Abstract
Streptococcus suis is an emerging zoonotic pathogen that can cause infections in pigs and humans, usually after ingestion of raw pork meat or wound contamination. We report the first S. suis meningitis and sepsis case in a human in Lithuania. 51 y.o. man with no relevant comorbidities, but with a history of alcohol abuse was admitted to the emergency department due to new-onset tonic-clonic seizures. The patient became agitated, aggressive and hypotensive, later sensible contact was lost (GCS of 8 points). Blood tests and cerebrospinal fluid (CSF) analysis were consistent with bacterial meningitis, thus ceftriaxone and ampicillin were empirically started. S. suis, susceptible to penicillin and ceftriaxone, was identified in blood and CSF cultures. The patient recovered without any immediate significant sequels, but later developed cognitive impairment. The route of infection for our patient was not clear because he had no contact with pigs or raw pork, although he lived in the countryside, helped farmers with non-pig related work, had some scabs on his shins and ate home-cooked pork. The paper presents the case report and review of the literature.
Keywords: Streptococcus suis, Meningitis, Sepsis
Highlights
-
•
First case of S.suis meningitis and sepsis in Lithuania.
-
•
Not all patients with S.suis infection have porcine exposure.
-
•
S.suis meningitis might have late sequels – cognitive impairment.
-
•
It is likely the prevalence of human infections with S. suis is underestimated.
Introduction
Streptococcus suis is an emerging zoonotic pathogen that causes severe invasive human infections such as meningitis, pneumonia, septicemia, and arthritis. Pig farmers, butchers, veterinarians, hunters, and people who eat raw pig meat meals are at risk of contracting S.suis infection [1], [2]. However, in Central Europe, a lot of cases arise with no known exposure to porcine or their products. Although European cases account for about 10 % of global prevalence, the incidence of S.suis infection is likely underestimated because this infection is not a notifiable disease [3]. To this date, there are no publications or reports from Lithuania, that show prevalence or other known S.suis human cases.
Case
Early 50′s male was admitted to the Emergency Department on the one of Autumn day in 2022 due to new-onset tonic-clonic seizure episode and disorientation. The patient did not have any comorbidities but had a history of alcohol abuse. In the past, he had a head injury, which resulted in moderate hearing loss and anisocoria. Before admission, the patient consumed alcohol in large quantities.
On physical examination, he was disoriented, non-febrile, his blood pressure (BP) was 151/88 mmHg, heart rate (HR) 92 bpm, Glasgow Coma Scale (GCS) of 11, horizontal nystagmus and anisocoria (R>L) were observed, finger-nose test was negative. A few scabs were observed on his shins. The rest of the physical examination was unremarkable. Blood test results showed a white blood cell count 8.6 × 109/l (88 % neutrophils) and CRP 236 mg/l. In cerebrospinal fluid (CSF), a pleocytosis as high as 15,197 leucocytes x106/l was detected with the predominance of polymorphonuclears up to 88.4 %, total protein 7.6 g/l, lactate 19.7 mmol/l, glucose 0.6 mmol/l (blood glucose level 8.00 mmol/l). Other lab test results are presented in Table 1. Two sets of blood cultures and CSF culture were taken. No abnormal findings were detected on the head CT scan.
Table 1.
Laboratory tests results.
| Date |
Normal range | 2022 09 22 | 2022 09 23 | 2022 09 27 | 2022 10 02 | 2022 10 05 | 2022 10 11 |
|---|---|---|---|---|---|---|---|
| Parameters | |||||||
| Creatinine (µmol/l) | 62.0 −110.0 | 98 | 74.5 | 44.6 | 63.5 | ||
| Glucose (mmol/l) | 3.33 −5.55 | 8.86 | 8.5 | 9.1 | 5.6 | ||
| Urea (mmol/l) | 2.50 −6.10 | 3.8 | 4.9 | 5.9 | 7.1 | ||
| Potassium (mmol/l) | 3.60 −5.00 | 3.53 | 5.1 | 3.7 | 4.6 | ||
| Sodium (mmol/l) | 137.0 −145.0 | 134 | 141.3 | 143 | 137.5 | ||
| CRP (mg/l) | 0.00 −10.00 | 236.6 | 325.8 | 46.5 | 218 | 59.5 | 18.9 |
| RBC (x1012/l) | 4.50 −5.90 | 4.38 | 3.9 | 3.86 | 4.13 | 3.73 | 3.83 |
| HGB (g/l) | 140.0 −175.0 | 143 | 127 | 114.1 | 129.6 | 120 | 121.3 |
| WBC (x109/l) | 4.00 −10.00 | 8.6 | 7.37 | 12.5 | 17.19 | 18.15 | 6.96 |
| NEU (x109/l) | 2.00 −6.93 | 7.6 | 6.14 | 8.74 | 13.64 | 15.18 | 4.22 |
| LYM (x109/l) | 1.00 −2.50 | 0.4 | 0.5 | 1.9 | 1.98 | 1.77 | 2 |
| PLT (x109/l) | 150.0 −350.0 | 55 | 39.5 | 167.1 | 459.3 | 636.6 | 577.7 |
| PCT-Q (µg/l) | 0.00 −0.077 | 48.8 | 1.19 | ||||
| D-dimer (µg/l) | 0.00 −0.50 | 8.58 | |||||
| HIV antibodies | Negative | Negative | |||||
| Blood culture | Negative | S. suis | Negative | ||||
| CSF culture | Negative | S. suis |
Subsequently, the general condition of the patient started to worsen rapidly: he became agitated, and aggressive, had generalized tremors and sensible contact was lost (GCS of 8 points). The patient developed hypotension (BP 71/50 mmHg) and acute respiratory failure, became febrile (39 °C), therefore was transferred to the intensive care unit and was treated empirically with ceftriaxone, ampicillin, dexamethasone, infusion therapy, and vasopressors. When respiratory failure progressed, the patient was intubated. Repeated blood cultures were taken on the second day of the treatment.
After two days Streptococcus suis, susceptible to penicillin and erythromycin, was isolated from CSF and blood cultures (the causative agent was identified in two bottles from different sets). The pathogen was grown in pure culture and identified with matrix assisted laser desorption ionization coupled to time-of-flight (MALDI-TOF) mass spectrometry. Later blood cultures came negative. However, therapy with ceftriaxone was continued, as CRP increased up to 325.8 mg/l (Table 1) and no clinical improvement was observed. On the 7th day of the hospitalisation, the patient’s clinical condition started to improve, he became fully conscious, was extubated, and his inflammatory markers started to decrease (Table 1). Brain CT scan was repeated but no abnormal lesions were detected (Fig. 1). Abdominal and heart ultrasounds did not show any significant alterations.
Fig. 1.
Head CT scan – no acute lesions observed.
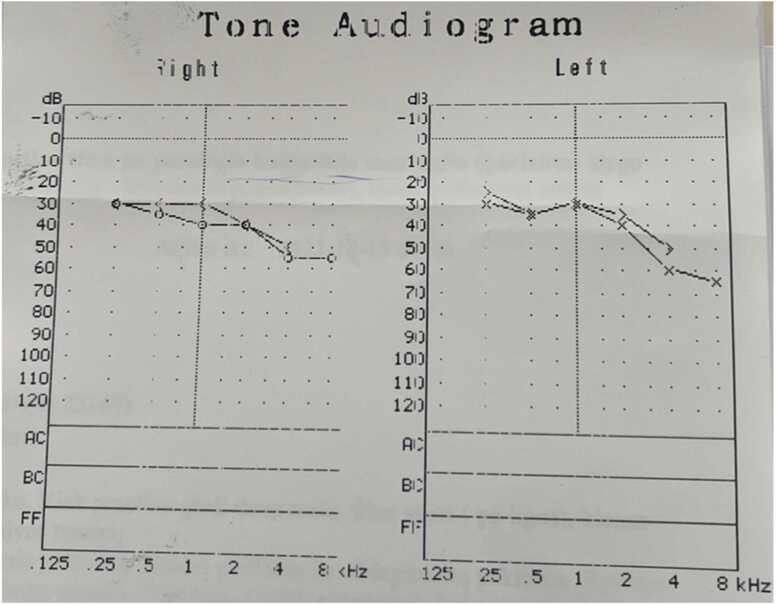
The patient was transferred to the Department of Infectious Diseases, where antibiotic therapy with ceftriaxone was continued. The patient started rehabilitation for persisting ataxia and an unsteady gait. The patient was evaluated for risk factors for the S. suis infection: he denied eating raw pork, but ate homemade pork dishes; the patient lives in the countryside but did not have any contact with domesticated or wild animals, nevertheless sometimes helps farmers with non-pig-related work. While continuing the treatment the elevation of repeated inflammatory markers was observed (Table 1), but no other cause, except ongoing S. suis infection, was identified. Inflammatory markers decreased by continuing ceftriaxone therapy. Audiometry was performed and showed moderate symmetrical bilateral neurosensory hearing loss, but the patient denied any change in hearing, therefore the changes were attributed to the previous head trauma (Fig. 2).
Fig. 2.
Audiometry showing medium neurosensoric bilateral hearing injury.
The patient received combined antibacterial treatment with ceftriaxone + ampicillin for 5 days, followed by 15 days of ceftriaxone therapy. On discharge, the patient’s gait and ataxia significantly improved, and he did not have any physical or cognitive complaints. The patient did not show for the follow-up visit, but family members, contacted by phone a few months after discharge, claimed, that he is not abusing alcohol anymore and does not feel any change in hearing. One year later after infection, the patient had an ischemic stroke in ACM sin. basin, but even before the stroke the patient became forgetful, started to show symptoms of cognitive impairment, and was being investigated for early-onset dementia. Cognitive impairment could likely be a long-term sequel after S.suis meningitis combined with unhealthy lifestyle choices before the disease.
Discussion
Streptococcus suis is a group of heterogenous gram-positive facultative anaerobes with spherical/ovoid shapes, that typically appear as diplococci or short chains [4]. S. suis naturally inhabits the upper respiratory, alimentary and genital tracts of pigs and hogs and might cause severe bacterial disease in these animals for example septicemia, meningitis, endocarditis and polyarthritis, that could be fatal, especially in piglets after weaning [4]. S. suis infection is considered to be a zoonosis. Most studies associated S. suis infection with pigs, occupational contact with porcine or related products, and consumption of undercooked or raw pork meals. Typically, infection results from ingestion of contaminated undercooked meat or wound infection, but there is also some evidence of an airborne infection route [4]. Most S. suis infections are sporadic, although two epidemics in China in 1998 and 2005 have been reported so far. Currently, S. suis is an important cause of bacterial meningitis in adults in Vietnam, Thailand and China [1]. Some cases have been reported in Europe as well, most of them in Netherlands, Germany, and France [3]. In neighboring countries Poland, Belarus, Finland and Sweden, there is evidence of S. suis circulation among pigs [5], [6], [7]. Moreover, in Poland there were 21 patients identified with invasive S. suis infection in the period from 2000 to 2013, and the authors postulated that the true prevalence of this infection in humans could be underestimated [8]. To our best knowledge, this is the first human S. suis meningitis and sepsis case identified in Lithuania. There is no national surveillance for S. suis infections in pigs or serological data in Lithuania, but there are some data of S. suis causing infections in pigs [9].
S. suis to this date has a total of 29 serotypes, from which serotype 2 (SS2) is the most prevalent [4]. Other serotypes that cause human infection are serotypes 4, 5, 9, 14, 16, 21, 24 and 31. SS2 was responsible for the two epidemics in China. A study in New Zealand found that a high ratio of farmers and meat inspectors were seropositive to SS2, suggesting sub-clinical infections in the past [10]. In Poland, SS2 was the most prevalent in the region, followed by serotypes 3, 4, 7 and 8 [5], [8].
S. suis infection can cause several clinical syndromes. The most common is meningitis, followed by sepsis, arthritis, endocarditis, rarely other manifestations [1]. S. suis infection can also cause streptococcal toxic shock syndrome (STSS), which was the most prevalent form of infection during the epidemics in China. Predisposing conditions for the development of clinically overt infection are alcoholism, splenectomy, diabetes, malignancies, and structural heart diseases [11].
Normally the incubation period is about 1 to 4.8 days [11]. S. suis infections are more likely to occur during the summer months or the rainy season in the endemic countries [11]. The clinical presentation of S. suis meningitis is most commonly characterized by fever, headache, neck stiffness, altered consciousness [2], and petechiae rash, and is clinically indistinguishable from other causes of bacterial meningitis. Hearing loss, which is usually permanent, is the most common sequel of this infection. In some cases, ataxia and tinnitus develop, also patients are at risk of developing long-term cognitive impairment [1], [2]. The fatality rate of S. suis meningitis is lower compared to bacterial meningitis of other aetiology (2.9 % [2] vs 30.4 % [12]), however, the case-fatality rate of other forms of this infection is higher [11].
Identification of S. suis can be done in several ways. Most commonly the bacteria are detected in CSF or blood cultures. The bacteria can grow on media used for common blood or CSF pathogens, although S. suis can be mistaken for other bacteria if laboratories are less experienced and less aware of this pathogen [13]. In some studies where CSF cultures were retrospectively reevaluated, S. suis was identified instead of previously determined bacteria [14], showing that the prevalence of this infection could be inaccurate and underestimated. S. suis can also be identified by polymerase chain reaction (PCR) test. After identification, it is recommended to do serotyping, based on the antigenicity of S. suis capsular polysaccharide. Also, several molecular typing techniques are used to study the genetic diversity of S.suis. In our case, serotyping and further investigations of S. suis were not done.
Empiric antibiotic therapy should be started as soon as possible, as in other bacterial meningitis cases. S. suis is usually susceptible to cephalosporins [4], [11], [15]. The resistance to tetracycline, lincosamides, aminoglycosides and macrolides is common [11], [15], although our isolate stain was susceptible to erythromycin. Similar results were found in Lithuania when antimicrobial susceptibility of isolated S. suis from pigs was tested [9], 97 % of tested isolates were susceptible to third-generation cephalosporins and 70 % to erythromycin. Other studies suggest that the most prevalent resistance profile is determined by prevalent macrolide-lincosamide-streptogramin B genes erm(B) tet(O), which could be acquired by horizontal antibiotic resistance gene transfer [15], but antibiotic resistance might vary depending on the geographical origin and from whom it was collected (human, pig or boar). Typically, it is recommended to treat S. suis bacterial meningitis for 14 days. Sometimes relapses occur, but they are successfully treated with the continuation of antibiotic therapy [11]. The use of dexamethasone has shown positive effects on other bacterial meningitis, especially S. pneumoniae, therefore it is used in empirical meningitis treatment when the cause of meningitis is still unknown. The data suggest that dexamethasone reduces hearing loss in S. suis [2], [11], although the effect on mortality is yet to be established.
At this moment, there are no vaccines for S. suis infection for humans, approved by the European Medicines Agency. The best disease control measure is to prevent transmission, by using protective gear (e.g., wearing gloves) while dealing with pigs or bores, following general sanitary and hygienic standards for slaughtered porcine, and implementing public health interventions in settings where raw or undercooked pork consumption is a common practice [4].
Conclusion
This paper reports the first Streptococcus suis meningitis and sepsis in Lithuania in a patient without any contact with pigs, who ate home-prepared pork dishes. The patient had an important risk factor – a history of alcohol abuse. The presentation of the disease was typical for bacterial meningitis and sepsis, and by giving ceftriaxone, dexamethasone and symptomatic treatment, the patient had recovered. No immediate sequels were noted, but subsequently patient started to develop cognitive impairment. S. suis is a rare causative agent of human infections in the European region, but is widely prevalent in pigs. It is likely that human infections with S. suis could be underestimated in Lithuania which calls for improved diagnostic capabilities and surveillance of this infection in both humans and pigs.
Consent
Studies on patients or volunteers require ethics committee approval and fully informed written consent which should be documented in the paper.
Ethical approval
The patient gave consent to publish this case report without revealing his identity. In the manuscript there are no identifiable information.
Funding
The preparation of this case report was not funded and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The manuscript is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals.
CRediT authorship contribution statement
Roberta Vaikutytė-Ramanauskienė: Writing – original draft, Conceptualization. Tautvydas Puslys: Writing – original draft, Conceptualization. Evelina Pukenyte: Writing – review & editing. Aukse Mickiene: Writing – review & editing, Conceptualization.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest. Aukse Mickiene received consulting fees and an honorarium for lectures outside the submitted work.
References
- 1.Huong V.T.L., et al. Epidemiology, clinical manifestations, and outcomes of streptococcus suis infection in humans. Emerg Infect Dis. 2014;vol. 20(7):1105–1114. doi: 10.3201/eid2007.131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Samkar A., Brouwer M.C., Schultsz C., van der Ende A., van de Beek D. Streptococcus suis meningitis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;vol. 9(10) doi: 10.1371/journal.pntd.0004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brizuela J., et al. Molecular epidemiology of underreported emerging zoonotic pathogen streptococcus suis in Europe. Emerg Infect Dis. 2024;vol. 30(3):413–422. doi: 10.3201/eid3003.230348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hlebowicz M., Jakubowski P., Smiatacz T. Streptococcus suis meningitis: epidemiology, clinical presentation and treatment. Mary Ann Liebe Inc. 2019 doi: 10.1089/vbz.2018.2399. [DOI] [PubMed] [Google Scholar]
- 5.Bojarska A., et al. Diversity of serotypes and new cps loci variants among Streptococcus suis isolates from pigs in Poland and Belarus. Vet Microbiol. 2020;vol. 240 doi: 10.1016/j.vetmic.2019.108534. [DOI] [PubMed] [Google Scholar]
- 6.Werinder A., Aspán A., Backhans A., Sjölund M., Guss B., Jacobson M. Streptococcus suis in Swedish grower pigs: occurrence, serotypes, and antimicrobial susceptibility. Acta Vet Scand. Jun. 2020;vol. 62(1) doi: 10.1186/s13028-020-00533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sihvonen L., Kur D.N., Henrichsen J. Streptococcus Suis Isolated from Pigs in Finland. Acta Vet Scand. 1988;vol. 29:9–13. doi: 10.1186/BF03548386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bojarska A., et al. Streptococcus suis in invasive human infections in Poland: clonality and determinants of virulence and antimicrobial resistance. Eur J Clin Microbiol Infect Dis. 2016;vol. 35(6):917–925. doi: 10.1007/s10096-016-2616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.M. Ruzauskas and I. Klimienė, Survey of antimicrobial susceptibility among some pathogenic and commensal bacteria isolated from pigs in Lithuania.” [Online]. Available: 〈https://www.researchgate.net/publication/215831831〉.
- 10.Robertson I.D., Blackmore D.K. Occupational exposure to Streptococcus suis type 2. Epidem Inf. 1989;vol. 103:157–164. doi: 10.1017/s0950268800030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayanakorn A., Goh B.H., Lee L.H., Khan T.M., Saokaew S. Risk factors for Streptococcus suis infection: A systematic review and meta-analysis. Sci Rep. 2018;vol. 8(1) doi: 10.1038/s41598-018-31598-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh D.Y., et al. Sex-based differences in bacterial meningitis in adults: Epidemiology, clinical features, and therapeutic outcomes. J Infect Public Health. 2021;vol. 14(9):1218–1225. doi: 10.1016/j.jiph.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Goyette-Desjardins G., Auger J.P., Xu J., Segura M., Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. Jun. 2014;vol. 3 doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.C. Dejthevaporn, R. Witoonpanich, and K. Donsakul, Streptococcus suis infection: Clinical features and diagnostic pitfalls, 2003. [Online]. Available: 〈https://www.researchgate.net/publication/10569087〉. [PubMed]
- 15.Wang C.-Z., et al. Antibiotic resistance patterns and molecular characterization of Streptococcus suis isolates from Swine and humans in China. Microbiol Spectr. 2023;vol. 11(3) doi: 10.1128/spectrum.00309-23. [DOI] [PMC free article] [PubMed] [Google Scholar]