Abstract
Background/Aim
Cigarette smoke has been shown to induce a phenotype in humans known as “acquired cystic fibrosis”. This occurs because the cystic fibrosis transmembrane conductance regulator (CFTR) functions are impaired systemically due to the deleterious effects of smoke components. Elucidation of cigarette smoke effects on the tracheal epithelium is important. The aim of this study was to develop an ex vivo sheep tracheal model to investigate tracheal ion function. In this model, the epithelial sodium channel (ENaC) is inhibited after exposure to cigarette smoke extract (CSE) as a proof of principle.
Materials and Methods
Tracheas were isolated from healthy sheep and the tracheal epithelium was surgically excised. Tissues were mounted in Ussing chambers and the short circuit current (Isc) was measured after incubation with 5% CSE in PBS or PBS alone for 30 min. The function of ENaC was investigated by the addition of amiloride (10–5M) apically. Western blot analysis was performed to assess differences in ENaC quantity after CSE exposure. Some specimens were stained with H&E for detection of histological alterations.
Results
The amiloride effect on normal epithelium led to a significant decrease in Isc [ΔI=33±5.92 μA/cm2; p<0.001 versus control experiments (ΔI=1.44±0.71 μA/cm2)]. After incubation with CSE, ENaC Isc was significantly reduced (ΔI=14.80±1.96 μA/cm2; p<0.001). No differences in αENaC expression were observed between CSE-exposed and normal tracheal epithelium. Histological images post CSE incubation revealed decreases in the height of the epithelium, with basal cell hyperplasia and loss of ciliated cells.
Conclusion
Reduced ENaC inhibition by amiloride after CSE incubation could be due to alterations in the tracheal epithelium.
Keywords: Amiloride, cigarette smoke extract, electrophysiology, epithelial sodium channel, tracheal epithelium, Ussing system
Cigarette smoke significantly contributes to sustained inflammation, lung injury, and increased respiratory-associated morbidity (1-5). One of the critical molecular mediators of lung fluid clearance is the amiloride sensitive epithelial sodium channel (ENaC) (6). ENaC is found in airway epithelial cells and functions in an orchestrated fashion with the cystic fibrosis transmembrane regulator (CFTR) to maintain the height of the airway surface liquid at a desired level, i.e., 7-10 microns (7). In patients with CF, the compromised function of CFTR, along with the sustained function of ENaC, leads to a dehydrated ASL, with reduced height, compromised mucociliary clearance, and thus, biofilm development and sustained infections (8-10). There is considerable evidence supporting the deleterious effects of cigarette smoke on ENaC function (11). These span from reduction in mRNA expression of the αENaC subunit, to increased ENaC open channel probability in human cells due to reactive oxygen species-mediated increase of ENaC channel residence time on the cell membrane (12). Components of cigarette smoke, such as formaldehyde and crotonaldehyde, have variable effects; for instance, they reduce ENaC activity (13,14).
More concrete evidence that indicates that smokers exhibit an acquired CF phenotype due to CFTR blockade by cigarette smoke byproducts, mainly acrolein, has been published in the last decade (15). Apart from studies in murine nasal cells showing compromised CFTR function due to acrolein, studies in human cells and samples from smokers, ex-smokers, and controls, have demonstrated that there is systemic dysfunction of CFTR (16). Furthermore, therapeutical exploitation of such findings with provision of CFTR activators or potentiators (such as roflumilast or ivacaftor) have additionally verified the claims for an acquired form of CF (17-19).
Sheep tracheal epithelium, despite its similarity to human tracheal properties, is not extensively utilized for experimentation. Nonetheless, it represents an ideal model for experiments involving whole tissues (20). To this end, this study aimed to establish an ex vivo model for the study of the effects of cigarette smoke extract (CSE) on ion transport, providing the inhibition of ENaC as a proof of principle.
Materials and Methods
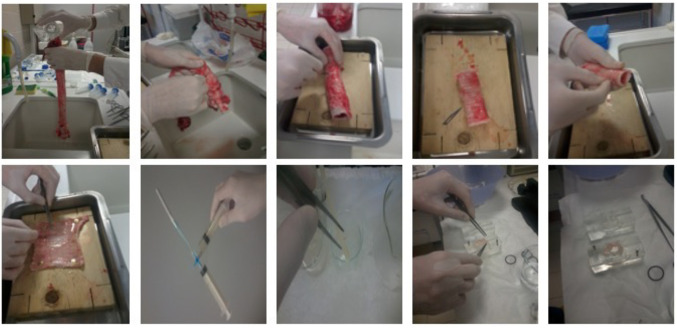
Tissue isolation. Intact tracheas from adult sheep were obtained from the local abattoir immediately after the death of the animals, kept on ice in PBS, and transferred to the laboratory within 60 min. In a bath with PBS buffer, the anterior region of the trachea was cut longitudinally, and the epithelial surface of the posterior membranous region of the trachea was excised with a scalpel in order to obtain specimens of nearly 3×3 cm, as demonstrated in Figure 1. Specimens were then placed in ice cold PBS until used for the experiment.
Figure 1. Stepwise demonstration of tracheal specimen preparation. Steps that demonstrate the sequence of sheep tracheal tissue handling, posterior tracheal membrane excision, and its treatment with Kreb’s Ringer bicarbonate buffer or cigarette smoke extract (CSE). Using forceps, the intact membrane was mounted onto a Ussing chamber for trans-epithelial resistance recordings during exposure to Kreb’s Ringer bicarbonate buffer or CSE complemented Kreb’s Ringer bicarbonate buffer.
CSE preparation and tissue CSE exposure. Smoke from two commercially available cigarettes (Marlboro Brand) was bubbled into 5 ml of Krebs Ringer bicarbonate (KR) solution [balanced at pH 7.4 and consisted of (in mM) 117.5 NaCl, 1.15 NaH2PO4, 24.99 NaHCO3, 5.65 KCl, 1.18 MgSO4, 2.52 CaCl2, and 5.55 glucose], through a system of syringes connected by a 3-way stop valve. CSE was prepared fresh and used immediately for each experiment at a concentration of 5% in KR solution. Tracheal epithelial specimens were cut into equal halves and incubated on ice, either in KR solution bubbled with air or in 5% CSE-KR for 30 min. In Figure 1 the series of steps from trachea washing to tissue mounting in Ussing chambers is demonstrated.
Ussing chamber experiments and histology. The specimens were then mounted on Ussing chambers (Dipl.-Ing. K. Mussler Scientific Instruments, Aachen, Germany) with an opening surface area of 1 cm2 and bathed continuously with oxygenated KRB solution (95% O2/5% CO2 at 37˚C) for 2 h to allow for equilibration. Two pairs of Ag/AgCl electrodes monitored the trans-epithelial electrical resistance (RTE; Ω*cm2) under open-circuit every 60 s. The voltage responses to applied current pulses of a given amplitude (50 μA) and duration (200 ms) were measured and resistance was calculated automatically by Ohm’s law (21). Experiments were conducted in computer-controlled chambers (Clamp version 2.14 software: AC Micro-Clamp). RTM was measured in the basal state (end of equilibration time of 120 min) and after the addition of the ENaC inhibitor amiloride (10–5M). RTE was calculated by automatically deducting the initially measured resistance of the solution. After Ussing chambers experiments, the tracheal epithelial specimens were fixed in 4% formaldehyde solution. After the dehydration process, they were embedded in paraffin wax. Tissue specimens were orientated and 3 μm sections were cut using a microtome for hematoxylin–eosin staining. Stained slides were subsequently examined under a light microscope.
Protein extraction and western blot. After treatments with KR or 5%CSE-KR for 30 min, tracheal epithelial specimens were soaked in RIPA buffer (applied to the mucosal surface) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The lysates were centrifuged at 20,000 × g at 4˚C for 10 min. The protein content of the supernatant was measured by using the microBCA method (Thermo Scientific, Waltham, MA, USA). Equal amounts of protein (10 μg) were separated by 4-12% SDS–PAGE Bis-Tris polyacrylamide gel and then transferred to nitrocellulose membranes. Membranes were probed with anti-αENaC (cat# sc-22239, Santacruz Biotech, Dallas, TX, USA) and anti-β-actin (cat.#:13E5, Cell Signaling Technology, Danvers, MA, USA) antibodies, followed by incubation with appropriate IgG-HRP. Protein bands were revealed using chemiluminescence HRP substrate (Thermo Scientific) and captured by X-ray films. The experiment was performed on eight tracheal samples. Western blot images were subject to a gel analyzer algorithm on ImageJ software (National Institutes of Health). The area of each peak for α-ENaC or β-actin was measured. The band intensity from each sample for α-ENaC protein was normalized to β-actin correspondingly as a ratio.
Statistical analyses. Statistical analyses were performed using Graphpad Prism 9.0 (San Diego, CA, USA). Electrophysiology experiments were analyzed with the Kruskal-Wallis and Dunn’s post-hoc test. α-ENaC to β-actin expression ratio from eight paired samples (KR vs. KR+CSE) were subjected to Wilcoxon matched-pairs signed rank test to determine two-tailed p-value. All values shown are means±standard error of the mean (m±SEM).
Results
Amiloride sensitive currents in sheep tracheal epithelium are partially inhibited by CSE exposure. The basal transepithelial resistance (RTE; Ω•cm2) of the sheep tracheal epithelium was 32.63±7.92 Ω•cm2 (n=9) and the basal short circuit current (Isc) 43.05±12.14 μΑ/cm2 (n=9). Specimens of sheep tracheal epithelium were mounted in Ussing chambers, and after equilibration, incubated with 10–5M amiloride on the apical side in order to block the amiloride sensitive ENaC current. The absolute difference of Isc was ΔΙ=31.75±4.96 μΑ/cm2, suggesting the occurrence of a significant amiloride sensitive sodium current (p<0.005). This absolute difference of Isc was significantly inhibited (ΔΙ=14.80±1.96 μΑ/cm2; p<0.05) when the tissue specimens were exposed to 5% CSE for 30 min prior to mounting in the Ussing System; however, it remained significantly high when compared to controls (p<0.005), suggesting residual amiloride-sensitive sodium channel function (Figure 2).
Figure 2. Sheep tracheal epithelium amiloride sensitive current without/with CSE exposure. The transepithelial resistance characteristics of sheep tracheal tissue during exposure to Kreb’s Ringer bicarbonate buffer, amiloride, or 5% cigarette smoke extract (CSE) as indicated below each bar. ***p<0.001 compared to Kreb’s Ringer bicarbonate control solution. #p<0.05 compared to Amiloride treatment.

Exposure of tracheal epithelium specimens to CSE did not alter the expression of α-ENaC expression. We observed that there was no statistically significant difference in the expression of α-ENaC subunit proteins in tracheas exposed to CSE (KR+CSE) compared to tracheas receiving no CSE treatment (KR), as shown following densitometric analysis in Figure 3A (KR 1.3±0.3 compared to KR+CSE 1.0±0.22, p=0.55). For illustration purposes, we show α-ENaC and β-actin expression from paired tracheal epithelial lysates obtained from two separate sheep in Figure 3B.
Figure 3. Sheep tracheal epithelium α-ENaC without/with cigarette smoke extract (CSE) exposure. A) Western blot densitometric analysis of α-ENaC normalized to β-Actin from protein samples extracted from sheep tracheal tissue treated in vitro with or without 5% CSE in Kreb’s Ringer bicarbonate buffer. B) Demonstration of the expression of α-ENaC subunit and β-Actin as a loading control –/+ indicates treatment with 5% CSE in Kreb’s Ringer bicarbonate buffer.

CSE exposure leads to a reduction in epithelial thickness. We evaluated histological changes in sheep tracheal epithelial specimens mounted in Ussing chambers after exposure to CSE. Figure 4A shows intact sheep tracheal rings stained with hematoxylin and eosin, illustrating the epithelium, lamina propria, submucosa, and cartilage. In Figure 4B, the epithelium of the isolated tracheal specimen is shown. In Figure 4C, a nontreated specimen of tracheal epithelium is demonstrated after being mounted for 2 h in Ussing chamber, whereas in Figure 4D a corresponding specimen that was incubated for 30 min with 5% CSE prior to mounting in the Ussing chamber is shown. Histopathology showed normal tracheal mucosa lined with respiratory type epithelium. The lamina propria and submucosa contained seromucinous glands and lymphoid infiltrations were also observed in the lamina propria. The tracheal mucosa was 2 to 3 cells layers thick with a luminal lining composed of tall cuboidal ciliated cells and Clara cells with dome-shaped apices. Chronic inflammatory infiltration was seen in the lamina propria. After treatment with KR buffer + 5% CSE, the tracheal mucosa appeared attenuated lined by cuboidal epithelial cells with shortened and decreased cilia. Basal cell hyperplasia and a decreased number of subepithelial inflammatory cells were also observed.
Figure 4. Histological features of the sheep tracheal epithelium. A) H&E staining of the sheep tracheal cross section, normal mucosa, submucosa and cartilage. B) H&E staining of the sheep tracheal posterior epithelium incubated in Krebs Ringer bicarbonate (KR) buffer. Histopathology shows normal tracheal mucosa lined by respiratory type epithelium. The lamina propria and submucosa contain seromucinous glands. Lymphoid infiltrations are also observed in the lamina propria. C) H&E staining of the sheep tracheal posterior epithelium post Ussing chamber experiment in KR buffer. Tracheal mucosa is 2 to 3 cells layers thick with luminal lining comprised of tall cuboidal ciliated cells and Clara cells with dome-shaped apices. Chronic inflammatory infiltration is seen in the lamina propria. D) H&E staining of the sheep tracheal posterior epithelium after Ussing chamber experiment in KR buffer + 5% CSE shows attenuated tracheal mucosa lined by cuboidal epithelial cells with shortened and decreased cilia. Basal cell hyperplasia and a decreased number of subepithelial inflammatory cells are also observed. Scale bar applies to all figures.
Discussion
Research on cigarette smoking has attracted new interest following the description of the acquired CF phenotype. This phenotype involves the dysfunction of CFTR due to chemical interactions with the channels, rather than mutations in the CFTR gene, occurring systemically (15,16,19). However, these results are pharmacologically reversible and treatable (17,18). However, new smoking products such as e-cigarette liquid flavorings also have similar deleterious effects on CFTR (22).
Given that the airway epithelium maintains its surface liquid phase due to the orchestrated function of CFTR, ENaC, and Na-K—ATPase to exploit the vectorial sodium transport, it is important to also study the effects of cigarette smoke on ENaC (23). In this pilot study, we established that isolated sheep tracheal epithelium specimens can be used for the study of cigarette smoke effects, by means of CSE exposure, with respect to tracheal epithelial permeability and ion transport relative to sodium. Our main findings showed that the function of ENaC was partially inhibited by CSE, and this was not accompanied by a reduction in the expression of the α-ENaC subunit, but rather by the morphological compromise of the microvillus component of the airway epithelium. Indeed, in kidney principal cells, it has been described that ENaC resides in the microvilli of the apical membrane, and its function depends on their cholesterol content (24). More importantly, both chemical cholesterol extraction and cholesterol biosynthesis by lovastatin led to ENaC function down-regulation, while intracellular cholesterol induced ENaC function (24,25). In rat and human preparations like ours, CSE exposure was also accompanied by reduction in microvillar densities that corroborate our findings (26,27). Therefore, the CSE effects on the microvilli of tracheal airway epithelium could, in part, explain the reduction in the amiloride-sensitive sodium transport (28). Morphological alterations of the tracheal mucosa correlated with smoke exposure have been reported only rarely in a few previous studies, and they have not been thoroughly explained (29). In our study, the reduced ENaC inhibition by amiloride might be presumably the effect of the altered cellular composition of the tracheal epithelium or vice versa. However, the potential contribution of the reduction of other subunits of ENaC cannot be ruled out. Further large-scale studies might contribute to a better understanding of the underlying pathophysiology of smoke-related ENaC dysfunction.
Conclusion
ENaC inhibition by amiloride shows that it is functional in the normal sheep tracheal epithelium. Following CSE incubation, ENaC currents are significantly reduced, potentially correlating with the observed changes in the tracheal epithelium. Further validation of the ex vivo model is needed.
Conflicts of Interest
The Authors have no conflicts of interest to report in relation to this study.
Authors’ Contributions
Conceptualization: S.G.Z.; Data curation: R.M.J., A.G., M.I.,SGZ; Formal analysis: R.M.J., M.I., E.S., S.G.Z.; Investigation: R.M.J., A.G.; Methodology: R.M.J., A.D.G., B.M., SGZ; Project administration: C.H., K.I.G., SGZ; Resources: C.H., K.I., S.G.Z.; Software: A.D.G., B.M.; Supervision: S.G.Z.; Validation: A.D.G., B.M.; Visualization: R.M.J., M.I., S.G.Z.; Writing – original draft: R.M.J., B.M., S.G.Z.; Writing – review & editing: A.G, M.I., E.S., C.H., K.I.G.
Acknowledgements
The Authors would like to thank Dr. Dimitrios Magouliotis, Dr. Theodoros Karampitsakos, and Mr. Ioannis Makantasis for their assistance with tissue collection, western blots, and technical support, respectively. The Authors would also like to thank Dr. Morsal Sabihi for providing a native speaker assessment of the English writing of the manuscript.
Funding
This study received no external funding.
References
- 1.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol. 2014;192(11):5226–5235. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotlyarov S. The role of smoking in the mechanisms of development of chronic obstructive pulmonary disease and atherosclerosis. Int J Mol Sci. 2023;24(10):8725. doi: 10.3390/ijms24108725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calfee CS, Matthay MA, Kangelaris KN, Siew ED, Janz DR, Bernard GR, May AK, Jacob P, Havel C, Benowitz NL, Ware LB. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med. 2015;43(9):1790–1797. doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gan H, Hou X, Zhu Z, Xue M, Zhang T, Huang Z, Cheng ZJ, Sun B. Smoking: a leading factor for the death of chronic respiratory diseases derived from Global Burden of Disease Study 2019. BMC Pulm Med. 2022;22(1):149. doi: 10.1186/s12890-022-01944-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garmendia J, Morey P, Bengoechea JA. Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur Respir J. 2012;39(2):467–477. doi: 10.1183/09031936.00061911. [DOI] [PubMed] [Google Scholar]
- 6.Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40(1):31–39. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y, Namkung W, Nielson DW, Lee JW, Finkbeiner WE, Verkman AS. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na+ and Cl- channel function. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1131–L1140. doi: 10.1152/ajplung.00085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivença DV, Fonseca LL, Voit EO, Pinto FR. Thickness of the airway surface liquid layer in the lung is affected in cystic fibrosis by compromised synergistic regulation of the ENaC ion channel. J R Soc Interface. 2019;16(157):20190187. doi: 10.1098/rsif.2019.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rose V, Molloy K, Gohy S, Pilette C, Greene CM. Airway epithelium dysfunction in cystic fibrosis and COPD. Mediators Inflamm. 2018;2018:1309746. doi: 10.1155/2018/1309746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305(8):L530–L541. doi: 10.1152/ajplung.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Ferro TJ, Chu S. Cigarette smoke condensate inhibits ENaC -subunit expression in lung epithelial cells. Eur Respir J. 2007;30(4):633–642. doi: 10.1183/09031936.00014107. [DOI] [PubMed] [Google Scholar]
- 12.Downs CA, Kreiner LH, Trac DQ, Helms MN. Acute effects of cigarette smoke extract on alveolar epithelial sodium channel activity and lung fluid clearance. Am J Respir Cell Mol Biol. 2013;49(2):251–259. doi: 10.1165/rcmb.2012-0234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, Li H, Wu S, Zhao R, Du D, Ding Y, Nie H, Ji HL. Formaldehyde impairs transepithelial sodium transport. Sci Rep. 2016;6:35857. doi: 10.1038/srep35857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Chang J, Cui Y, Zhao R, Ding Y, Hou Y, Zhou Z, Ji HL, Nie H. Novel mechanisms for crotonaldehyde-induced lung edema. Oncotarget. 2017;8(48):83509–83522. doi: 10.18632/oncotarget.17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander NS, Blount A, Zhang S, Skinner D, Hicks SB, Chestnut M, Kebbel FA, Sorscher EJ, Woodworth BA. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122(6):1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188(11):1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert JA, Raju SV, Tang LP, McNicholas CM, Li Y, Courville CA, Farris RF, Coricor GE, Smoot LH, Mazur MM, Dransfield MT, Bolger GB, Rowe SM. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. 2014;50(3):549–558. doi: 10.1165/rcmb.2013-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid A, Baumlin N, Ivonnet P, Dennis JS, Campos M, Krick S, Salathe M. Roflumilast partially reverses smoke-induced mucociliary dysfunction. Respir Res. 2015;16:135. doi: 10.1186/s12931-015-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raju SV, Lin VY, Liu L, McNicholas CM, Karki S, Sloane PA, Tang L, Jackson PL, Wang W, Wilson L, Macon KJ, Mazur M, Kappes JC, DeLucas LJ, Barnes S, Kirk K, Tearney GJ, Rowe SM. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol. 2017;56(1):99–108. doi: 10.1165/rcmb.2016-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham A, Steel DM, Alton EW, Geddes DM. Second-messenger regulation of sodium transport in mammalian airway epithelia. J Physiol. 1992;453:475–491. doi: 10.1113/jphysiol.1992.sp019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arsenopoulou ZV, Taitzoglou IA, Molyvdas PA, Gourgoulianis KI, Hatzoglou C, Zarogiannis SG. Silver nanoparticles alter the permeability of sheep pleura and of sheep and human pleural mesothelial cell monolayers. Environ Toxicol Pharmacol. 2017;50:212–215. doi: 10.1016/j.etap.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17(1):57. doi: 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J, Chen Z, Zhao R, Nie HG, Ji HL. Ion transport mechanisms for smoke inhalation-injured airway epithelial barrier. Cell Biol Toxicol. 2020;36(6):571–589. doi: 10.1007/s10565-020-09545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai YJ, Liu BC, Wei SP, Chou CF, Wu MM, Song BL, Linck VA, Zou L, Zhang S, Li XQ, Zhang ZR, Ma HP. Depletion of cholesterol reduces ENaC activity by decreasing phosphatidylinositol-4,5-bisphosphate in microvilli. Cell Physiol Biochem. 2018;47(3):1051–1059. doi: 10.1159/000490170. [DOI] [PubMed] [Google Scholar]
- 25.Zhai YJ, Wu MM, Linck VA, Zou L, Yue Q, Wei SP, Song C, Zhang S, Williams CR, Song BL, Zhang ZR, Ma HP. Intracellular cholesterol stimulates ENaC by interacting with phosphatidylinositol-4,5-bisphosphate and mediates cyclosporine A-induced hypertension. Biochim Biophys Acta Mol Basis Dis. 2019;1865(7):1915–1924. doi: 10.1016/j.bbadis.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Brekman A, Walters MS, Tilley AE, Crystal RG. FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro. Am J Respir Cell Mol Biol. 2014;51(5):688–700. doi: 10.1165/rcmb.2013-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Li Y, Hua C, Jia P, Xing Y, Xue B, Tian X, Yang Y, Zhang J, Qiao L, Liu H, Li X, Xie F. Establishment of rapid risk assessment model for cigarette smoke extract exposure in chronic obstructive pulmonary disease. Toxicol Lett. 2019;316:10–19. doi: 10.1016/j.toxlet.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Henke K, Balcerzak I, Czepil E, Bem A, Piskorska E, Olszewska-Słonina D, Woźniak A, Szewczyk-Golec K, Hołyńska-Iwan I. 30-min exposure to tobacco smoke influences airway ion transport-an in vitro study. Curr Oncol. 2023;30(7):7007–7018. doi: 10.3390/curroncol30070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter CA, Misra M, Maronpot RR. Tracheal morphologic and protein alterations following short-term cigarette mainstream smoke exposure to rats. J Toxicol Pathol. 2012;25(3):201–207. doi: 10.1293/tox.25.201. [DOI] [PMC free article] [PubMed] [Google Scholar]