define other potential sources of AGT and AngII, such as interstitial fibroblast-like cells, glomerular cells, or adipocytes (31,33-37), and to elucidate their compensatory roles and detailed mechanisms in kidney injury and fibrosis during obstructive nephropathy (32,38-41).
Conclusion
Collectively, our findings demonstrate the roles of liver- or PT-derived AGT and its downstream signaling in the progression of kidney injury and fibrosis during obstructive nephropathy. This highlights the complexity of the signaling pathways involved and the compensatory mechanisms associated with them. Understanding these mechanisms is crucial for developing effective therapeutics for treating kidney injury and fibrosis in cases of UUO.
Conflicts of Interest
All the Authors declare no competing interests in relation to this study.
Authors’ Contributions
H.S.J. and B.J.P. conception and design of research; H.S.J., M.R.N., L.H., and J.K. performed experiments; H.S.J. and B.J.P analyzed data; H.S.J. and B.J.P. interpreted the results of the experiments; H.S.J. prepared the figures; H.S.J. drafted the manuscript; H.S.J. and B.J.P. edited and revised the manuscript. All Authors approved the final version of the manuscript.
Acknowledgements
The Authors thank Dr. Volker H. Haase (Vanderbilt University) for providing the transgenic mouse with the Pepck-Cre recombinase.
Funding
This study is supported by NIH grant DK-120846 (BJP) and AHA postdoctoral fellowship Grant 15POST25130003 (HSJ).
References
- 1.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20(3):259–272. doi: 10.1007/s10741-014-9460-9. [DOI] [PubMed] [Google Scholar]
- 4.Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, Wanner C, Anders HJ. Chronic kidney disease. Nat Rev Dis Primers. 2017;3(1):17088. doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 5.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 6.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016;15(8):568–588. doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha AD, Agarwal R. Clinical pharmacology of antihypertensive therapy for the treatment of hypertension in CKD. Clin J Am Soc Nephrol. 2019;14(5):757–764. doi: 10.2215/CJN.04330418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT Jr, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55(3):441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol. 2017;28(4):1040–1049. doi: 10.1681/ASN.2016070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol. 2012;2(4):2733–2752. doi: 10.1002/cphy.c120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramkumar N, Kohan DE. Proximal tubule angiotensinogen modulation of arterial pressure. Curr Opin Nephrol Hypertens. 2013;22(1):32–36. doi: 10.1097/MNH.0b013e328359dbed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23(7):1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navar LG. Translational studies on augmentation of intratubular renin-angiotensin system in hypertension. Kidney Int Suppl (2011) 2013;3(4):321–325. doi: 10.1038/kisup.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66(5):2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang HS, Noh MR, Ha L, Kim J, Padanilam BJ. Proximal tubule cyclophilin D mediates kidney fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2021;321(4):F431–F442. doi: 10.1152/ajprenal.00171.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noh MR, Jang HS, Salem FE, Ferrer FA, Kim J, Padanilam BJ. Epoxyeicosatrienoic acid administration or soluble epoxide hydrolase inhibition attenuates renal fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2023;324(2):F138–F151. doi: 10.1152/ajprenal.00052.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang HS, Kim JI, Noh M, Rhee MH, Park KM. Regulator of G protein signaling 2 (RGS2) deficiency accelerates the progression of kidney fibrosis. Biochim Biophys Acta. 2014;1842(9):1733–1741. doi: 10.1016/j.bbadis.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Jang HS, Noh MR, Plumb T, Lee K, He JC, Ferrer FA, Padanilam BJ. Hepatic and proximal tubule angiotensinogen play distinct roles in kidney dysfunction, glomerular and tubular injury, and fibrosis progression. Am J Physiol Renal Physiol. 2022;323(4):F435–F446. doi: 10.1152/ajprenal.00029.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang HS, Padanilam BJ. Simultaneous deletion of Bax and Bak is required to prevent apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;309(6):F540–F550. doi: 10.1152/ajprenal.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HS, Noh MR, Jung EM, Kim WY, Southekal S, Guda C, Foster KW, Oupicky D, Ferrer FA, Padanilam BJ. Proximal tubule cyclophilin D regulates fatty acid oxidation in cisplatin-induced acute kidney injury. Kidney Int. 2020;97(2):327–339. doi: 10.1016/j.kint.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285(53):41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3(4):258–267. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 25.Olearczyk J, Gao S, Eybye M, Yendluri S, Andrews L, Bartz S, Cully D, Tadin-Strapps M. Targeting of hepatic angiotensinogen using chemically modified siRNAs results in significant and sustained blood pressure lowering in a rat model of hypertension. Hypertens Res. 2014;37(5):405–412. doi: 10.1038/hr.2013.155. [DOI] [PubMed] [Google Scholar]
- 26.Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, Hoorn EJ, Foster D, Danser AJ. Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension. 2019;73(6):1249–1257. doi: 10.1161/HYPERTENSIONAHA.119.12703. [DOI] [PubMed] [Google Scholar]
- 27.Bovée DM, Ren L, Uijl E, Clahsen-van Groningen MC, Van Veghel R, Garrelds IM, Domenig O, Poglitsch M, Zlatev I, Kim JB, Huang S, Melton L, Lu X, Hoorn EJ, Foster D, Danser AJ. Renoprotective effects of small interfering RNA targeting liver angiotensinogen in experimental chronic kidney disease. Hypertension. 2021;77(5):1600–1612. doi: 10.1161/HYPERTENSIONAHA.120.16876. [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Navar LG, Satou R, Gonzalez-Villalobos RA. The increasing complexity of the intratubular renin-angiotensin system. J Am Soc Nephrol. 2012;23(7):1130–1132. doi: 10.1681/ASN.2012050493. [DOI] [PubMed] [Google Scholar]
- 30.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60(6):1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302(2):R244–R251. doi: 10.1152/ajpregu.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo JL, Kobori H, Li XC, Satou R, Katsurada A, Navar LG. Augmentation of angiotensinogen expression in the proximal tubule by intracellular angiotensin II via AT1a/MAPK/NF-кB signaling pathways. Am J Physiol Renal Physiol. 2016;310(10):F1103–F1112. doi: 10.1152/ajprenal.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada H, Inoue T, Kanno Y, Kobayashi T, Watanabe Y, Kopp JB, Carey RM, Suzuki H. Interstitial fibroblast-like cells express renin-angiotensin system components in a fibrosing murine kidney. Am J Pathol. 2002;160(3):765–772. doi: 10.1016/S0002-9440(10)64898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species—ERK/JNK pathways. Hypertens Res. 2010;33(11):1174–1181. doi: 10.1038/hr.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki M, Fukusumi Y, Kayaba M, Kitazawa Y, Takamura S, Narita I, Kawachi H. Possible role for glomerular-derived angiotensinogen in nephrotic syndrome. J Renin Angiotensin Aldosterone Syst. 2016;17(4):1470320316681223. doi: 10.1177/1470320316681223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. Am J Physiol Endocrinol Metab. 2007;292(5):E1280–E1287. doi: 10.1152/ajpendo.00277.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Soltani-Bejnood M, Quignard-Boulange A, Massiera F, Teboul M, Ailhaud G, Kim JH, Moustaid-Moussa N, Voy BH. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J Biomed Biotechnol. 2006;2006(5):27012. doi: 10.1155/JBB/2006/27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z, Li W, Han J, Zou C, Huang W, Yu W, Shan X, Lum H, Li X, Liang G. Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2) Sci Rep. 2017;7:44911. doi: 10.1038/srep44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majid DSA, Mahaffey E, Castillo A, Prieto MC, Navar LG. Angiotensin II-induced renal angiotensinogen formation is enhanced in mice lacking tumor necrosis factor-alpha type 1 receptor. Physiol Rep. 2021;9(16):e14990. doi: 10.14814/phy2.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang HS, Kim JI, Kim J, Park JW, Park KM. Angiotensin II removes kidney resistance conferred by ischemic preconditioning. Biomed Res Int. 2014;2014:602149. doi: 10.1155/2014/602149. [DOI] [PMC free article] [PubMed] [Google Scholar]