Abstract
Background/Aim
In current literature, there is a notable lack of studies investigating the role of radiation-sensitive protein 51 (RAD-51) in pterygium diagnosis. Nevertheless, reports indicate elevated expression levels of RAD-51 among recurrent pterygium cases compared to those with primary pterygium. However, the genomic involvement of RAD-51 has yet to be explored in any population. This study aimed to assess the contribution of RAD-51 genotypes to pterygium risk in a representative Taiwanese population.
Materials and Methods
RAD-51 rs1801320 genotyping was successfully conducted in a Taiwanese cohort comprising 140 pterygium cases and 280 non-pterygium controls using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technology.
Results
The distribution of RAD-51 rs1801320 genotypes (GG, CG, and CC) in the pterygium group (70.0%, 25.7%, and 4.3%, respectively) did not significantly differ from that in the non-pterygium group (73.6%, 23.6%, and 2.8% for GG, CG, and CC genotypes, respectively; p for trend=0.6337). Carriers of the variant CG and CC RAD-51 rs1801320 genotypes exhibited 1.15- and 1.58-fold increased pterygium risk, respectively (95%CI=0.72-1.84 and 0.53-4.67, p=0.6552 and p=0.5914, respectively). In the dominant model, there appeared to be a slight association between variant genotypes CG and CC and pterygium risk (OR=1.19, 95%CI=0.76-1.87, p=0.0223). Allelic analysis revealed that the RAD-51 rs1801320 variant C allele was not significantly linked to pterygium risk (17.1% versus 14.6%, OR=1.20, 95%CI=0.82-1.78, p=0.3991).
Conclusion
Variant genotypes at RAD-51 rs1801320 were firstly identified to associate with susceptibility to pterygium among Taiwanese individuals. Nonetheless, these findings warrant validation in larger and more diverse populations.
Keywords: Association, genotype, polymorphism, pterygium, RAD-51
Pterygium presents as a common ocular surface ailment characterized by irregular epithelial and fibrovascular proliferation, infiltration, and restructuring of the extracellular matrix (ECM) (1,2). This fibrovascular proliferation, leading to an abnormal wing-shaped growth, bears resemblance to the excessive growth observed in neoplastic formations (3). The migration of these abnormal, wedge-shaped tissues from the bulbar conjunctiva to the cornea also shares certain characteristics of tumorigenesis seen in solid cancers (4). The multifaceted nature of pterygium, influenced by various factors including heat, dust, atmospheric particles, immunological cytokines, rearrangement of the extracellular matrix, UV radiation, and growth factors, contributes to its complex etiology (5-13). Additionally, several studies have indicated the significant role of genetic variations in predisposing individuals to pterygium (14-17). However, a practical and readily accessible marker for pterygium remains notably elusive.
From a molecular standpoint, cellular DNA repair pathways become activated to uphold genetic stability and integrity upon exposure to various endogenous or exogenous DNA-damaging agents. Failure to rectify these lesions may culminate in genomic instability and the gradual accrual of mutations, which constitutes a hallmark of cancer (18,19). Among the diverse types of DNA damage, DNA double-strand breaks (DSBs) pose a particular menace to cells. Two primary repair pathways, namely non-homologous end joining (NHEJ) and homologous recombination (HR), undertake the task of mending DSBs (20,21). Generally, NHEJ represents an error-prone repair mechanism that juxtaposes the broken ends, while HR executes a precise and error-free repair process (22,23).
From a molecular perspective, the DSB repair protein RAD-51 homolog 1, encoded by RAD-51 and situated at chromosome locus 15q15.1, assumes a critical role in preserving genetic stability and integrity under the assault of various DNA-damaging agents (24). This genomic region, notable for its propensity for loss of heterozygosity in malignancies, such as breast, colorectal, and lung cancers, holds significance in elucidating cancer progression. RAD-51, comprising 339 amino acids in humans, is indispensable for HR during the repair of DSBs (25-27). Overexpression of RAD-51 has been documented across a spectrum of cancers, encompassing (28-33), pancreatic (34,35), head and neck (36), prostate (37), soft tissue sarcoma (38) and esophageal cancer (39). Remarkably, RAD-51 overexpression has been detected in non-small cell lung cancer, with renal cell carcinoma representing the sole exception, demonstrating under-expression (40,41). In genomic investigations, a widely scrutinized polymorphism is the G to C polymorphism in RAD-51’s promoter region, denoted as rs1801320 (G-135C) (42). Literature underscores the association of RAD-51 rs1801320 genotypes with susceptibility to diverse cancers, including breast (43-49), laryngeal (50), colorectal (51,52), prostate (42), ovarian (53-55), cervical (56), endometrial (57,58), and glioblastoma malignancies (59).
Regarding pterygium, there is a lack of literature exploring genetic variations of RAD-51 in relation to this condition. Building upon this gap, the present study endeavors to examine the potential association between RAD-51 rs1801320 genotypes, a single-nucleotide polymorphic (SNP) site, and the susceptibility to pterygium within a representative Taiwanese cohort comprising 140 pterygium cases and 280 non-pterygium controls.
Materials and Methods
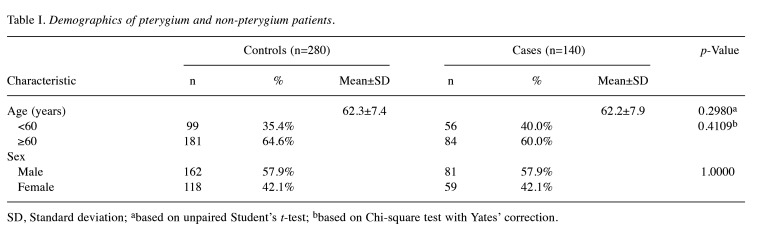
Recruitment of pterygium and non-pterygium population. The research concepts, association hypotheses, and experimental protocols employed in this study have been sanctioned by the Institutional Review Board of Changhua Christian Hospital (number: 151225). Furthermore, written informed consent has been procured from either one or both parents of each participant. A cohort comprising 140 individuals diagnosed with pterygium, alongside a double size of non-pterygium control subjects, was enrolled for the study. Each participant willingly completed a questionnaire and furnished peripheral blood samples for genotyping. Non-pterygium control subjects were chosen based on the absence of pterygium, endometriosis, myoma, or any malignancy. Demographic characteristics of all participants are delineated in Table I.
Table I. Demographics of pterygium and non-pterygium patients.
SD, Standard deviation; abased on unpaired Student’s t-test; bbased on Chi-square test with Yates’ correction.
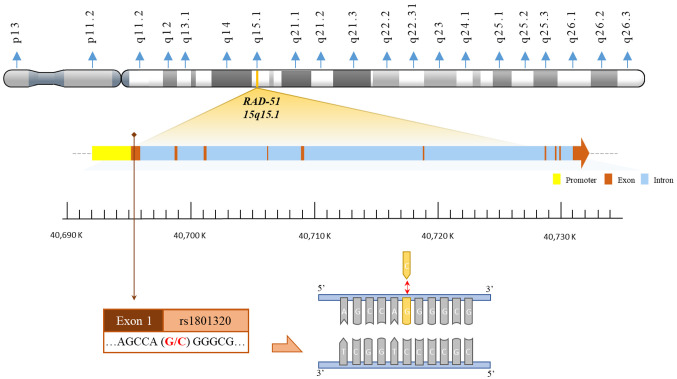
RAD-51 rs1801320 genotyping procedures. Genomic DNA isolated from peripheral blood leukocytes of both patient cohorts and controls was extracted utilizing the QIAamp Blood Mini Kit (Blossom, Taipei, Taiwan, ROC) and processed following established methodologies (60-63). Polymerase chain reaction (PCR) cycling conditions for RAD-51 rs1801320 genotyping were as follows: an initial denaturation step at 94˚C for 5 min, followed by 35 cycles of denaturation at 94˚C for 30 s, annealing at 55˚C for 30 s, and extension at 72˚C for 30 s, with a final extension step at 72˚C for 10 min. The forward and reverse primers for RAD-51 rs1801320 were 5’-CAGGATCAAGCTCTCGAGCT-3’ and 5’-GGTGTTGCCTATAAAGGCTC-3’, respectively. Subsequently, PCR products underwent digestion by the restriction enzyme PspG I (New England BioLabs, Ipswich, MA, USA). The G-allele contigs were cleaved into 333- and 281-base pair fragments, while the C-allele contigs remained unaltered, presenting as 614-base pair products. Genotypic analysis was conducted independently and blindly by laboratory personnel, with repeated data demonstrating 100% concordance. The physical map illustrating the locations of RAD-51 rs1801320 is presented in Figure 1.
Figure 1. The physical map of RAD-51 rs1801320 polymorphic site. The polymorphic site rs1801320 is located within exon 1 of the RAD-51 gene. Specifically, rs1801320 represents a G to C polymorphism.
RAD-51 rs1801320 statistical analyzing methodologies. Age comparison between the pterygium patient and non-pterygium control groups was presented as the mean±standard deviation (SD), with unpaired Student’s t-test employed for analysis. Evaluation of the impact of RAD-51 rs1801320 polymorphisms on pterygium risk was conducted using Pearson’s chi-square test. Associations were further assessed through odds ratios (ORs) accompanied by their 95% confidence intervals (CIs). Statistical significance was considered when the resulting p-value was below 0.05.
Results
Comparison of age and sex distributions between the pterygium patient and non-pterygium control groups. Firstly, it is imperative to scrutinize the age distributions across the pterygium and non-pterygium cohorts. The mean ages of both groups were evaluated, revealing no notable disparity between them (p=0.2980). This observation persisted even upon stratification of data by employing a threshold age of 60 years (p=0.4109). Secondly, it is noteworthy that, as a component of our recruitment strategy, we meticulously matched the pterygium and non-pterygium groups, thereby ensuring equitable distribution of sex across these cohorts (p=1.0000).
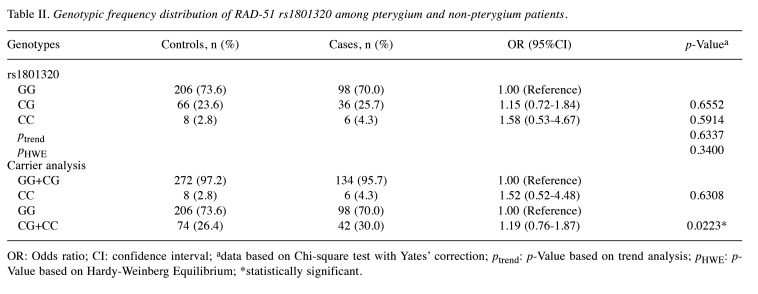
Association of RAD-51 rs1801320 genotypes and pterygium risk. Table II presents the distribution of RAD-51 rs1801320 genotypes within the pterygium and non-pterygium cohorts. Notably, the RAD-51 rs1801320 genotypes did not display divergent distributions between the pterygium and non-pterygium groups (p for trend=0.6337). Specifically, the heterozygous variant CG and homozygous variant CC of RAD-51 rs1801320 did not exhibit a significant association with pterygium risk (OR=1.15 and 1.58, 95%CI=0.72-1.84 and 0.53-4.67, p=0.6552 and 0.5914, respectively). Confirmation of the negative association of RAD-51 rs1801320 polymorphic variants with pterygium risk was obtained in the recessive model (OR=1.52, 95%CI=0.52-4.48, p=0.6308). Notably, a borderline association of RAD-51 rs1801320 genotype with pterygium risk was observed in the dominant model (p=0.0223; lower section of Table II). Carriers of the CG and CC variants at RAD-51 rs1801320 exhibited a 1.19-fold increased risk of pterygium development compared to those with the GG genotype (95%CI=0.76-1.87; lower section of Table II).
Table II. Genotypic frequency distribution of RAD-51 rs1801320 among pterygium and non-pterygium patients.
OR: Odds ratio; CI: confidence interval; adata based on Chi-square test with Yates’ correction; ptrend: p-Value based on trend analysis; pHWE: p-Value based on Hardy-Weinberg Equilibrium; *statistically significant.
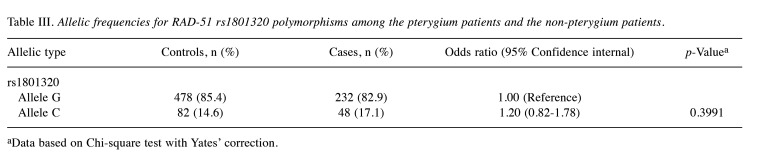
Association of RAD-51 rs1801320 allelic frequencies and pterygium risk. Allelic frequency analysis results suggested that the presence of the variant C allele at RAD-51 rs1801320 does not exhibit an association with pterygium risk (17.1% versus 14.6%, OR=1.20, 95%CI=0.82-1.78, p=0.3991) (Table III).
Table III. Allelic frequencies for RAD-51 rs1801320 polymorphisms among the pterygium patients and the non-pterygium patients.
aData based on Chi-square test with Yates’ correction.
Discussion
Among ophthalmologists, there remains a lack of consensus regarding the optimal comprehension of pterygium etiology and its management. This divergence is partly ascribed to the complexity of involved risk factors and the lack of a dependable marker for tailoring personalized therapeutic approaches. In 2007, Tsai and his colleagues have revealed the genotypes at Ku70 promoter T-991C (rs5751129) can serve as novel predictor for pterygium risk (64). RAD-51 is known to play a central role in HR during the repair of DNA DSBs. Although little is known about the role of RAD-51 protein in pterygium, it has been reported that the expression level of RAD-51 was higher in the peripheral blood lymphocytes from patients with recurrent pterygium in comparison to those patients with primary pterygium (65). As mentioned in the introduction part, the genomic contribution of RAD-51 to pterygium remains unrevealed and we are the first team to assess the impact of RAD-51 rs1801320 genotype on pterygium risk. Among the non-pterygium healthy controls, the percentages of wild-type GG and variant CG and CC genotypes were 73.6%, 23.6%, and 2.8%, respectively, and fitted well with the Hardy-Weinberg equilibrium (p=0.6337, Table II). The percentages of wild-type G and variant C alleles at RAD-51 rs1801320 in Taiwan population were 85.4% and 14.6%, respectively (Table III). In the global 1000 Genomes Project, the percentages of wild-type G and variant C alleles at RAD-51 rs1801320 for East Asians were 85.0% and 15.0%, respectively, based on a sample size of 1170 subjects (66). The adherence to Hardy-Weinberg Equilibrium indicated that our collection of non-pterygium samples can be representative of the whole Taiwanese population without sampling bias.
Although our data show that the C allele of RAD-51 rs1801320 does not appear to be a significant contributor to individual pterygium susceptibility, however, it’s noteworthy that RAD-51 rs1801320 variant CG and CC genotypes were more prevalent in the pterygium group compared to the non-pterygium group (25.7% versus 23.6% and 4.3% versus 2.8%, Table II). More important, in the dominant model, CG or CC carriers at RAD-51 rs1801320 exhibited a significantly higher risk of developing pterygium than GG carriers (Table II). These finding raises considerable interest, and larger pterygium populations could be beneficial to validate the diagnostic role for RAD-51 rs1801320 genotypes. To the best of our knowledge, our current study is the first to demonstrate the potential contribution of RAD-51 rs1801320 variant genotypes to pterygium susceptibility on a global scale.
There are several directions we may extend our study from the current findings. First, we did not extend our stratification analysis to investigate the impact of other factors (such as age and sex) combined with RAD-51 rs1801320 genotype on pterygium risk since the sample size for variant CC genotype were only 8 and 6 for the control and case groups, respectively. It is very essential to enlarge the sample size. Second, it is reported that the X-ray repair cross complementary 1 (XRCC-1) codon 194 polymorphism was associated with a decreased risk of developing pterygium, but the codon 399 polymorphism was associated with an elevation of pterygium risk (67). Since pterygium is a UV-related disease, we may figure out the role of those proteins and genes involved in the nucleotide excision and single strand break repair pathways as some literatures suggested (68-70). In addition to RAD-51, a significant elevated expression of XRCC-2 and XRCC-3 was found among recurrent pterygium patients, compared to those patients with primary pterygium (65). Third, lower level of RAD-51 expression in pterygium patients compared to the non-pterygium control group, providing another piece of clue to involvement of DSB repair pathway in pterygium in addition to the current study (65). Up to now, the knowledge for the involvement of Ku80, DNA-PKcs, ligase 4 and other DSB genes are still lacking.
In summary, this study initially investigated the genotypic impact of RAD-51 rs1801320 within a Taiwanese pterygium cohort and identified potential associations between RAD-51 rs1801320 variant genotypes and increased susceptibility to pterygium among individuals in Taiwan. It is pertinent to validate these findings in larger and more diverse population cohorts before clinical practices.
Conflicts of Interest
All the Authors declare no conflicts of interest regarding this study.
Authors’ Contributions
Research design: Hsia NY, Hu PS, Bau DT, Chang WS; patient and questionnaire summaries: Hsia NY, Hu PS, Chuang CL, Chen HC; experimental work: Wang YC, Tsai CW, Chang WS; statistical analysis: Chuang CL, Mong MC, Chen JC, Wang YC; manuscript writing: Hu PS, Bau DT, Chang WS; manuscript checking and discussing: Hsia NY, Hu PS, Chuang CL, Mong MC, Chen HC, Tsai CW, Wang YC, Chen JC, Bau DT, Chang WS.
Acknowledgements
The Authors are grateful to Yu-Cheng Luo, Yu-Hsin Yen, Yu-Ting Chin and Hou-Yu Shih for their excellent technical assistance. All the participants in this study are appreciated. This study is supported with grants from Changhua Christian Hospital (111-CCH-IRP-025), Taichung Armed Forces General Hospital (TCAFGH_D_112012) and China Medical University and Asia University (CMU111-ASIA-02 and ASIA-112-CMUH-10). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Batur M, Seven E, Tekin S, Ozer MD, Demir MS, Yasar T. The role of anterior segment optical coherence tomography in the evaluation of the pterygium. Photodiagnosis Photodyn Ther. 2023;43:103704. doi: 10.1016/j.pdpdt.2023.103704. [DOI] [PubMed] [Google Scholar]
- 2.He Q, Cai Y, Huang J, He X, Han W, Chen W. Impairment of autophagy promotes human conjunctival fibrosis and pterygium occurrence via enhancing the SQSTM1-NF-ĸB signaling pathway. J Mol Cell Biol. 2023;15(1):mjad009. doi: 10.1093/jmcb/mjad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ Open. 2013;3(11):e003787. doi: 10.1136/bmjopen-2013-003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Fang X, Lin Z, Xie Z, Wu H, Ou S. Histopathology-based diagnosis of Mooren’s ulcer concealed beneath the pterygium on eye. J Histotechnol. 2022;45(4):195–201. doi: 10.1080/01478885.2022.2137666. [DOI] [PubMed] [Google Scholar]
- 5.Asokan R, Venkatasubbu RS, Velumuri L, Lingam V, George R. Prevalence and associated factors for pterygium and pinguecula in a South Indian population. Ophthalmic Physiol Opt. 2012;32(1):39–44. doi: 10.1111/j.1475-1313.2011.00882.x. [DOI] [PubMed] [Google Scholar]
- 6.Viso E, Gude F, Rodríguez-Ares MT. Prevalence of pinguecula and pterygium in a general population in Spain. Eye (Lond) 2011;25(3):350–357. doi: 10.1038/eye.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang QF, Xu L, Jin XY, You QS, Yang XH, Cui TT. Epidemiology of pterygium in aged rural population of Beijing, China. Chin Med J (Engl) 2010;123(13):1699–1701. [PubMed] [Google Scholar]
- 8.Cajucom-Uy H, Tong L, Wong TY, Tay WT, Saw SM. The prevalence of and risk factors for pterygium in an urban Malay population: The Singapore Malay Eye Study (SiMES) Br J Ophthalmol. 2010;94(8):977–981. doi: 10.1136/bjo.2008.150847. [DOI] [PubMed] [Google Scholar]
- 9.West S, Munoz B. Prevalence of pterygium in Latinos: Proyecto VER. Br J Ophthalmol. 2009;93(10):1287–1290. doi: 10.1136/bjo.2008.152694. [DOI] [PubMed] [Google Scholar]
- 10.Shiroma H, Higa A, Sawaguchi S, Iwase A, Tomidokoro A, Amano S, Araie M. Prevalence and risk factors of pterygium in a southwestern island of Japan: the Kumejima Study. Am J Ophthalmol. 2009;148(5):766–771.e1. doi: 10.1016/j.ajo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. Prevalence and risk factors of pterygium and pinguecula: the Tehran Eye Study. Eye (Lond) 2009;23(5):1125–1129. doi: 10.1038/eye.2008.200. [DOI] [PubMed] [Google Scholar]
- 12.Durkin SR, Abhary S, Newland HS, Selva D, Aung T, Casson RJ. The prevalence, severity and risk factors for pterygium in central Myanmar: the Meiktila Eye Study. Br J Ophthalmol. 2008;92(1):25–29. doi: 10.1136/bjo.2007.119842. [DOI] [PubMed] [Google Scholar]
- 13.Nemesure B, Wu SY, Hennis A, Leske MC, Barbados Eye Studies Group Nine-year incidence and risk factors for pterygium in the Barbados Eye Studies. Ophthalmology. 2008;115(12):2153–2158. doi: 10.1016/j.ophtha.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Hu PS, Chang WS, Chou AK, Hsia NY, Hung YW, Lin CW, Wu CW, Huang CY, Wu MF, Liao CH, Tsai CW, Bau DT, Gong CL. The Association of MMP-8 Genotypes with Pterygium. In Vivo. 2018;32(1):41–46. doi: 10.21873/invivo.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu PS, Wang YC, Liao CH, Hsia NY, Wu MF, Yang JS, Yu CC, Chang WS, Bau DT, Tsai CW. The association of MMP7 genotype with pterygium. In Vivo. 2020;34(1):51–56. doi: 10.21873/invivo.11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CB, Hsia NY, Wang YC, Wang ZH, Chin YT, Huang TL, Yu CC, Chang WS, Tsai CW, Yin MC, Bau DT. The significant association of MMP-1 genotypes with Taiwan pterygium. Anticancer Res. 2020;40(2):703–707. doi: 10.21873/anticanres.14000. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CB, Hsia NY, Wang ZH, Yang JS, Hsu YM, Wang YC, Chang WS, Bau DT, Yin MC, Tsai CW. The contribution of MMP-9 genotypes to pterygium in Taiwan. Anticancer Res. 2020;40(8):4523–4527. doi: 10.21873/anticanres.14457. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Canela A, Maman Y, Jung S, Wong N, Callen E, Day A, Kieffer-Kwon KR, Pekowska A, Zhang H, Rao SSP, Huang SC, Mckinnon PJ, Aplan PD, Pommier Y, Aiden EL, Casellas R, Nussenzweig A. Genome organization drives chromosome fragility. Cell. 2017;170(3):507–521.e18. doi: 10.1016/j.cell.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bau DT, Fu YP, Chen ST, Cheng TC, Yu JC, Wu PE, Shen CY. Breast cancer risk and the DNA double-strand break end-joining capacity of nonhomologous end-joining genes are affected by BRCA1. Cancer Res. 2004;64(14):5013–5019. doi: 10.1158/0008-5472.CAN-04-0403. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Gong Z. Regulation of DNA double-strand break repair pathway choice: a new focus on 53BP1. J Zhejiang Univ Sci B. 2021;22(1):38–46. doi: 10.1631/jzus.B2000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali A, Xiao W, Babar ME, Bi Y. Double-stranded break repair in mammalian cells and precise genome editing. Genes (Basel) 2022;13(5):737. doi: 10.3390/genes13050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Guen T, Ragu S, Guirouilh-Barbat J, Lopez BS. Role of the double-strand break repair pathway in the maintenance of genomic stability. Mol Cell Oncol. 2014;2(1):e968020. doi: 10.4161/23723548.2014.968020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi E, Matsuda Y, Hori T, Yasuda N, Tsuji S, Mori M, Yoshimura Y, Yamamoto A, Morita T, Matsushiro A. Chromosome mapping of the human (RECA) and mouse (Reca) homologs of the yeast RAD51 and Escherichia coli recA genes to human (15q15.1) and mouse (2F1) chromosomes by Direct R-banding fluorescence in situ hybridization. Genomics. 1994;19(2):376–378. doi: 10.1006/geno.1994.1074. [DOI] [PubMed] [Google Scholar]
- 25.Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E, Hummerich J, Muller DJ, Stangl AP, Schramm J, Wiestler OD, von Deimling A. Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene. 1996;12(5):973–978. [PubMed] [Google Scholar]
- 26.van Wijk LM, Nilas AB, Vrieling H, Vreeswijk MPG. RAD51 as a functional biomarker for homologous recombination deficiency in cancer: a promising addition to the HRD toolbox. Expert Rev Mol Diagn. 2022;22(2):185–199. doi: 10.1080/14737159.2022.2020102. [DOI] [PubMed] [Google Scholar]
- 27.Tsang ES, Munster PN. Targeting RAD51-mediated homologous recombination as a treatment for advanced solid and hematologic malignancies: opportunities and challenges ahead. Onco Targets Ther. 2022;15:1509–1518. doi: 10.2147/OTT.S322297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maacke H, Opitz S, Jost K, Hamdorf W, Henning W, Kruger S, Feller AC, Lopens A, Diedrich K, Schwinger E, Sturzbecher HW. Over-expression of wild-type Rad51 correlates with histological grading of invasive ductal breast cancer. Int J Cancer. 2000;88(6):907–913. doi: 10.1002/1097-0215(20001215)88:6<907::aid-ijc11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Martin RW, Orelli BJ, Yamazoe M, Minn AJ, Takeda S, Bishop DK. RAD51 Up-regulation bypasses BRCA1 function and is a common feature of BRCA1-deficient breast tumors. Cancer Res. 2007;67(20):9658–9665. doi: 10.1158/0008-5472.CAN-07-0290. [DOI] [PubMed] [Google Scholar]
- 30.Lok BH, Carley AC, Tchang B, Powell SN. RAD52 inactivation is synthetically lethal with deficiencies in BRCA1 and PALB2 in addition to BRCA2 through RAD51-mediated homologous recombination. Oncogene. 2013;32(30):3552–3558. doi: 10.1038/onc.2012.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (Amst) 2008;7(5):686–693. doi: 10.1016/j.dnarep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbano R, Copetti M, Perrone G, Pazienza V, Muscarella LA, Balsamo T, Storlazzi CT, Ripoli M, Rinaldi M, Valori VM, Latiano TP, Maiello E, Stanziale P, Carella M, Mangia A, Pellegrini F, Bisceglia M, Muda AO, Altomare V, Murgo R, Fazio VM, Parrella P. High RAD51 mRNA expression characterize estrogen receptor-positive/progesteron receptor-negative breast cancer and is associated with patient’s outcome. Int J Cancer. 2011;129(3):536–545. doi: 10.1002/ijc.25736. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa K, Ogawa T, Baer R, Hemmi H, Honda K, Yamauchi A, Inamoto T, Ko K, Yazumi S, Motoda H, Kodama H, Noguchi S, Gazdar AF, Yamaoka Y, Takahashi R. Abnormal expression of BRCA1 and BRCA1-interactive DNA-repair proteins in breast carcinomas. Int J Cancer. 2000;88(1):28–36. [PubMed] [Google Scholar]
- 34.Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62(10):2890–2896. [PubMed] [Google Scholar]
- 35.Maacke H, Jost K, Opitz S, Miska S, Yuan Y, Hasselbach L, Lüttges J, Kalthoff H, Stürzbecher H. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene. 2000;19(23):2791–2795. doi: 10.1038/sj.onc.1203578. [DOI] [PubMed] [Google Scholar]
- 36.Connell PP, Jayathilaka K, Haraf DJ, Weichselbaum RR, Vokes EE, Lingen MW. Pilot study examining tumor expression of RAD51 and clinical outcomes in human head cancers. Int J Oncol. 2006;28(5):1113–1119. [PubMed] [Google Scholar]
- 37.Mitra A, Jameson C, Barbachano Y, Sanchez L, Kote-Jarai Z, Peock S, Sodha N, Bancroft E, Fletcher A, Cooper C, Easton D, IMPACT Steering Committee and IMPACT and EMBRACE Collaborators, Eeles R, Foster CS. Overexpression of RAD51 occurs in aggressive prostatic cancer. Histopathology. 2009;55(6):696–704. doi: 10.1111/j.1365-2559.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannay JA, Liu J, Zhu QS, Bolshakov SV, Li L, Pisters PW, Lazar AJ, Yu D, Pollock RE, Lev D. Rad51 overexpression contributes to chemoresistance in human soft tissue sarcoma cells: a role for p53/activator protein 2 transcriptional regulation. Mol Cancer Ther. 2007;6(5):1650–1660. doi: 10.1158/1535-7163.MCT-06-0636. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Yu H, Luo RZ, Zhang Y, Zhang MF, Wang X, Jia WH. Elevated expression of Rad51 is correlated with decreased survival in resectable esophageal squamous cell carcinoma. J Surg Oncol. 2011;104(6):617–622. doi: 10.1002/jso.22018. [DOI] [PubMed] [Google Scholar]
- 40.Qiao GB, Wu YL, Yang XN, Zhong WZ, Xie D, Guan XY, Fischer D, Kolberg HC, Kruger S, Stuerzbecher HW. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer. 2005;93(1):137–143. doi: 10.1038/sj.bjc.6602665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Li Y, Xu H, Wang K, Li N, Li J, Sun T, Xu Y. Increased expression of SET domain-containing proteins and decreased expression of Rad51 in different classes of renal cell carcinoma. Biosci Rep. 2016;36(3):BSR20160122. doi: 10.1042/BSR20160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowacka-Zawisza M, Wiśnik E, Wasilewski A, Skowrońska M, Forma E, Bryś M, Różański W, Krajewska WM. Polymorphisms of homologous recombination RAD51, RAD51B, XRCC2, and XRCC3 genes and the risk of prostate cancer. Anal Cell Pathol (Amst) 2015;2015:828646. doi: 10.1155/2015/828646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jara L, Acevedo ML, Blanco R, Castro VG, Bravo T, Gómez F, Waugh E, Peralta O, Cabrera E, Reyes JM, Ampuero S, González-Hormazábal P. RAD51 135G>C polymorphism and risk of familial breast cancer in a South American population. Cancer Genet Cytogenet. 2007;178(1):65–69. doi: 10.1016/j.cancergencyto.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 44.Romanowicz-Makowska H, Smolarz B, Zadrozny M, Westfal B, Baszczynski J, Polac I, Sporny S. Single nucleotide polymorphisms in the homologous recombination repair genes and breast cancer risk in Polish women. Tohoku J Exp Med. 2011;224(3):201–208. doi: 10.1620/tjem.224.201. [DOI] [PubMed] [Google Scholar]
- 45.Vral A, Willems P, Claes K, Poppe B, Perletti G, Thierens H. Combined effect of polymorphisms in Rad51 and Xrcc3 on breast cancer risk and chromosomal radiosensitivity. Mol Med Rep. 2011;4(5):901–12. doi: 10.3892/mmr.2011.523. [DOI] [PubMed] [Google Scholar]
- 46.Smolarz B, Zadrożny M, Duda-Szymańska J, Makowska M, Samulak D, Michalska MM, Mojs E, Bryś M, Forma E, Romanowicz-Makowska H. RAD51 genotype and triple-negative breast cancer (TNBC) risk in Polish women. Pol J Pathol. 2013;1:39–43. doi: 10.5114/pjp.2013.34602. [DOI] [PubMed] [Google Scholar]
- 47.Tulbah S, Alabdulkarim H, Alanazi M, Parine NR, Shaik J, Pathan AA, Al-Amri A, Khan W, Warsy A. Polymorphisms in RAD51 and their relation with breast cancer in Saudi females. Onco Targets Ther. 2016;9:269–277. doi: 10.2147/OTT.S93343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salim Al Zoubi MS, Al-Eitan LN, Rababa’h DM, Al-Batayneh K, Farzand R, Quinn GA, Tambuwala MM, Bakshi HA. RAD51-UTR haplotype genetic polymorphisms and susceptibility to breast cancer in women from Jordanian population. Exp Oncol. 2023;43(2):149–154. doi: 10.32471/exp-oncology.2312-8852.vol-43-no-2.16338. [DOI] [PubMed] [Google Scholar]
- 49.Gupta P, Sambyal V, Guleria K, Uppal MS, Sudan M. Association of RAD51, XRCC1, XRCC2, and XRCC3 polymorphisms with risk of breast cancer. Genet Test Mol Biomarkers. 2023;27(7):205–214. doi: 10.1089/gtmb.2023.0012. [DOI] [PubMed] [Google Scholar]
- 50.Romanowicz-Makowska H, Smolarz B, Gajęcka M, Kiwerska K, Rydzanicz M, Kaczmarczyk D, Olszewski J, Szyfter K, Błasiak J, Morawiec-Sztandera A. Polymorphism of the DNA repair genes RAD51 and XRCC2 in smoking- and drinking-related laryngeal cancer in a Polish population. Arch Med Sci. 2012;8(6):1065–1075. doi: 10.5114/aoms.2012.32417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krupa R, Sliwinski T, Wisniewska-Jarosinska M, Chojnacki J, Wasylecka M, Dziki L, Morawiec J, Blasiak J. Polymorphisms in RAD51, XRCC2 and XRCC3 genes of the homologous recombination repair in colorectal cancer—a case control study. Mol Biol Rep. 2011;38(4):2849–2854. doi: 10.1007/s11033-010-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hridy AU, Shabnaz S, Asaduzzaman MD, Shahriar M, Bhuiyan MA, Islam MS, Hossen SMM, Emran TB. Genetic variations of RAD51 and XRCC2 genes increase the risk of colorectal cancer in bangladeshi population. Asian Pac J Cancer Prev. 2020;21(5):1445–1451. doi: 10.31557/APJCP.2020.21.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smolarz B, Makowska M, Samulak D, Michalska MM, Mojs E, Romanowicz H, Wilczak M. Association between polymorphisms of the DNA repair gene RAD51 and ovarian cancer. Pol J Pathol. 2013;4:290–295. doi: 10.5114/pjp.2013.39338. [DOI] [PubMed] [Google Scholar]
- 54.Smolarz B, Michalska MM, Samulak D, Romanowicz H, Wójcik L. Polymorphism of DNA repair genes via homologous recombination (HR) in ovarian cancer. Pathol Oncol Res. 2019;25(4):1607–1614. doi: 10.1007/s12253-019-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gowtham Kumar G, Paul SFD, Martin J, Manickavasagam M, Sundersingh S, Ganesan N, Ramya R, Usha Rani G, Andrea Mary F. Association between RAD51, XRCC2 and XRCC3 gene polymorphisms and risk of ovarian cancer: a case control and an in silico study. Mol Biol Rep. 2021;48(5):4209–4220. doi: 10.1007/s11033-021-06434-6. [DOI] [PubMed] [Google Scholar]
- 56.Ivy SC, Shabnaz S, Shahriar M, Jafrin S, Aka TD, Aziz MA, Islam MS. Association of RAD51 and XRCC2 gene polymorphisms with cervical cancer risk in the Bangladeshi women. Asian Pac J Cancer Prev. 2021;22(7):2099–2107. doi: 10.31557/APJCP.2021.22.7.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michalska MM, Samulak D, Romanowicz H, Smolarz B. Association of polymorphisms in the 5’ untranslated region of RAD51 gene with risk of endometrial cancer in the Polish population. Arch Gynecol Obstet. 2014;290(5):985–991. doi: 10.1007/s00404-014-3305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das AP, Chaudhary N, Tyagi S, Agarwal SM. Meta-analysis of 49 SNPs covering 25,446 cases and 41,106 controls identifies polymorphisms in hormone regulation and DNA repair genes associated with increased endometrial cancer risk. Genes (Basel) 2023;14(3):741. doi: 10.3390/genes14030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franceschi S, Tomei S, Mazzanti CM, Lessi F, Aretini P, La Ferla M, De Gregorio V, Pasqualetti F, Zavaglia K, Bevilacqua G, Naccarato AG. Association between RAD 51 rs1801320 and susceptibility to glioblastoma. J Neurooncol. 2016;126(2):265–270. doi: 10.1007/s11060-015-1974-z. [DOI] [PubMed] [Google Scholar]
- 60.Yang MD, Lin KC, Lu MC, Jeng LB, Hsiao CL, Yueh TC, Fu CK, Li HT, Yen ST, Lin CW, Wu CW, Pang SY, Bau DT, Tsai FJ. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine (Taipei) 2017;7(2):10. doi: 10.1051/bmdcn/2017070203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen LH, Li CH, Wang SC, Chiu KL, Wu MF, Yang JS, Tsai CW, Chang WS, Hsia TC, Bau DT. Association of matrix metalloproteinase-1 promoter polymorphisms with asthma risk. In Vivo. 2024;38(1):365–371. doi: 10.21873/invivo.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu CK, Mong MC, Tzeng HE, Yang MD, Chen JC, Hsia TC, Hsia NY, Tsai CW, Chang WS, Chen CP, Bau DT. The significant contribution of interleukin-16 genotypes, smoking, alcohol drinking, and Helicobacter pylori infection to gastric cancer. In Vivo. 2024;38(1):90–97. doi: 10.21873/invivo.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng Y, Ke TW, Yueh TC, Chin YT, Wang YC, Hung YC, Mong MC, Yang YC, Wu WT, Chang WS, Gu J, Bau DT, Tsai CW. The contribution of DNA ligase 4 polymorphisms to colorectal cancer. In Vivo. 2024;38(1):127–133. doi: 10.21873/invivo.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai YY, Bau DT, Chiang CC, Cheng YW, Tseng SH, Tsai FJ. Pterygium and genetic polymorphism of DNA double strand break repair gene Ku70. Mol Vis. 2007;13:1436–1440. [PubMed] [Google Scholar]
- 65.Lekawa-Ilczuk A, Antosz H, Rymgayllo-Jankowska B, Zarnowski T. Expression of double strand DNA breaks repair genes in pterygium. Ophthalmic Genet. 2011;32(1):39–47. doi: 10.3109/13816810.2010.524907. [DOI] [PubMed] [Google Scholar]
- 66.dbSNP rs1801320. Available at: https://www.ncbi.nlm.nih.gov/snp/rs1801320. [Last accessed on May 1, 2024]
- 67.Chiang CC, Tsai YY, Bau DT, Cheng YW, Tseng SH, Wang RF, Tsai FJ. Pterygium and genetic polymorphisms of the DNA repair enzymes XRCC1, XPA, and XPD. Mol Vis. 2010;16:698–704. [PMC free article] [PubMed] [Google Scholar]
- 68.Liu T, Liu Y, Xie L, He X, Bai J. Progress in the pathogenesis of pterygium. Curr Eye Res. 2013;38:1191–1197. doi: 10.3109/02713683.2013.823212. [DOI] [PubMed] [Google Scholar]
- 69.Van Acker SI, Van den Bogerd B, Haagdorens M, Siozopoulou V, Ní Dhubhghaill S, Pintelon I, Koppen C. Pterygium-the good, the bad, and the ugly. Cells. 2021;10(7):1567. doi: 10.3390/cells10071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J, Tao T, Yu Y, Xu N, Du W, Zhao M, Jiang Z, Huang L. Expression profiling suggests the involvement of hormone-related, metabolic, and Wnt signaling pathways in pterygium progression. Front Endocrinol (Lausanne) 2022;13:943275. doi: 10.3389/fendo.2022.943275. [DOI] [PMC free article] [PubMed] [Google Scholar]