Abstract
Background/Aim
Esophagectomy for esophageal carcinoma (EC) is known to lead to deterioration of respiratory function (RF) due to thoracotomy and mediastinal lymph node dissection. This study aimed to evaluate the impact of transmediastinal esophagectomy (TME) on pulmonary function.
Patients and Methods
We retrospectively analyzed the data of 102 patients with EC who underwent transthoracic esophagectomy (TTE) or TME and underwent RF tests within three months postoperatively at Kyoto Prefectural University of Medicine between 2014 and 2022. Perioperative pulmonary functions were evaluated based on vital capacity (VC) and forced expiratory volume in one second (FEV1.0).
Results
Among 102 patients undergoing esophagectomy, 12 (11.8%) patients were included in the TTE group, and the remaining 90 (88.2%) patients were included in the TME group. Neoadjuvant treatments were significantly more common in the TTE group (p=0.011), with more advanced tumor stages (p=0.017). The TME group had significantly lower estimated blood loss (p=0.015). RF after esophagectomy showed a decrease in VC, and VC of predicted (%VC). The decrease rate in VC, %VC, and FEV1.0 was significantly greater in the TTE group than in the TME group.
Conclusion
TME is a surgical procedure with a less severe postoperative decline in RF than TTE.
Keywords: Esophageal cancer, esophagectomy, respiratory function
Esophageal carcinoma (EC) is the sixth most common cause of cancer-associated death worldwide despite improvements in survival outcomes due to advances in multimodal treatment strategies (1). Esophagectomy is characterized by its high incidence of postoperative morbidity and deterioration in respiratory function (RF) due to thoracotomy and mediastinal lymph node (LN) dissection (2), with decreased vital capacity (VC) (3), low forced expiratory volume in one second (FEV1.0) (4), and reduced lung diffusing capacity (5) reportedly associated with postoperative pulmonary complications. Therefore, preoperative evaluation of pulmonary functions using spirometry testing is widely employed to select surgical candidates and predict the development of postoperative pulmonary complications (6,7). Although histopathological findings are the most powerful prognostic determinants for patients with EC (8), assessment of preoperative physiological status is crucial for optimizing clinical outcomes in patients with EC undergoing esophagectomy, which is a highly invasive procedure involving considerable morbidity (9).
Recently, minimally invasive esophagectomy (MIE) was shown to be associated with a lower frequency of postoperative complications compared with conventional open esophagectomy, and a trend toward MIE is observable worldwide (10). Esophagectomy with transcervical and transhiatal mediastinal LN dissection, transmediastinal esophagectomy (TME), was recently developed as radical esophagectomy without thoracotomy for EC, with the significant benefit of reducing pulmonary complications compared with transthoracic esophagectomy (TTE) (11). Although TME can be more safely applied to older patients, patients with comorbidities, and those who have difficulty opening the chest due to adhesions or poor pulmonary function, its impact on postoperative RF remains unclear. This study aimed to evaluate the impact of TME on pulmonary function.
Patients and Methods
Patients. This study was conducted per the ethical principles of Kyoto Prefectural University of Medicine and the Declaration of Helsinki. Informed consent was obtained from all participants through our hospital website on the basis of opt-outs. The Ethical Review Board of the Kyoto Prefectural University of Medicine approved the experimental protocol (ERB-C-1414-1). This study included 542 patients who underwent curative esophagectomy for EC at Kyoto Prefectural University of Medicine Hospital in Japan between January 2014 and December 2022. Of these, 102 patients with EC who underwent TTE or TME and underwent RF tests within three months postoperatively after surgery were enrolled, and the data were retrospectively analyzed.
Clinical and pathological staging was performed using the 8th edition of the Union for International Cancer Control tumor, nodes, and metastases (TNM) staging (12). The patients underwent esophagectomy with LN dissection with or without neoadjuvant treatment following the guidelines of the Japan Esophageal Society (13). Postoperative pulmonary complications and anastomotic leak defined as Grade 2 or higher according to the Clavien-Dindo classification were determined as positive. An otolaryngologist evaluated postoperative recurrent laryngeal nerve paralysis.
Evaluation of perioperative RF. Perioperative pulmonary function tests were performed using spirometry. In all cases, spirometry was performed in a sitting position using a FUDAC-77 device (Fukuda Denshi, Tokyo, Japan) in our RF laboratory. Preoperative spirometry was performed during the patient’s first visit to the outpatient clinic. For patients who received preoperative treatment, the test was performed before preoperative treatment. Postoperative spirometry was performed at the first outpatient visit after discharge. The median time to postoperative RF testing was 43 days, with a range of 24-90 days. VC of predicted (%VC) and FEV1.0/forced VC ratio (FEV1.0%) were used to assess ventilatory function.
Surgical procedure. For TME, patients were placed in the supine position with both arms fixed to the trunk and both lower limbs abducted. Esophagectomy with radical lymphadenectomy was performed using transcervical and transhiatal approaches with single-port mediastinoscopy and laparoscopy. The details of the surgeon’s position, skin incision, port placement, and procedures were previously described (11). For TTE, patients were placed in the left lateral decubitus position, and the thoracic procedure was performed with an open approach.
Statistical analysis. Data were analyzed using JMP version 10 (ASA Institute, Cary, NC, USA). Chi-square and Fisher’s exact probability tests were used to compare categorical variables between groups, whereas Student’s t-tests and Mann–Whitney U-tests were used for unpaired continuous data. Survival curves were estimated using the Kaplan–Meier method, and differences were evaluated using the log-rank test. Statistical significance was set at p<0.05.
Results
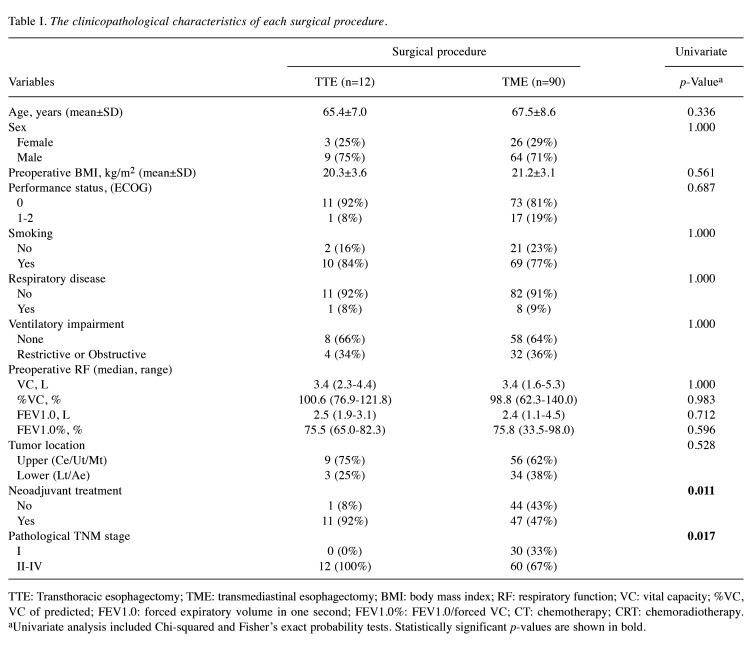
Differences in clinicopathologic characteristics and preoperative RF in TTE and TME. Table I shows the differences in the clinicopathological characteristics and preoperative RF between the surgical procedures. Twelve (11.8%) patients were included in the TTE group, and the remaining 90 (88.2%) patients were included in the TME group. Univariate analysis revealed that neoadjuvant treatments were significantly more common in the TTE group than in the TME group (p=0.011), with a more frequent advanced tumor stage at the diagnosis of EC (p=0.017). There were no correlations between the two groups regarding age, sex, body mass index (BMI), performance status, tumor location, smoking status, incidence of respiratory disease, or ventilatory impairment. The median RF before esophagectomy was not significantly different between the TTE and TME groups.
Table I. The clinicopathological characteristics of each surgical procedure.
TTE: Transthoracic esophagectomy; TME: transmediastinal esophagectomy; BMI: body mass index; RF: respiratory function; VC: vital capacity; %VC, VC of predicted; FEV1.0: forced expiratory volume in one second; FEV1.0%: FEV1.0/forced VC; CT: chemotherapy; CRT: chemoradiotherapy. aUnivariate analysis included Chi-squared and Fisher’s exact probability tests. Statistically significant p-values are shown in bold.
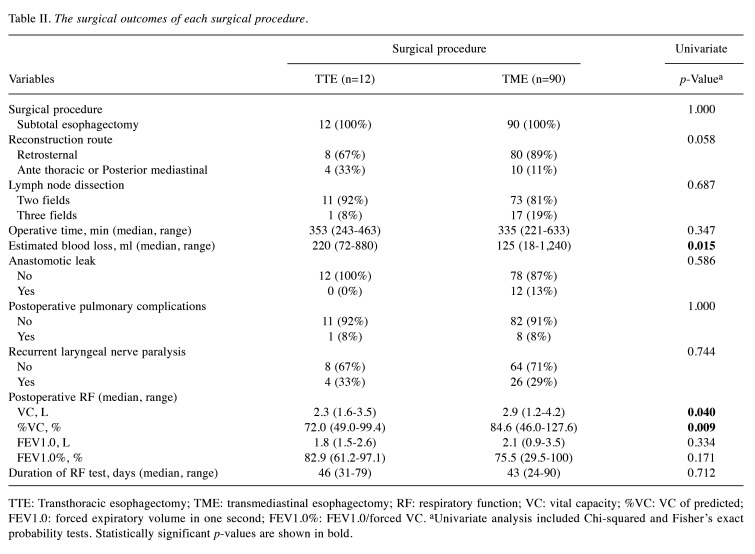
Differences in surgical characteristics and postoperative RF in TTE and TME. Table II shows the differences in surgical characteristics and postoperative RF between the surgical procedures. All patients underwent subtotal esophagectomy. The retrosternal route was more often the reconstructive route in the TME group (p=0.005). Estimated blood loss was significantly lower in the TME group (p=0.015). The two groups did not differ significantly regarding lymph dissection, operative time, or postoperative complications. Postoperative RF was significantly lower in the TTE group for VC (p=0.040) and %VC (p=0.009); FEV1.0 and FEV1.0% were not significantly different between the two groups. The median time to RF after esophagectomy was not significantly different between the TTE and TME groups.
Table II. The surgical outcomes of each surgical procedure.
TTE: Transthoracic esophagectomy; TME: transmediastinal esophagectomy; RF: respiratory function; VC: vital capacity; %VC: VC of predicted; FEV1.0: forced expiratory volume in one second; FEV1.0%: FEV1.0/forced VC. aUnivariate analysis included Chi-squared and Fisher’s exact probability tests. Statistically significant p-values are shown in bold.
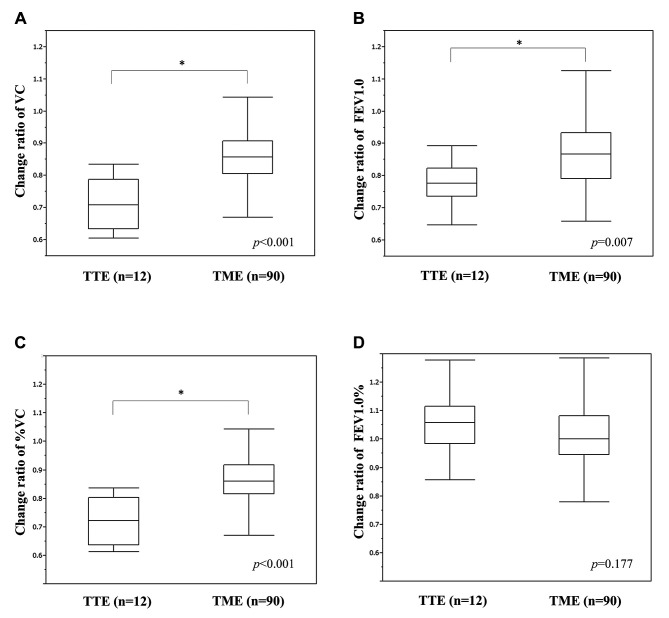
Impact of operative procedure on RF after esophagectomy. Figure 1 shows the change ratio in perioperative RF in patients stratified by group. RF after esophagectomy showed a decrease in VC, %VC, and FEV1.0. Comparing the two groups, the decrease in VC, %VC, and FEV1.0 was statistically significantly greater in the TTE group than in the TME group. The difference in FEV1.0% between the two groups was not significant, with no postoperative functional decline.
Figure 1. Perioperative changes in respiratory function (RF) after esophageal carcinoma surgery according to surgical techniques. Patients were classified into transthoracic esophagectomy (TTE) and transmediastinal esophagectomy (TME) groups and evaluated by calculating the change ratio of: A) vital capacity (VC) with postoperative VC/preoperative VC; B) %VC with postoperative %VC/preoperative %VC; C) forced expiratory volume in one second (FEV1.0) with postoperative FEV1.0/preoperative FEV1.0; and D) FEV1.0% with postoperative FEV1.0%/preoperative FEv1.0%.*p<0.05.
Discussion
This study revealed several novel findings, including that TME is associated with a less severe decline in postoperative respiratory function compared to TTE. The presence of impaired RF has been reported to be associated with mortality in the general population (14) and with decreased survival in patients undergoing surgery for upper gastrointestinal malignancies (15-17). The degree of decline in RF after esophagectomy varies significantly depending on surgical technique, and it has been reported that respiratory deterioration persists even five years postoperatively (18,19). Functional destruction of respiratory muscles by thoracotomy or laparotomy leads to a persistent deterioration of postoperative RF, which was also evident in this study in the TTE group. MIE by right thoracoscopic approach resulted in a smaller thoracic wound, avoided chest wall fractures, and few pulmonary manipulations compared to TTE (20), but decreased postoperative RF (19). In contrast, transhiatal esophagectomy (THE) has been reported to have a mild postoperative RF decline, thought to be due to the absence of a thoracotomy and preservation of respiratory muscles (19). However, as a disadvantage in oncologic relevance in EC, the extent of mediastinal LN dissection is limited in THE (21). TME in this study is a radical surgical approach without thoracotomy applicable to all operable EC and comparable to conventional TTE in radical surgery for EC (22,23). Therefore, TME may be useful as a minimally invasive approach for preserving RF in radical surgery for EC.
The poor long-term prognosis due to postoperative complications is not limited to EC but has been reported in many other types of cancer (24,25). Pulmonary complications in EC have a significant postoperative morbidity due to the procedure and play an important role in perioperative management. The effectiveness of TME in reducing respiratory complications was first reported by Mori et al. (26), and some studies have reported the possibility of fewer respiratory complications than with TTE (27). Our findings highlight the advantages of TME over TTE in maintaining postoperative RF. Patients who underwent TME had less pronounced reductions in VC, %VC, and FEV1.0 compared to those who underwent TTE. Some reports suggest that preoperative low RF is associated with increased postoperative pulmonary complications in EC (28), suggesting that the minimally invasive nature of TME, which avoids open thoracotomy and one-lung ventilation, may contribute to better preservation of postoperative RF, thereby reducing postoperative pulmonary complications. These results are consistent with those of previous studies highlighting the benefits of minimally invasive approaches in reducing postoperative complications after esophagectomy (26,27).
Limitations of this study include its retrospective nature and single-center cohort, which may limit the generalizability of the findings. Additionally, the lack of long-term follow-up data precludes an assessment of the durability of the observed differences in RF between TME and TTE. Future prospective studies with larger multicenter cohorts and longer follow-up periods are warranted to validate our findings and further elucidate the optimal surgical approach for preserving RF in patients undergoing esophagectomy.
In conclusion, our study showed that TME was associated with a less severe decline in RF compared to TTE in patients undergoing esophagectomy for EC.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
All Authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S Sumiyoshi, A Shiozaki, and H Fujiwara. The first draft of the manuscript was written by S Sumiyoshi and all Authors commented on previous versions of the manuscript. All Authors read and approved the final manuscript.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Davakis S, Charalabopoulos A, Kyros E, Sakarellos P, Tsourouflis G, Dimitroulis D, Nikiteas N. Minimally invasive transcervical esophagectomy with mediastinal lymphadenectomy for cancer. A comparison with standardized techniques. Anticancer Res. 2022;42(2):675–680. doi: 10.21873/anticanres.15526. [DOI] [PubMed] [Google Scholar]
- 3.Ida S, Watanabe M, Yoshida N, Baba Y, Umezaki N, Harada K, Karashima R, Imamura Y, Iwagami S, Baba H. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol. 2015;22(13):4432–4437. doi: 10.1245/s10434-015-4559-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg. 2011;91(5):1494–1501. doi: 10.1016/j.athoracsur.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Goense L, Meziani J, Bülbül M, Braithwaite SA, Van Hillegersberg R, Ruurda JP. Pulmonary diffusion capacity predicts major complications after esophagectomy for patients with esophageal cancer. Dis Esophagus. 2019;32(3):doy082. doi: 10.1093/dote/doy082. [DOI] [PubMed] [Google Scholar]
- 6.Smetana GW. Preoperative Pulmonary Evaluation. N Engl J Med. 1999;340(12):937–944. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Nishikawa K, Tanishima Y, Ishikawa Y, Kurogochi T, Yuda M, Tanaka Y, Matsumoto A, Yano F, Eto K. Risk stratification of postoperative pneumonia in patients undergoing subtotal esophagectomy for esophageal cancer. Anticancer Res. 2022;42(6):3023–3028. doi: 10.21873/anticanres.15787. [DOI] [PubMed] [Google Scholar]
- 8.Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson-Hansen C, Blackstone EH, Worldwide Esophageal Cancer Collaboration Investigators Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29(8):897–905. doi: 10.1111/dote.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Sugihara K, Mori M. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260(2):259–266. doi: 10.1097/SLA.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa S, Uchi Y, Ando T, Hayashi K, Aoki T. Essential updates 2020/2021: Recent topics in surgery and perioperative therapy for esophageal cancer. Ann Gastroenterol Surg. 2023;7(3):346–357. doi: 10.1002/ags3.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiwara H, Shiozaki A, Konishi H, Kosuga T, Komatsu S, Ichikawa D, Okamoto K, Otsuji E. Perioperative outcomes of single-port mediastinoscope-assisted transhiatal esophagectomy for thoracic esophageal cancer. Dis Esophagus. 2017;30(10):1–8. doi: 10.1093/dote/dox047. [DOI] [PubMed] [Google Scholar]
- 12.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours, 8th Edition. Wiley. 2016 [Google Scholar]
- 13.Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Nemoto K, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20(3):343–372. doi: 10.1007/s10388-023-00993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burney PGJ, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66(1):49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- 15.Sugawara K, Yamashita H, Yajima S, Uemura Y, Okumura Y, Nishida M, Yagi K, Aikou S, Seto Y. Preoperative restrictive pulmonary dysfunction influences the survival after gastrectomy for elderly patients with gastric carcinoma. Surg Today. 2020;50(9):1065–1073. doi: 10.1007/s00595-020-01983-y. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara K, Mori K, Okumura Y, Yagi K, Aikou S, Uemura Y, Yamashita H, Seto Y. Preoperative low vital capacity influences survival after esophagectomy for patients with esophageal carcinoma. World J Surg. 2020;44(7):2305–2313. doi: 10.1007/s00268-020-05450-0. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara K, Oka D, Hara H, Yoshii T, Fukuda T. Survival impacts of impaired lung functions and comorbidities on elderly esophageal cancer patients. World J Surg. 2023;47(12):3229–3239. doi: 10.1007/s00268-023-07195-y. [DOI] [PubMed] [Google Scholar]
- 18.Kosumi K, Yoshida N, Okadome K, Eto T, Kuroda D, Ohuchi M, Kiyozumi Y, Nakamura K, Izumi D, Tokunaga R, Harada K, Mima K, Sawayama H, Ishimoto T, Iwatsuki M, Baba Y, Miyamoto Y, Watanabe M, Baba H. Minimally invasive esophagectomy may contribute to long-term respiratory function after esophagectomy for esophageal cancer. Diseases of the Esophagus. 2018;31(6) doi: 10.1093/dote/dox153. [DOI] [PubMed] [Google Scholar]
- 19.Otani T, Ichikawa H, Hanyu T, Ishikawa T, Kano Y, Kanda T, Kosugi S, Wakai T. Long-Term trends in respiratory function after esophagectomy for esophageal cancer. J Surg Res. 2020;245:168–178. doi: 10.1016/j.jss.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Motoyama S, Sato Y, Wakita A, Kawakita Y, Nagaki Y, Imai K, Minamiya Y. Extensive lymph node dissection around the left laryngeal nerve achieved with robot-assisted thoracoscopic esophagectomy. Anticancer Res. 2019;39(3):1337–1342. doi: 10.21873/anticanres.13246. [DOI] [PubMed] [Google Scholar]
- 21.Donohoe CL, O’Farrell NJ, Ravi N, Reynolds JV. Evidence-based selective application of transhiatal esophagectomy in a high-volume esophageal center. World J Surg. 2012;36(1):98–103. doi: 10.1007/s00268-011-1307-0. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara H, Shiozaki A, Konishi H, Otsuji E. Transmediastinal approach for esophageal cancer: A new trend toward radical surgery. Asian J Endosc Surg. 2019;12(1):30–36. doi: 10.1111/ases.12687. [DOI] [PubMed] [Google Scholar]
- 23.Tokairin Y, Nakajima Y, Kawada K, Hoshino A, Okada T, Ryotokuji T, Ogo T, Okuda M, Kume Y, Kawamura Y, Yamaguchi K, Nagai K, Kawano T, Kinugasa Y. A feasibility study of mediastinoscopic radical esophagectomy for thoracic esophageal cancer from the viewpoint of the dissected mediastinal lymph nodes validated with thoracoscopic procedure: a prospective clinical trial. Esophagus. 2019;16(2):214–219. doi: 10.1007/s10388-018-00656-7. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 25.Gamboa AC, Lee RM, Turgeon MK, Varlamos C, Regenbogen SE, Hrebinko KA, Holder-Murray J, Wiseman JT, Ejaz A, Feng MP, Hawkins AT, Bauer P, Silviera M, Maithel SK, Balch GC. Impact of postoperative complications on oncologic outcomes after rectal cancer surgery: An analysis of the us rectal cancer consortium. Ann Surg Oncol. 2021;28(3):1712–1721. doi: 10.1245/s10434-020-08976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori K, Yamagata Y, Aikou S, Nishida M, Kiyokawa T, Yagi K, Yamashita H, Nomura S, Seto Y. Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis Esophagus. 2016;29(5):429–434. doi: 10.1111/dote.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa K, Akashi Y, Hisakura K, Kim J, Owada Y, Ohara Y, Enomoto T, Furuya K, Moue S, Miyazaki Y, Doi M, Shimomura O, Takahashi K, Hashimoto S, Oda T. Clinical advantage of transmediastinal esophagectomy in terms of postoperative respiratory complications. Int J Clin Oncol. 2023;28(6):748–755. doi: 10.1007/s10147-023-02328-8. [DOI] [PubMed] [Google Scholar]
- 28.Elliott JA, O’Byrne L, Foley G, Murphy CF, Doyle SL, King S, Guinan EM, Ravi N, Reynolds JV. Effect of neoadjuvant chemoradiation on preoperative pulmonary physiology, postoperative respiratory complications and quality of life in patients with oesophageal cancer. Br J Surg. 2019;106(10):1341–1351. doi: 10.1002/bjs.11218. [DOI] [PubMed] [Google Scholar]