Abstract
Background/Aim
Non-B non-Hodgkin lymphomas (NHL) represent over 30 T/NK lymphoma types. The majority of them are T-cell lymphoblastic lymphomas (TLL) and anaplastic large cell lymphomas (ALCL). Other rare non-B NHLs represent a diverse group of neoplasms, usually excluded from clinical trials. This study analyzed outcomes in pediatric patients with non-B NHL in a single oncology center with particular emphasis on patients with rare NHLs.
Patients and Methods
We retrospectively analyzed data from patients <18 years with newly diagnosed non-B NHL treated at the Department of Pediatric Hematology and Oncology in Bydgoszcz between 2002 and 2022. The probability of 5-year overall survival (pOS) and event-free survival (pEFS) were calculated for the entire cohort and patients with TLL and ALCL. The clinical course for patients with rare non-B NHL was described in detail.
Results
Twenty-six children were eligible for analysis. Fourteen patients were diagnosed with ALCL, nine with TLL, and three with rare NHL types (subcutaneous panniculitis-like T-cell lymphoma, extranodal NK/T-cell lymphoma and hydroa vacciniforme-like lympho-proliferative disease associated lymphoma). For the entire group, the 5-year pOS was 83.7% and the 5-year pEFS was 72.4%. For TLL and ALCL, the outcomes were comparable with those achieved in clinical trials. Patients with rare NHL were treated according to individualized therapy recommendations based on physicians’ expertise and available case report descriptions.
Conclusion
There is a lack of knowledge on optimal therapeutic strategies for rare NHLs. It is crucial to create trials dedicated to uncommon NHLs and establish therapy guidelines for these patients.
Keywords: Non-Hodgkin lymphoma, non-B NHL, children, rare malignancies
Non-Hodgkin lymphomas (NHL) represent a heterogeneous group of lymphoid malignancies. According to the fifth edition of the World Health Organization Classification, non-B NHL comprises over 30 T/NK lymphoid proliferation and lymphoma types, many with subtypes (1). However, in the pediatric population, approximately 90% of patients with non-B NHL are diagnosed with T-cell lymphoblastic lymphomas (TLL) and anaplastic large cell lymphomas (ALCL) (2,3). The remaining 10% represent a diverse group of neoplasms with very low incidences, characterized by limited understanding of their pathogenesis and biology. This has resulted in a lack of standardized diagnostic and therapeutic approaches, as well as insufficient knowledge of long-term outcomes (4,5).
In the Children's Oncology Group's (COG) 2023 research blueprint, the primary goal of the COG Committee was to provide optimal cures for all children with NHL (6). Currently, for rare peripheral T-cell NHL, the 5-year event-free survival (EFS) ranges from 47±7% to 61±11% and the 5-year overall survival (OS) ranges from 56±7% to 65±11% (7,8). Priorities in NHL therapy include developing clinical trials for rare NHL types and expanding studies on their biology to facilitate targeted and personalized therapies (5,6,9). Although further studies are necessary, adhering to specific clinical protocols can be challenging, particularly for oncology centers with limited funding or those located in developing countries.
In this study we retrospectively analyze outcomes in pediatric patients diagnosed with non-B NHL in a single oncology center with particular emphasis on the challenges and opportunities, that pediatric oncologists face in therapy of children with rare tumors.
Patients and Methods
Design of the study. In this study, we retrospectively analyzed outcomes for pediatric non-B NHL patients, who received treatment at a single oncology center in Poland between 2002 and 2022, with particular emphasis on patients with rare types of lymphomas. The study was approved by Ethics Committee of Collegium Medicum, Nicolas Copernicus University, Bydgoszcz (KB 577/2021).
Patients. The medical records of patients diagnosed with non-B NHL at the Department of Pediatric Hematology and Oncology in Bydgoszcz were analyzed. Patients <18 years with newly diagnosed non-B NHL were eligible for the study. Children with missing or incomplete data were excluded from the analysis.
Diagnosis. The diagnosis was established on the basis of histological analysis from a tissue sample and confirmed by the national reference laboratory. Staging workup included medical interview information, laboratory test results (complete blood count, lactate dehydrogenase (LDH) activity, cerebrospinal fluid (CSF) analysis) and imaging test results (ultrasonography of the abdomen and peripheral lymph nodes, computed tomography (CT) of the neck, thorax, abdomen and pelvis).
Definitions. Progression was defined as reappearance or increase in size of residual lesions or appearance of new manifestations during treatment or up to three months after completion of the treatment. Relapse was defined as reappearance or appearance of new manifestations more than three months after therapy completion. Bone marrow progression/relapse was diagnosed in case of >25% lymphoma cells in the bone marrow. Central nervous system (CNS) progression/relapse was diagnosed, if lymphoma cells were present in the CNS with a cell count of ≥5/µl or in case of appearance of an intra-cerebral tumor. The diagnosis of relapse and progression were proved by biopsy and histology.
An event was defined as death due to any cause, progression, relapse, or secondary malignancy. Overall survival was calculated as the time in years from the first day of treatment to death of any cause or last follow-up contact for alive patients. Event free survival was calculated as the time in years from the first day of treatment to the event.
Statistical analysis and language corrections. The probability of OS (pOS) and the probability of EFS (pEFS) were calculated using the Kaplan–Meier method and compared using the log-rank test. Statistical calculations were performed using MedCalc 20.100 software (MedCalc Software, Mariakerke, Belgium). The language was corrected using the TRINKA grammar checker on the www.trinka.ai website.
Results
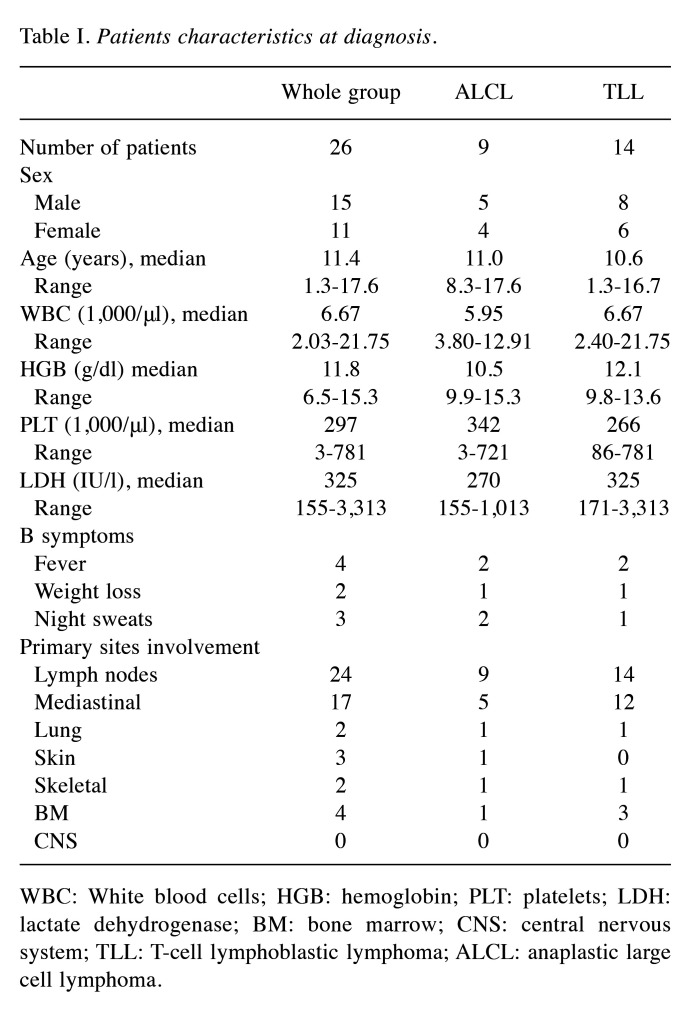
Demographics. During the analyzed period, 32 pediatric patients were treated for non-B NHL. Six children were excluded from the analysis because of incomplete or missing data. The median age at diagnosis was 11.0 years (range=1.3-17.8 years). One patient was diagnosed with immunodeficiency before NHL diagnosis, and none was diagnosed with cancer predisposition syndrome. Patient characteristic is shown in Table I.
Table I. Patients characteristics at diagnosis.
WBC: White blood cells; HGB: hemoglobin; PLT: platelets; LDH: lactate dehydrogenase; BM: bone marrow; CNS: central nervous system; TLL: T-cell lymphoblastic lymphoma; ALCL: anaplastic large cell lymphoma.
Diagnosis and therapy. In the analyzed cohort, the following histological types were diagnosed: TLL (n=14), ALCL (n=9), subcutaneous panniculitis-like T-cell lymphoma (SPTCL) (n=1), extranodal NK/T-cell lymphoma (ENKL) (n=1), and hydroa vacciniforme-like lymphoproliferative disease associated lymphoma (HV-LPDL) (n=1).
Patients treated for TLL and ALCL received therapy according to standardized therapy protocols. Thirteen patients diagnosed with TLL were treated according to the EURO-LB02 protocol and one with the LBL 2018 protocol (10). All patients diagnosed with ALCL were treated according to the ALCL99 protocol (11). Among the analyzed group three patients were diagnosed with rare types of NHL (SPTCL, ENKL and HV-LPDL).
A girl diagnosed with SPTCL was treated with six cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. She presented with laboratory and clinical symptoms of hemophagocytic lymphohistiocytosis at diagnosis, which resolved after the first treatment course. She presented also a significant decrease of subcutaneous lymphoma lesions after the two first cycles of chemotherapy and a complete remission after the fourth cycle. She received two additional consolidation cycles of CHOP regimens and has remained free of disease during the 16-year follow-up.
A boy diagnosed with ENKL presented with lymphoma lesions in the nasal structures, skin of the cheeks, and upper lip at diagnosis. He underwent four cycles of CHOP therapy, resulting in a significant reduction in tumor lesions. The boy did not agree to local radiotherapy as a consolidation therapy; therefore, he received bexarotene as a maintenance therapy. After the completion of maintenance treatment, magnetic resonance imaging (MRI) results showed enhancement at the primary involved sites. He received an additional three cycles of CHOP chemotherapy, achieving complete remission as confirmed by positron emission tomography scan results. However, 12 months post-therapy completion, he was diagnosed with relapse and died nine months later due to disease progression.
A case of a boy with common variable immunodeficiency complicated by systemic lymphoma, developed from hydroa vacciniforme-like lymphoproliferative disorder was described previously (12). In this case, systemic chemotherapy combined with antiviral drugs was unsuccessful. Finally, he received treatment with third-party donor Epstein Barr virus-specific cytotoxic T-cells (tabelecleucel), followed by allogenic hematopoietic stem cell transplantation (HSCT). He remains in lymphoma remission up to the date of the article publication.
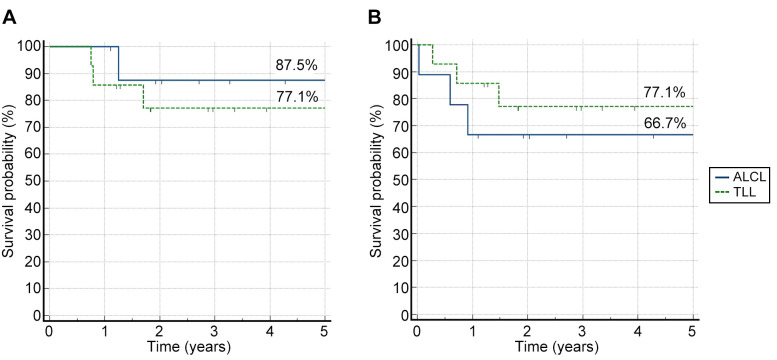
Outcomes. For the entire group, the 5-year pOS was 83.7% and the 5-year pEFS was 72.4%. For TLL and ALCL, the 5-year pOS and pEFS were comparable to those achieved in clinical trials (Figure 1) (10,11).
Figure 1. Five-year probability of overall survival and probability of event-free survival for non-Hodgkin lymphoma subtypes. A) The 5-year probability of overall survival for T-cell lymphoblastic lymphoma (TLL) and anaplastic large cell lymphoma (ALCL); B) The 5-year probability of event-free survival for TLL and ALCL.
Three patients presented with progression during the frontline therapy. Among them one was diagnosed with ALCL and two with TLL. The patient with progressed ALCL was successfully treated with a second cycle of induction therapy according to the ALCL99 protocol. Two patients with progressed TLL were treated with salvage chemotherapy, but both subsequently died due to disease progression.
Three patients were diagnosed with relapse. The patient with relapsed ENKL was described above. Another two patients with relapsed disease were primarily diagnosed with ALCL. Both presented with relapse in peripheral lymph nodes within the first year after completion of frontline therapy. The first patient, diagnosed in 2005, received salvage chemotherapy. However, the treatment was unsuccessful, and he died from disease progression. The second patient with relapsed ALCL, treated in 2020, received salvage chemotherapy combined with an anti-CD30 antibody-drug conjugate brentuximab vedotin (BV), followed by allogenic hematopoietic stem cell transplantation (allo-HSCT). He has remained free of disease up to the date of the article publication. Five patients died, and the most common reason for death was disease progression (n=4, 80.0%). One patient died because of treatment toxicity (infection).
Discussion
This study is a comprehensive, single-center analysis of therapeutic outcomes in pediatric patients with non-B-cell non-Hodgkin lymphomas. Although the analysis includes relatively small number of patients, it reflects the challenges in the treatment of children with non-B NHL. According to the Polish Central Statistical Office’s annual report from 2021, there are approximately 1100 new cases of pediatric cancer diagnosed in Poland each year (13). Non-Hodgkin lymphomas represent 7% of them, with an annual incidence 0.9 per 100,000 children, what makes the whole group of NHL an ultrarare disease (13,14). In our department, over a period of 20 years, only 32 patients were diagnosed with non-B NHL, which gives a number of little higher than 1.5 patient per year in a region inhabited by 2.09 million people (15).
TLL and ALCL constitute the vast majority of pediatric non-B NHL cases (2,3). Despite extensive research on prognostic factors in TLL, only disease stage has proven to be applicable in risk group stratification. There is also a lack of new drugs or other therapeutic options for pediatric patients with TLL (16). The opposite situation is observed in the therapy of ALCL, with multiple therapeutic options emerging in recent years. Although the 5-year OS in pediatric patients with ALCL has reached over 90%, the 5-year EFS remains at an unacceptable level of 70%, despite several modifications in frontline treatment (17). Recent advances in understanding ALCL biology resulted in the introduction of BV to standard chemotherapy, primarily in refractory or relapsed ALCL, and subsequently as a part of frontline therapy (18-20). Another therapeutic option for patients with relapsed or refractory ALK-positive ALCL are ALK inhibitors. Although their role in children's therapy is still under investigation, they show promising results in adult patients, particularly in refractory ALCL (17,20). In relapsed disease, allo-HSCT remains the treatment of choice (21,22). However, the high incidence of treatment related mortality raises questions about the potential role of alternative treatments (21).
As TLL and ALCL represent the majority of pediatric non-B NHL cases, it is not surprising that most efforts are directed toward the therapy development for these patients (2,16,18). Moreover, pediatric patients with rare types of non-B NHL are generally excluded from clinical trials (4,23). In a single oncology center, treatment decisions are usually made by individual physicians or therapeutic teams, often relying on limited available data on outcomes derived from case reports. Many patients receive CHOP-based regimens as a potentially efficient therapeutic option, but the results vary according to the NHL type (4,23,24). The challenges in treatment of rare types NHL have been addressed by COG by initiating the Rare and Cutaneous NHL registry (COG protocol ANHL 04B1) (5). The first report from the registry published in 2016 highlighted the lack of treatment guidelines and variety of regimens are administered in therapy of patients with rare non-B NHL types, starting from observation or surgery alone to HSCT (5).
Other limitations of therapeutic options for NHL patients are funding issues, particularly for newly discovered or experimental therapies (25). Our analysis demonstrated how the oncology center's financial status influenced changes in therapeutic approaches. The first two patients with rare types of NHL (SPTCL and ENKL) were treated in the early 2000s, a period marked by limited funding in our country. Therefore, they received only CHOP-based regimens, resulting in varied outcomes. The last patient with HV-LPDL was treated in the early 2020s, when Poland become a developed country that directly impacted funding opportunities for oncology centers, including our Department. Therefore, the patient was able to receive innovative treatment, which proved to be successful in his case. A similar situation can be observed in the therapy of relapsed ALCL patients. The first patient, treated in 2005, received only standard salvage chemotherapy and subsequently died due to disease progression. In contrast, the second patient, diagnosed with relapse in 2020, was treated with chemotherapy combined with BV and followed by allo-HSCT, which proved to be successful in his case.
Despite funding concerns, we must also consider, that smaller institutions may be discouraged from participating in large studies due to the disease's rarity (25). Therefore, we need to provide therapy recommendations adapted to the local capabilities. Addressing those needs in the United States and Canada, Priya Mayahan et al. described the multidisciplinary monthly Rare Tumor Tele Tumor Boards created in 2018 (26). During these meetings, pediatric rare solid tumor cases were discussed to determine the best diagnostic and therapeutic options (26). The multidisciplinary approach facilitates therapeutic approaches including not only systemic chemotherapy or targeted treatment but also surgery, radiation, and the consideration of other non-medical aspects (25-28). A similar solution could be applied to pediatric patients with rare NHL types, on a local, national, or international level. Besides providing access to multidisciplinary consultation, this approach offers patients and their families the chance to receive treatment close to home, thereby reducing the financial and personal costs associated with treatment (29).
In conclusion, non-B NHL represent a heterogeneous group of rare diseases with varied therapeutic approaches and outcomes. Although we observe a significant progress in therapy for ALCL, results for TLL treatment remain unsatisfactory. Moreover, many rare non-B NHL types are excluded from clinical trials and further research. In these cases, treatment decisions often rely on individual center expertise and available case reports. However, there is a lack of comprehensive literature and knowledge on optimal therapeutic strategies.
Many individual oncology centers feel the pressure to demonstrate outcomes comparable to those achieved in international trials. However, their unique value lies in the deep understanding of challenges they face every day. While celebrating successes is important, it is equally crucial to identify institutional limitations, to create new directions for therapy development.
Conflicts of Interest
The Authors have no competing interests to declare that are relevant to the content of this article.
Authors’ Contributions
Joanna S. and Jan S. contributed to the study conception and design. Material preparation, data collection and analysis were performed by Joanna S., Anna J. and Paweł T. The first draft of the manuscript was written by Joanna S. and all authors commented on previous versions of the manuscript. All Authors read and approved the final manuscript.
Acknowledgements
The Authors would like to thank the physicians, nurses, and other team members from the Department of Pediatric Hematology and Oncology in Bydgoszcz for their continuous excellent care of children with malignancies.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720–1748. doi: 10.1038/s41375-022-01620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minard-Colin V, Brugières L, Reiter A, Cairo MS, Gross TG, Woessmann W, Burkhardt B, Sandlund JT, Williams D, Pillon M, Horibe K, Auperin A, Le Deley MC, Zimmerman M, Perkins SL, Raphael M, Lamant L, Klapper W, Mussolin L, Poirel HA, Macintyre E, Damm-Welk C, Rosolen A, Patte C. Non-Hodgkin lymphoma in children and adolescents: Progress through effective collaboration, current knowledge, and challenges Ahead. J Clin Oncol. 2015;33(27):2963–2974. doi: 10.1200/JCO.2014.59.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandlund JT. Non-Hodgkin lymphoma in children. Curr Hematol Malig Rep. 2015;10(3):237–243. doi: 10.1007/s11899-015-0277-y. [DOI] [PubMed] [Google Scholar]
- 4.Xavier AC, Suzuki R, Attarbaschi A. Diagnosis and management of rare paediatric Non-Hodgkin lymphoma. Best Pract Res Clin Haematol. 2023;36(1):101440. doi: 10.1016/j.beha.2023.101440. [DOI] [PubMed] [Google Scholar]
- 5.O’Suoji C, Welch JJ, Perkins SL, Smith LM, Weitzman S, Simko SJ, Galardy PJ, Bollard CM, Gross TG, Termuhlen AM. Rare pediatric Non-Hodgkin lymphomas: a report from Children’s Oncology Group study ANHL 04B1. Pediatr Blood Cancer. 2016;63(5):794–800. doi: 10.1002/pbc.25881. [DOI] [PubMed] [Google Scholar]
- 6.El-Mallawany NK, Alexander S, Fluchel M, Hayashi RJ, Lowe EJ, Giulino-Roth L, Wistinghausen B, Hermiston M, Allen CE, COG NHL Committee Children’s Oncology Group’s 2023 blueprint for research: Non-Hodgkin lymphoma. Pediatr Blood Cancer. 2023;70 Suppl 6(Suppl 6):e30565. doi: 10.1002/pbc.30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kontny U, Oschlies I, Woessmann W, Burkhardt B, Lisfeld J, Salzburg J, Janda A, Attarbaschi A, Niggli F, Zimmermann M, Reiter A, Klapper W. Non-anaplastic peripheral T-cell lymphoma in children and adolescents – a retrospective analysis of the NHL-BFM study group. Br J Haematol. 2015;168(6):835–844. doi: 10.1111/bjh.13216. [DOI] [PubMed] [Google Scholar]
- 8.Mellgren K, Attarbaschi A, Abla O, Alexander S, Bomken S, Bubanska E, Chiang A, Csóka M, Fedorova A, Kabickova E, Kapuscinska-Kemblowska L, Kobayashi R, Krenova Z, Meyer-Wentrup F, Miakova N, Pillon M, Plat G, Uyttebroeck A, Williams D, Wróbel G, Kontny U, European Intergroup for Childhood Non-Hodgkin Lymphoma (EICNHL) and the international Berlin-Frankfurt-Münster (i-BFM) Group Non-anaplastic peripheral T cell lymphoma in children and adolescents—an international review of 143 cases. Ann Hematol. 2016;95(8):1295–1305. doi: 10.1007/s00277-016-2722-y. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo M, Hashimoto K, Kogo R, Jiromaru R, Hongo T, Manako T, Nakagawa T. Utility of precision oncology using cancer genomic profiling for head and neck malignancies. In Vivo. 2023;37(5):2147–2154. doi: 10.21873/invivo.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landmann E, Burkhardt B, Zimmermann M, Meyer U, Woessmann W, Klapper W, Wrobel G, Rosolen A, Pillon M, Escherich G, Attarbaschi A, Beishuizen A, Mellgren K, Wynn R, Ratei R, Plesa A, Schrappe M, Reiter A, Bergeron C, Patte C, Bertrand Y. Results and conclusions of the European Intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica. 2017;102(12):2086–2096. doi: 10.3324/haematol.2015.139162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mussolin L, Le Deley MC, Carraro E, Damm-Welk C, Attarbaschi A, Williams D, Burke A, Horibe K, Nakazawa A, Wrobel G, Mann G, Csóka M, Uyttebroeck A, Fernández-Delgado Cerdá RF, Beishuizen A, Mellgren K, Burkhardt B, Klapper W, Turner SD, D’Amore ESG, Lamant L, Reiter A, Woessmann W, Brugières L, Pillon MPOBOTEIFCNL Prognostic factors in childhood anaplastic large cell lymphoma: Long term results of the international alcl99 trial. Cancers. 2020;12(10):2747. doi: 10.3390/cancers12102747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grześk E, Kołtan S, Dąbrowska A, Urbańczyk A, Małdyk J, Małkowski B, Bogiel T, Dębski R, Czyżewski K, Wysocki M, Styczyński J. Case report: Cellular therapy for hydroa vacciniforme-like lymphoproliferative disorder in pediatric common variable immunodeficiency with chronic active Epstein-Barr virus infection. Front Immunol. 2022;13:915986. doi: 10.3389/fimmu.2022.915986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didkowska JWU, Barańska K, Miklewska M, Michałek I, Olasek P. Cancer in Poland in 2021. Polish National Cancer Registry, pp. 25-28, 2023. Available at: https://onkologia.org.pl/sites/default/files/publications/2024-01/0_krn-2023-book-2024-01-22.pdf. [Last accessed on May 31, 2024]
- 14.Potrykowska A, Strzelecki Z, Szymborski J, Witkowski J. Zachorowalność i umieralność na nowotwory a sytuacja demograficzna polski. Rządowa Rada Ludnościowa, 2014. Available at: https://bip.stat.gov.pl/download/gfx/bip/pl/defaultstronaopisowa/805/1/1/zachorowalnosc_na_nowotwory.pdf. [Last accessed on May 31, 2024]
- 15.Statystyczny GU. Stan i struktura demograficzna ludności oraz liczba budynków i mieszkań w województwie kujawsko-pomorskim – wyniki ostateczne NSP 2021. Warszawa, 2022. Available at: https://bydgoszcz.stat.gov.pl/opracowania-biezace/opracowania-sygnalne/narodowy-spis-powszechny-2021/stan-i-struktura-demograficzna-ludnosci-oraz-liczba-budynkow-i-mieszkan-w-wojewodztwie-kujawsko-pomorskim-wyniki-ostateczne-nsp-2021,4,1.html. [Last accessed on May 31, 2024]
- 16.Burkhardt B, Hermiston ML. Lymphoblastic lymphoma in children and adolescents: reviewof current challenges and future opportunities. Br J Haematol. 2019;185(6):1158–1170. doi: 10.1111/bjh.15793. [DOI] [PubMed] [Google Scholar]
- 17.Larose H, Burke GAA, Lowe EJ, Turner SD. From bench to bedside: the past, present and future of therapy for systemic paediatric ALCL, ALK+ Br J Haematol. 2019;185(6):1043–1054. doi: 10.1111/bjh.15763. [DOI] [PubMed] [Google Scholar]
- 18.Lowe EJ, Reilly AF, Lim MS, Gross TG, Saguilig L, Barkauskas DA, Wu R, Alexander S, Bollard CM. Brentuximab vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: results of COG trial ANHL12P1. Blood. 2021;137(26):3595–3603. doi: 10.1182/blood.2020009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy P, Harford J, O’Marcaigh A, Malone A, Evans P, Sills A, Storey L, Rooney S, Betts D, O’Sullivan MJ, McDermott M, Bond J, Trinquand A, Smith OP. Ongoing excellent outcomes with reduced toxicities following integration of molecular targeted therapies in pediatric anaplastic large cell lymphoma. Leuk Lymphoma. 2021;62(8):1995–1999. doi: 10.1080/10428194.2021.1894644. [DOI] [PubMed] [Google Scholar]
- 20.Prokoph N, Larose H, Lim MS, Burke GAA, Turner SD. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): Current standard and beyond. Cancers (Basel) 2018;10(4):99. doi: 10.3390/cancers10040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strullu M, Thomas C, Le Deley MC, Chevance A, Kanold J, Bertrand Y, Jubert C, Dalle JH, Paillard C, Baruchel A, Lamant L, Michel G, Brugières L. Hematopoietic stem cell transplantation in relapsed ALK+ anaplastic large cell lymphoma of children and adolescent: A study on behalf of the SFCE and SFGM-TC. Blood. 2013;122(21):2120. doi: 10.1038/bmt.2015.57. [DOI] [PubMed] [Google Scholar]
- 22.Jung EH, Shin DY, Hong J, Kim I, Yoon SS, Koh Y, Byun JM. Identification of an optimal population for allogeneic hematopoietic stem cell transplantation in patients with mature T and NK cell neoplasms. In Vivo. 2021;35(4):2379–2390. doi: 10.21873/invivo.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceppi F, Pope E, Ngan B, Abla O. Primary cutaneous lymphomas in children and adolescents. Pediatr Blood Cancer. 2016;63(11):1886–1894. doi: 10.1002/pbc.26076. [DOI] [PubMed] [Google Scholar]
- 24.Oschlies I, Simonitsch-Klupp I, Maldyk J, Konovalov D, Abramov D, Myakova N, Lisfeld J, Attarbaschi A, Kontny U, Woessmann W, Klapper W. Subcutaneous panniculitis-like T-cell lymphoma in children: A detailed clinicopathological description of 11 multifocal cases with a high frequency of haemophagocytic syndrome. Br J Dermatol. 2015;172(3):793–797. doi: 10.1111/bjd.13440. [DOI] [PubMed] [Google Scholar]
- 25.Schultz KAP, Chintagumpala M, Piao J, Chen KS, Shah R, Gartrell RD, Christison-Lagay E, Pashnakar F, Berry JL, O’Neill AF, Vasta LM, Flynn A, Mitchell SG, Seynnaeve BK, Rosenblum J, Potter SL, Kamihara J, Rodriguez-Galindo C, Hawkins DS, Laetsch TW. Rare tumors: Opportunities and challenges from the Children’s Oncology Group perspective. EJC Paediatr Oncol. 2023;2:100024. doi: 10.1016/j.ejcped.2023.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan P, Navai S, Smith V, Potter S, Venkatramani R. Advancing the care of pediatric rare solid tumors: a virtual rare tumor board. Pediatr Hematol Oncol. 2022;39(7):678–680. doi: 10.1080/08880018.2022.2042436. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa T, Someya M, Tsuchiya T, Kitagawa M, Fukushima Y, Gocho T, Mafune S, Okuda R, Kaguchi J, Ohguro A, Kamiyama R, Ashina A, Toshima Y, Hirohashi Y, Torigoe T, Sakata KI. Identification and quantification of radiotherapy-related protein expression in cancer tissues using the Qupath software and prediction of treatment response. In Vivo. 2024;38(3):1470–1476. doi: 10.21873/invivo.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eismann J, Elsayad K, Rolf D, Sarif I, Wardelmann E, BerssenbrÜgge H, Lenz G, Eich HT. Intensity-modulated radiotherapy in patients with aggressive extranodal Non-Hodgkin lymphoma of the head and neck. Anticancer Res. 2021;41(10):5131–5135. doi: 10.21873/anticanres.15330. [DOI] [PubMed] [Google Scholar]
- 29.Adashek JJ, Kurzrock R. Home-run trials for rare cancers: giving the right drug(s) to the right patients at the right time and in the right place. NPJ Precis Oncol. 2023;7(1):129. doi: 10.1038/s41698-023-00487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]