Summary
Background
Incidentally, the non-invasive prenatal test (NIPT) shows chromosomal aberrations suspicious of a maternal malignancy, especially after genome-wide testing. The aim of this study is to determine how many cases of cancer in pregnancy are diagnosed or missed with NIPT and whether in retrospect subtle changes in NIPT results could have detected cancer.
Methods
We identified Dutch patients diagnosed in 2017–2021 with pregnancy-associated cancer from the International Network on Cancer, Infertility and Pregnancy (INCIP) Registry, who underwent NIPT in the Dutch NIPT implementation study (TRIDENT-2). We retrospectively assessed how many of these women showed a malignancy suspicious-NIPT, their tumour types and –stages, and the time interval between NIPT and cancer diagnosis.
Findings
Of 143 women with pregnancy-associated cancer, we included 65 patients that underwent an NIPT. Fifty-four women had a solid tumour and 11 a haematological malignancy. Sixteen (24.6%) NIPTs were malignancy suspicious (15 genome-wide, one targeted). All 10 haematological cancer patients with genome-wide NIPT had a malignancy suspicious-NIPT, irrespective of the disease stage. Only five patients with a solid tumour had a genome-wide malignancy suspicious-NIPT (4/5 advanced cancer stage III or IV). The mean time between date of NIPT and cancer diagnosis was significantly shorter after a malignancy suspicious-NIPT compared to a non-suspicious-NIPT, respectively 49.9 days (± SD 31.8) and 100.7 days (± SD 74.9), p = 0.001.
Interpretation
All genome-wide NIPT in women with pregnancy-associated haematological malignancies were malignancy suspicious. Women with a solid tumour showed a malignancy suspicious-NIPT in only a minority of cases, mainly the advanced stages.
Funding
None.
Keywords: Pregnancy-associated cancer, Noninvasive prenatal test
Research in context.
Evidence before this study
The non-invasive prenatal test (NIPT) for foetal aneuploidy on cell-free DNA (cfDNA) of maternal plasma incidentally shows a malignancy suspicious-result. We and others have shown that most cases with multiple chromosomal aberrations in NIPT are diagnosed with cancer. We previously defined an expert-based tool for classifying malignancy suspicious-NIPT and a comprehensive set of recommendations for multidisciplinary diagnostic follow-up of pregnant women with a malignancy suspicious-NIPT. The incidence of cancer in pregnancy is about 1:1.500 as deducted from cancer in pregnancy registries such as the International Network on Cancer, Infertility and Pregnancy (INCIP), while NIPT can detect cancer only in 1:15.000 pregnancies as concluded from implementation studies utilizing NIPT for foetal aneuploidy screening, such as the TRIDENT-2 study. A knowledge gap exists in the fraction of women with pregnancy-associated cancer showing a malignancy suspicious-NIPT, and whether a malignancy suspicious-NIPT result is associated with certain types of cancer and if it will lead to an earlier diagnosis. We aimed to determine the fraction of women with pregnancy associated cancer who showed a malignancy suspicious-NIPT. To explore this research question we used an unbiased sample of data of two Dutch registries, on pregnancy in cancer and NIPT results respectively. Available literature data was extracted from PubMed up to the 15th of April 2024. Search terms used were “pregnancy [MeSH]”, “Neoplasms [MeSH]”, “Noninvasive Prenatal Testing [MeSH]”, “NIPT”. Reference lists of relevant articles on this topic were examined and possible relevant papers were added.
Added value of this study
This study is the first to evaluate prenatal cfDNA testing by NIPT in a group of women with pregnancy-associated cancer not knowing to have cancer at the time of NIPT request. We found that about a quarter of women with pregnancy-associated cancer had a malignancy suspicious-NIPT result, when tested with a genome-wide NIPT. In all women with haematological cancer in pregnancy, the genome-wide NIPT results were malignancy suspicious. The majority of women with solid tumours showed a non-suspicious NIPT, although NIPT could be suspicious in women with advanced stage solid tumours. We found a significantly shorter time to diagnosis in patients with a malignancy suspicious-NIPT compared to the group with a non-suspicious NIPT result. The knowledge from this study may contribute to an improved interpretation of NIPT results in relation to cancer diagnosis during pregnancy.
Implications of all the available evidence
The number of women referred to a clinic with pregnancy-associated cancer will increase as NIPT providers will be able to better recognize malignant-suspicious NIPT from foetal aneuploidy screening. Reporting malignancy suspicious-NIPT results may be a step forward in detecting cancers and could enable an earlier diagnosis and start of cancer therapy, especially for haematological malignancies and advanced solid tumours. Nevertheless, NIPT for foetal aneuploidy screening should not be considered a cancer-in-pregnancy test.
Introduction
Women who are diagnosed with cancer during pregnancy or within one or two years after childbirth are generally classified as having ‘pregnancy-associated cancer’.1,2 The incidence of cancer during pregnancy is about one in 1000–2000 pregnancies.1,3 In the Netherlands, patients with pregnancy-associated cancer are included in a multicentre registry of the International Network on Cancer, Infertility and Pregnancy (INCIP) by their treating physician, on a voluntary basis. The inclusion criteria for the INCIP registry study (UZ Leuven founded study S25470, study Part I.I.A.) are premenopausal women under 45 years of age with a histological proven cancer, either during or within 5 years after pregnancy.4 To date, it is not known how many women with pregnancy-associated cancer showed an NIPT (non-invasive prenatal test) result that is suspicious of a maternal malignancy (malignancy suspicious-NIPT).
In the Netherlands, between April 2017 and April 2023, the NIPT was only available within the TRIDENT-2 study. NIPT was offered to all pregnant women with a vital pregnancy, as a first-tier screening test for foetal aneuploidy, licensed by the Dutch Ministry of Health.5 Participants could choose between receiving NIPT results for all autosomes (genome-wide NIPT) or for chromosomes 13, 18, and 21 only (targeted NIPT). An exclusion criterion for the TRIDENT-2 study was a known current malignancy. The uptake of NIPT was 46% from April 2017 to March 2019.6 Chromosomal aberrations like copy number variations (CNVs), that originate from cancer-derived cell-free DNA (cfDNA), may incidentally be detected by NIPT and may be indicative of an underlying malignancy.7, 8, 9, 10
For this study, the INCIP registry was merged with the TRIDENT-2 registry, to identify women with pregnancy-associated cancer, who had undergone an NIPT. We aimed to determine the fraction of malignancy suspicious-NIPT in women with pregnancy-associated cancer, and which tumour types were found in these women. We also evaluated the time interval to cancer diagnosis for suspicious- and non-suspicious NIPTs. This knowledge may contribute to an improved interpretation of NIPT results in relation to cancer diagnosis during pregnancy.
Methods
Study design and study population
This is a retrospective cross-sectional study, that assessed the history of NIPT in women with pregnancy-associated cancer, defined as cancer during pregnancy or within two years after childbirth. We used data of Dutch patients who were included in both the INCIP and TRIDENT-2 study, with a cancer diagnosis between April 1, 2017 and April 1, 2021. In the 4-yr study period, INCIP included 143 Dutch women and TRIDENT-2 included 334,499 women. To identify eligible women, we linked the INCIP database with the TRIDENT-2 registry by family name, date of birth, expected date of delivery plus or minus seven days, and primary hospital where the cancer was diagnosed. If no match was found, we assumed that no NIPT was performed. Patients were pregnant at the moment of NIPT. We collated oncological information (date of cancer diagnosis, tumour type and -stage) from the INCIP database. The NIPT results and date of blood draw were obtained from the TRIDENT-2 database. Women found to undergo active treatment for cancer when requesting the NIPT were excluded, because this was an exclusion criterion for the TRIDENT-2 study. Women with a prior cancer diagnosis who had finished treatment with curative intent, were not excluded as they were assumed to be healthy at the time of NIPT.
NIPT data collection
We obtained detailed information of the NIPT analysis of the selected study population from the Dutch NIPT laboratories, located in Maastricht University Medical Centre (UMC), Amsterdam UMC, and Rotterdam Erasmus Medical Centre. NIPT sample processing, sequencing, WISECONDOR bioinformatical analyses, and assessment of the NIPT results in the TRIDENT-2 study were previously described.5,10
For genome-wide NIPT we had access to the bioinformatical data of all autosomes, whereas for targeted NIPT only of chromosomes 13, 18 and 21. NIPT-detected CNV's, defined as a windowed bin test Stouffer's z score ≥3 or ≤ −3 of at least 10 Mb,11, 12, 13, 14 were reviewed for regions or genes known to be associated with the diagnosed malignancy, using online resources on gene function and cancer (cyto)genetics, and scientific publications. The “Maastricht criteria” were used to standardize the NIPT results into three groups: no suspicion, mild suspicion and strong suspicion for a maternal malignancy (Supplemental Table S1).15
Analyses
We categorized the patients by tumour type and the type of NIPT (genome-wide versus targeted) and calculated, for both malignancy suspicious- and non-suspicious NIPTs, the mean time between the date of blood draw for NIPT during pregnancy and the date of cancer diagnosis. The two groups were compared using Two-Sample T-Test, with a p-value <0.05 considered as statistical significant. For this analysis, two patients with an initial non-suspicious NIPT result, but a suspicious NIPT result after review on the basis of the Maastricht criteria, were excluded, because this could have influenced the time of diagnosis. A third patient was excluded, because the actual date of disease recurrence was not known. The date of cancer diagnosis was set on zero for the patients with a cancer diagnosis within one week after NIPT.
Ethical approval
The study protocol was approved by the Medical Ethical Committee (METC) of the Maastricht University Medical Centre (METC 2021-2840) and the Ethics Committee of University Hospital Leuven (S66801). This study was classified as exempt from the Medical Research Involving Human Subjects Act (WMO).
Role of the funding source
There was no funding source for this study.
Results
Cancers in the study population
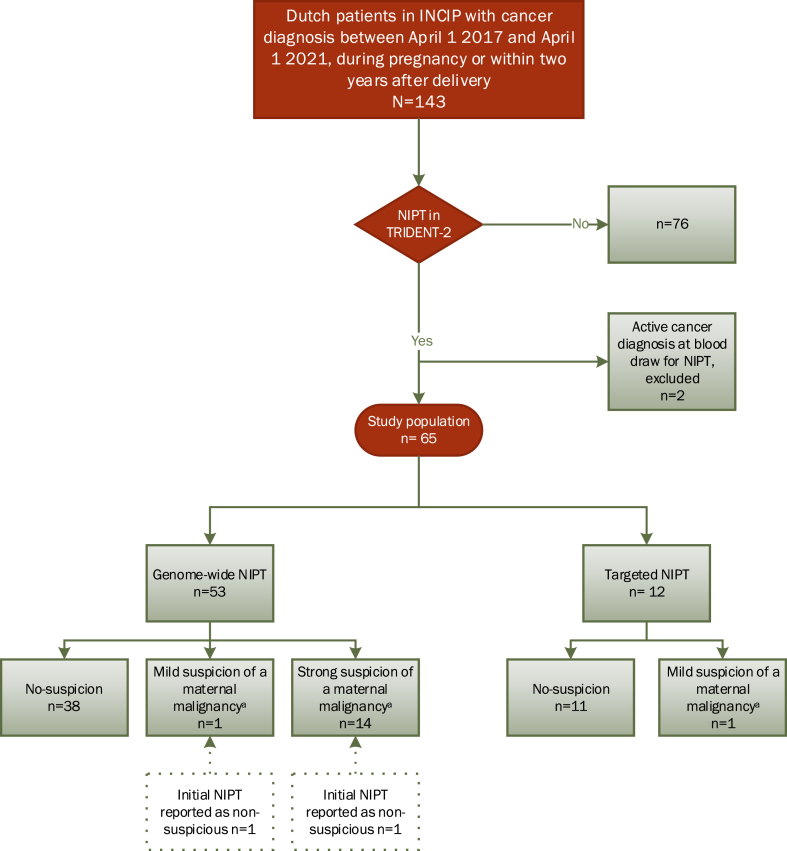
A total of 143 Dutch patients, with a cancer diagnosis during pregnancy or within two years after delivery, were identified from the INCIP registry (Fig. 1). An NIPT was performed in 67/143 (46.9%) patients. Two patients were excluded, because they were already diagnosed with cancer before venepuncture for NIPT and should have been excluded from TRIDENT-2. In three other patients the malignancy was diagnosed within one week after the NIPT; these were considered eligible, as there was no proven cancer diagnosis at the time of the NIPT request. Hence, the study population consisted of 65 patients.
Fig. 1.
Study population. The number of Dutch patients included in the INCIP study in the study period (n = 143), and the number of patients who had undergone an NIPT within the TRIDENT-2 study. In the TRIDENT-2 study 334499 NIPTs were performed between April 1 2017 and April 1 2021. aReviewed NIPT result according to the “Maastricht criteria”. Abbreviations: INCIP: International Network on Cancer, Infertility and Pregnancy. NIPT: non-invasive prenatal test. TRIDENT: Trials by Dutch Laboratories for Evaluation of Non-Invasive Prenatal Testing.
Cancer was diagnosed during pregnancy in 61/65 patients, at a median gestational age of 22+1 weeks (range, 11+1–38+3). In four patients, cancer was diagnosed postpartum, all within the first year after delivery. The most frequently diagnosed malignancies were breast cancer (n = 20), gynaecological cancers (n = 19) and haematological malignancies (n = 11) (Table 1, Supplemental Table S2). Tumour stages ranged from stage I to stage IV disease (Table 1, Supplemental Table S2).
Table 1.
Malignancy suspicious-NIPTs per tumour types and tumour stages.
| Tumour type | Total number of pregnancy-associated cancers | Total suspicious-NIPTs according to “Maastricht criteria” (%) | Stage Ib: number of suspicious-NIPTs per number of patients | Stage IIb: number of suspicious-NIPTs per number of patients | Stage IIIb: number of suspicious-NIPTs per number of patients | Stage IVb: number of suspicious-NIPTs per number of patients | Unknown stage: number of suspicious NIPT per number of patients |
|---|---|---|---|---|---|---|---|
| Breast | 20 | 2 (10%) | 0 of 2 | 1 of 12 | 0 of 3 | 1 of 3 | 0 of 0 |
| Gynaecological | 19 | 2 (10%) | 0 of 11 | 0 of 4 | 2 of 4 | 0 of 0 | 0 of 0 |
| Haematological | 11 | 10 (90%) | 3 of 3 | 3 of 3 | 2 of 2 | 2 of 3 | 0 of 0 |
| Melanoma | 6 | 0 (0%) | 0 of 3 | 0 of 1 | 0 of 1 | 0 of 0 | 0 of 1 |
| Nasopharyngeal | 2 | 0 (0%) | 0 of 0 | 0 of 1 | 0 of 0 | 0 of 1 | 0 of 0 |
| Thyroid | 2 | 0 (0%) | 0 of 0 | 0 of 0 | 0 of 1 | 0 of 0 | 0 of 1 |
| Othera | 5 | 2 (40%) | 0 of 1 | 0 of 1 | 0 of 0 | 1 of 2 | 1 of 1 |
| Total non-haematological | 54 | 6 (11%) | 0 of 17 | 1 of 19 | 2 of 9 | 2 of 6 | 1 of 3 |
| Total haematological | 11 | 10 (91%) | 3 of 3 | 3 of 3 | 2 of 2 | 2 of 3 | 0 of 0 |
| Total | 65 | 16 (30%) | 3 of 20 | 4 of 22 | 4 of 11 | 4 of 9 | 1 of 3 |
Abbreviations: NA: not applicable.
Consists of four different cancer types, colon carcinoma (stage IV), thymoma (stage IV), bladder cancer (stage I), brain tumour (stage II) and carcinoma of unknown primary (NA).
The TNM-, FIGO- or WHO-classification was used for solid tumours, and the Ann Arbor staging system was used for the haematological cancers.
Review and frequency of malignancy suspicious-NIPTs
The median gestational age at blood draw for the NIPT was 12+1 weeks (range 11+0–15+0). A genome-wide NIPT-analysis was performed in 53/65 (81.5%) and a targeted NIPT in 12/65 (18.5%) of the women.
Of the 65 women with pregnancy-associated cancers in our study population, 16 had a malignancy suspicious-NIPT result (24.6%; 16/65) and 49 had a normal NIPT result (75.5%; 49/65). Of the malignancy suspicious results, 15 were genome-wide and one was a targeted NIPT (Supplemental Table S2). A strong suspicion was shown in 14 patients and a mild suspicion in two (Fig. 1, Supplemental Table S2). The NIPT results of the four patients diagnosed with cancer postpartum, were all non-suspicious (Supplemental Table S2).
The NIPT results reviewed on the basis of the Maastricht criteria were congruent with the initial NIPT results in all targeted NIPTs and in 51 of 53 (96.2%) genome-wide NIPTs. In two patients (3.8%, NIPT-48 and NIPT-50), the NIPT malignancy-score was changed after review from non-suspicious to, respectively, “strong suspicion” and “mild suspicion” (Supplemental Table S2), based on the presence of known tumour-associated oncogenes in the CNVs, initially reported as possible foetal structural chromosomal aberrations. Of the 16 patients with a malignancy suspicious-NIPT, 7 were asymptomatic at the time of cancer diagnosis, 6 were symptomatic, and for 3 patients this was unknown. This is in contrast to the 49 patients with a non-suspicious NIPT where only one patient was asymptomatic, 42 were symptomatic, and for one patient this was unknown (Supplemental Table S3).
NIPT results per tumour type and stage
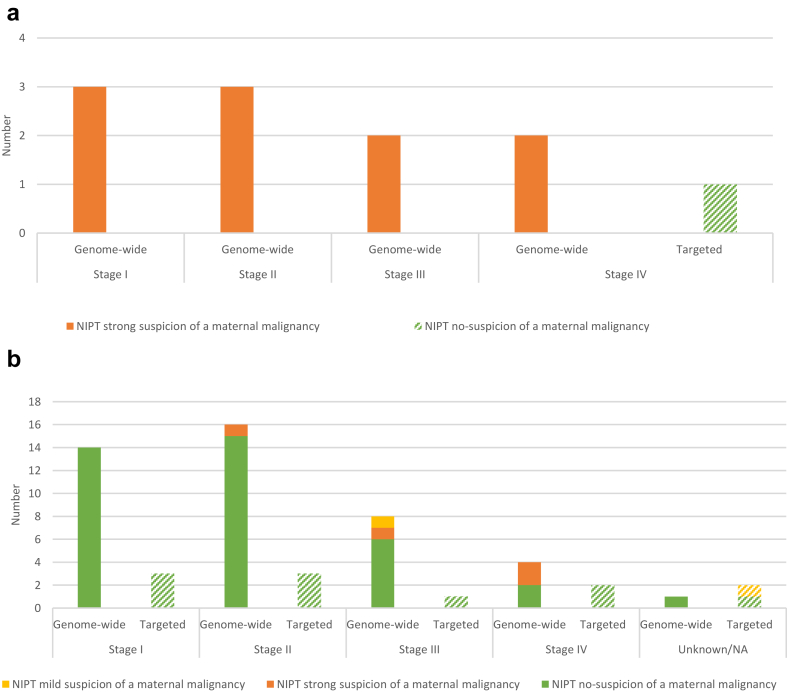
The NIPT results for patients with haematological malignancies and solid tumours differed per tumour stage, and whether genome-wide or targeted NIPT was performed (Fig. 2).
Fig. 2.
a) NIPT results for the haematological malignancies (n = 11) per Ann Arbor tumour stage at diagnosis. The y-axis represents the number of patients and the x-axis represents the different tumour stages divided into genome-wide (solid bars) and targeted (shaded bar) NIPT. The colours represent the reviewed NIPT results. Genome-wide NIPT was able to raise strong suspicion (orange bars) for all 10 haematological malignancies of all stages during pregnancy, whereas one postpartum diagnosed malignancy was tested normal with targeted NIPT (shaded green bar). See also Supplemental Table S2. Abbreviations: NIPT: Non-invasive prenatal test. b) NIPT results for the solid tumours (n = 54) per tumour stage at diagnosis using the TNM-, FIGO- or WHO classification. The y-axis represent the number of patients and the x-axis the different tumour stages divided into genome-wide (solid bars, n = 43) and targeted (shaded bars, n = 11) NIPT. The colours represent the reviewed NIPT results. NIPT was able to raise suspicion for malignancy only for 6 of 54 solid tumours, 5 of 43 with genome-wide NIPT and 1 of 11 with targeted NIPT. For solid tumours with stage I and II only 1 of 30 genome-wide NIPT results showed suspicion for a malignancy, see also Supplemental Table S2. Abbreviations: NIPT: Non-invasive prenatal test.
In pregnant patients with a haematological malignancy (Fig. 2a), the genome-wide NIPT result was suspicious of a malignancy in 10 out of 10 cases, irrespective of the stage of the disease. One patient whose haematological malignancy was diagnosed postpartum had a non-suspicious targeted NIPT result (NIPT-62).
For the solid tumours (Fig. 2b), only 5/43 (11.6%) of genome-wide NIPTs were malignancy suspicious. Of the five patients with a solid tumour and a genome-wide malignancy suspicious-NIPT, four were diagnosed with an advanced cancer stage III or IV (80.0%; 4/5) and one with a cancer stage II (20.0%; 1/5). One targeted NIPT (9.1%; 1/11) was mildly malignancy suspicious, concerning a patient with a carcinoma of unknown primary (CUP). For patients with stage I and II solid tumours, suspicion for a malignancy was shown in one of 30 genome-wide NIPTs (3.3%; 1/30) and none of the targeted NIPTs (0%; 0/6).
In Supplemental Figure S1, the NIPT analyses of three patients are highlighted as an example, showing a typical pattern of chromosomal gains and losses known for Hodgkin lymphoma, diffuse large B-cell lymphoma, and ovarian cancer respectively. For case NIPT-67 (cervical cancer), we found a borderline significant CNV gain of chromosome band 3q26 which did not meet the Maastricht criteria for ‘malignancy-suspicious’, but is a known hallmark of squamous cervical carcinomas (Supplemental Table S2).16
Time between NIPT and cancer diagnosis and symptoms of disease
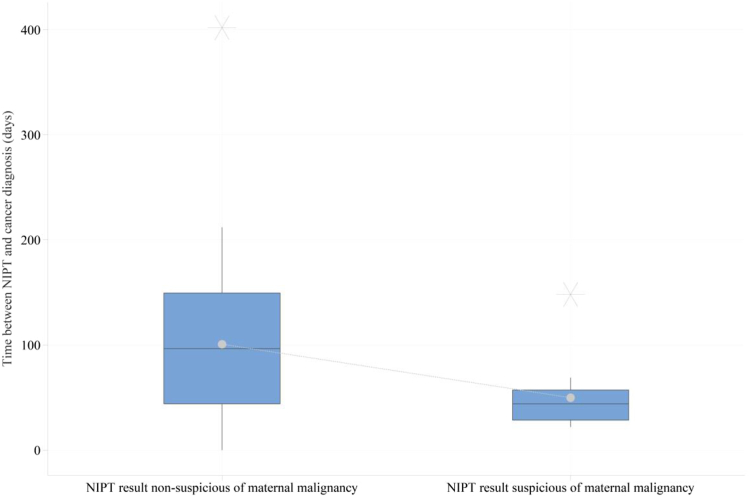
The mean time between the NIPT and the diagnosis of pregnancy-associated cancer was statistically significantly shorter for the group with a malignancy suspicious-NIPT result when compared with the group with a non-suspicious NIPT result, respectively 49.9 days (± SD 31.8) and 100.7 days (± SD 74.9), p = 0.001 (Fig. 3).
Fig. 3.
Time between NIPT and the cancer diagnosis. This figure shows the time in days between the blood draw for NIPT (t = 0) and the diagnosis of cancer. Time is plotted on the y-axis and on the x-axis the subgroups of patients with respectively a non-suspicious NIPT result (n = 48), and suspicious-NIPT result (n = 14). The number of NIPT results for genome-wide and targeted NIPT were taken together. The dotted line represents the connection of the mean. One patient, NIPT-18 (non-suspicious NIPT), was excluded in this analysis because the actual date of disease recurrence was not known to us. The time to cancer diagnosis was set on zero for the three patients with a cancer diagnosis within one week before NIPT. The asterisks denote the outliers.
Discussion
In this retrospective cross-sectional study, we examined how many women diagnosed with pregnancy-associated cancer, who are unaware of cancer at time of the blood draw for NIPT, have received a malignancy suspicious-NIPT result and what tumour types were associated with the aberrant NIPT result. It was reported before, that NIPT on cfDNA from maternal blood plasma may show chromosomal aberrations suspicious of a maternal malignancy in women not known with an active malignancy.7, 8, 9, 10,17, 18, 19, 20 To this end, no data on the reverse situation are available, the fraction of women with pregnancy-associated cancer showing a malignancy suspicious-NIPT, and whether a malignancy suspicious-NIPT result is associated with certain types of cancer. We have addressed this research question by combining the clinical data of 65 Dutch patients with pregnancy-associated cancer from the INCIP registry with the genomic NIPT data from the nationwide TRIDENT-2 study. We found that about a quarter of women with pregnancy-associated cancer had a malignancy suspicious-NIPT result, when tested with a genome-wide NIPT. A targeted NIPT sporadically revealed a suspicious result.
In all women with a pregnancy-associated haematological cancer, the genome-wide NIPT results were malignancy suspicious. As haematological malignancies arise from blood forming organs or lymph nodes, and maternal cfDNA in blood plasma predominantly originates from neutrophils, B- and T-lymphocytes,21,22 it is plausible that genomic instability occurring in neoplastic transformation of these cells can be detected relatively easy by copy number analysis of cfDNA in blood plasma.
In solid tumours, the NIPT was non-suspicious in the majority of cases. The suspicious ones mostly concerned advanced stage solid tumours. A previous study using NIPT in (pregnant) patients already known with breast cancer, showed malignancy-associated CNVs in 26% of, mostly symptomatic, patients.23 We found a lower detection rate for solid tumours in our study population. Possible explanations for the relatively low frequency of malignancy suspicious-NIPT results in the women with a solid tumour could be that these tumours often do not shed enough cfDNA to the blood stream, and, therefore, remain under the detection threshold of NIPT utilizing shallow sequencing depth. Levels of circulating tumour DNA (ctDNA) may rise in tumours with a higher rate of necrosis and cell proliferation,24 and in advanced disease.25
The tumour type distribution in this Dutch study population may (slightly) differ from the population-based cancer in pregnancy studies in Nordic countries26 or Australia, where for example melanomas are more common than in the INCIP database.1,27 This could have influenced the rate of malignancy suspicious-NIPTs in our study. Another limitation of our study is the relatively small sample size. However, selection bias is not assumed in the NIPT, as the uptake of NIPT in our study population (46.9%) is comparable with the earlier reported uptake of the general population in the Netherlands (45.9%).6 The low number of patients diagnosed with cancer postpartum (n = 4, 6.2%) in our study population is due to selection bias in the INCIP study, as a relatively small number of patients with a postpartum cancer diagnosis in the INCIP registry is currently registered, probably because tailored advice regarding the safety of oncological treatment on the unborn child is not needed anymore.28
For this study, we standardized the initial NIPT analyses by means of the “Maastricht Criteria”.15 Although no changes had occurred in the laboratory protocol or bioinformatics methods, in this way we eliminated differences in NIPT assessments between laboratories and discrepancies in interpretation over time. At the time of the initial assessment of the NIPTs, the “Maastricht criteria” were not yet available. In addition, we scrutinized the NIPT results for chromosomal aberrations known to be associated with the particular malignancy the patient was diagnosed with. In two patients the reviewed NIPT results were assessed differently compared to the initial results. In retrospect, the suspicion of a maternal malignancy could have been expressed more strongly for them. By utilizing uniform criteria, guidance is provided to obtain standardization in interpretation, reporting and registration.
If the detection of an underlying malignancy could significantly be advanced in time, the incidental malignancy-suspicious findings of an NIPT may be proven to be clinical relevant. Therefore, we performed explorative analyses on the time interval between NIPT and the date of cancer diagnosis. We found a shorter time interval in patients with a malignancy suspicious-NIPT compared to the group with a non-suspicious NIPT result, probably due to the fact that in patients with a malignancy suspicious-NIPT diagnostic examinations were initiated almost immediately. During the study period, the awareness has increased that a malignancy suspicious-NIPT result must be taken seriously, because it can lead to a cancer diagnosis. The awareness and related urgency to diagnose may probably further shorten the time interval between NIPT and cancer diagnosis in future cases of malignancy suspicious-NIPT.
The analysis of ctDNA in patients with cancer is rapidly evolving in the oncologic field, with presumably future implications for diagnostics, genomic profiling for treatment choices, monitoring treatment response and surveillance of recurrence.29 Besides the different applications, several platforms are used with differences in sequencing depth or breadth.30 From this point of view malignancy suspicious-NIPTs may be a step forward in detecting cancers. This study evaluates NIPT results in a sample of patients with known cancer status. It says nothing about whether NIPT is sensitive to use for cancer screening in a pregnancy cohort with unknown cancer-status. Thus, although cancer can occur as an incidental finding of NIPT for prenatal screening, NIPT should not be seen as a cancer test.10 Moreover, a malignancy is certainly not ruled out by a normal NIPT result. Communicating a suspicion of a malignancy in the NIPT report is important, because it could enable an earlier diagnosis and start of cancer therapy, especially for haematological malignancies.
In conclusion, this study evaluates prenatal cfDNA testing of a group of women with pregnancy-associated cancer, not knowing to have cancer at the time of NIPT request. All genome-wide NIPTs of women with a haematological malignancy were suspicious of a malignancy, irrespective of the disease stage. For the solid tumours the NIPT was most often non-suspicious, and chromosomal aberrations in the NIPT were almost exclusively seen in the advanced stages.
Contributors
The study was conceptualised by VTH, CH, CDS and MM. All authors designed the study, had access to all data and interpreted data, edited and reviewed the manuscript. All authors approved the final manuscript as submitted.
Data sharing statement
All data collected for this study are present in the paper or supplemental information or are available under a data use agreement and subject to the limitations of the informed consent document.
Declaration of interests
VTH participated in the advisory board of AstraZeneca, E Lilly and Novartis; received grants for the institution outside the present work, from AstraZeneca, E Lilly, Gilead, Novartis and Pfizer. All other authors declare no competing interests.
Acknowledgements
The authors would like to thank the TRIDENT-2 and INCIP study participants and investigators for their contribution. Sandra Geurts is acknowledged for her help with the statistical analyses. Patrick van Santvoort is acknowledged for providing data from Peridos, the national digital registration system for prenatal screening.
The Dutch NIPT Consortium: E.A. Sistermans, L. Henneman, A. Polstra, E. Voorhoeve, S.L. Zelderen-Bohla, E.M.J. Boon, M.P.R. Lombardi, C. Louwerens-Zintel, M. Smit, M.C. van Maarle, M.B. Tan-Sindhunata, K. van der Meij, H. Meij, C. Bax, E. Pajkrt, I.H. Linskens, L. Martin, J.T. Gitsels-van der Wal, S. van ‘t Padje (Amsterdam University Medical Centre); R.J.H. Galjaard, D. Van Opstal, M.I. Srebniak, F.M. Sarquis Jehee, I.H.I.M. Hollink, F. Sleutels, W. de Valk, W.H. Deelen, A.M.S. Joosten, K.E.M. Diderich, M.E. Redeker, A.T.J.I. Go, M.F.C.M Knapen, S. Galjaard, A.K.E. Prinsen, A.P.G. Braat (Erasmus Medical Centre, Rotterdam); M.V.E. Macville, S.J.C. Stevens, A. van der Wijngaard, L.H. Houben, M.A.A. van Esch-Lennarts, L. Hamers, A.G.P. Jetten, S.A.I. Ghesquiere, B. de Koning, M. Zamani Esteki, C.J. Heesterbeek, C.E.M. de Die-Smulders, H. Brunner, M.J. Pieters, A.B.C Coumans (Maastricht University Medical Centre); D.F.C.M. Smeets, B.H.W. Faas, D. Westra, M.M. Weiss, I. Derks-Prinsen, I. Feenstra, M. van Rij, E. Sikkel (Radboud University Medical Centre, Nijmegen); M.J.V. Hoffer, N.S. den Hollander, E.J.T. Verweij, M.C. Haak (Leiden University Medical Centre); R.F. Suijkerbuijk, B. Sikkema-Raddatz, I.M. van Langen, K. Bouman, L.K. Duin, G.H. Schuring-Blom, K.D. Lichtenbelt, M.N. Bekker (University Medical Centre, Utrecht); A.J.E.M. van der Ven (Royal Dutch Organization of Midwives (KNOV)); E. van Vliet-Lachotzki (Patient Alliance for Rare and Genetic Diseases (VSOP)); J. Pot (Dutch National Genetic Resource and Information Centre (Erfocentrum)).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101024.
Contributor Information
Catharina J. Heesterbeek, Email: karin.heesterbeek@mumc.nl.
The Dutch NIPT Consortium:
E.A. Sistermans, L. Henneman, A. Polstra, E. Voorhoeve, S.L. Zelderen-Bohla, E.M.J. Boon, M.P.R. Lombardi, C. Louwerens-Zintel, M. Smit, M.C. van Maarle, M.B. Tan-Sindhunata, K. van der Meij, H. Meij, C. Bax, E. Pajkrt, I.H. Linskens, L. Martin, J.T. Gitsels-van der Wal, R.J.H. Galjaard, D. Van Opstal, M.I. Srebniak, F.M. Sarquis Jehee, I.H.I.M. Hollink, F. Sleutels, W. de Valk, W.H. Deelen, A.M.S. Joosten, K.E.M. Diderich, M.E. Redeker, A.T.J.I. Go, M.F.C.M. Knapen, S. Galjaard, A.K.E. Prinsen, A.P.G. Braat, M.V.E. Macville, S.J.C. Stevens, A. van der Wijngaard, L.H. Houben, M.A.A. van Esch-Lennarts, L. Hamers, A.G.P. Jetten, S.A.I. Ghesquiere, B. de Koning, M. ZamaniEsteki, C.J. Heesterbeek, C.E.M. de Die-Smulders, H. Brunner, M.J. Pieters, A.B.C. Coumans, D.F.C.M. Smeets, B.H.W. Faas, D. Westra, M.M. Weiss, I. Derks-Prinsen, I. Feenstra, M. van Rij, E. Sikkel, M.J.V. Hoffer, N.S. den Hollander, E.J.T. Verweij, M.C. Haak, R.F. Suijkerbuijk, B. Sikkema-Raddatz, I.M. van Langen, K. Bouman, L.K. Duin, G.H. Schuring-Blom, K.D. Lichtenbelt, M.N. Bekker, A.J.E.M. van der Ven, E. van Vliet-Lachotzki, J. Pot, S. van ‘t Padje, I.M.C. Bakker, and E.J. Bradley
Appendix A. Supplementary data
References
- 1.Lee Y.Y., Roberts C.L., Dobbins T., et al. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994-2008: a population-based linkage study. BJOG. 2012;119(13):1572–1582. doi: 10.1111/j.1471-0528.2012.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson T.M., Johansson A.L.V., Hsieh C.C., Cnattingius S., Lambe M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114(3):568–572. doi: 10.1097/AOG.0b013e3181b19154. [DOI] [PubMed] [Google Scholar]
- 3.Pavlidis N.A. Coexistence of pregnancy and malignancy. Oncol. 2002;7(4):279–287. [PubMed] [Google Scholar]
- 4.International Network on Cancer Infertility and Pregnancy Cancer in pregnancy n.d. https://www.cancerinpregnancy.org Available from:
- 5.van der Meij K.R.M., Sistermans E.A., Macville M.V.E., et al. TRIDENT-2: national implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in The Netherlands. Am J Hum Genet. 2019;105(6):1091–1101. doi: 10.1016/j.ajhg.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Meij K.R.M., de Groot-van Mooren M., Carbo E.W.S., et al. Uptake of fetal aneuploidy screening after the introduction of the non-invasive prenatal test: a national population-based register study. Acta Obstet Gynecol Scand. 2021;100(7):1265–1272. doi: 10.1111/aogs.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne C.M., Hardisty E., Devers P., et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33(6):609–611. doi: 10.1002/pd.4100. [DOI] [PubMed] [Google Scholar]
- 8.Amant F., Verheecke M., Wlodarska I., et al. Presymptomatic identification of cancers in pregnant women during noninvasive prenatal testing. JAMA Oncol. 2015;1(6):814–819. doi: 10.1001/jamaoncol.2015.1883. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi D.W., Chudova D., Sehnert A.J., et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314(2):162–169. doi: 10.1001/jama.2015.7120. [DOI] [PubMed] [Google Scholar]
- 10.Heesterbeek C.J., Aukema S.M., Galjaard R.H., et al. Noninvasive prenatal test results indicative of maternal malignancies: a nationwide genetic and clinical follow-up study. J Clin Oncol. 2022:2426–2435. doi: 10.1200/JCO.21.02260. [DOI] [PubMed] [Google Scholar]
- 11.Straver R., Sistermans E.A., Holstege H., Visser A., Oudejans C.B., Reinders M.J. WISECONDOR: detection of fetal aberrations from shallow sequencing maternal plasma based on a within-sample comparison scheme. Nucleic Acids Res. 2014;42(5) doi: 10.1093/nar/gkt992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atlas of genetics and cytogenetics in oncology and haematology. http://atlasgeneticsoncology.org/
- 13.McKusick-Nathans Institue of Genetic Medicine JHUB, MD) Online mendelian inheritance in man, OMIM. ncbi.nlm.nih.gov/omim Available from:
- 14.Mitelman F., Johansson B., Mertens F. Mitelman database of chromosome aberrations and gene fusions in cancer 2022. https://mitelmandatabase.isb-cgc.org Available from:
- 15.Heesterbeek C.J., Lenaerts L., Tjan-Heijnen V.C.G., et al. Comprehensive recommendations for the clinical management of pregnant women with noninvasive prenatal test results suspicious of a maternal malignancy. JCO Oncol Pract. 2024 doi: 10.1200/OP.23.00594. [DOI] [PubMed] [Google Scholar]
- 16.Voutsadakis I.A. 3q26 amplifications in cervical squamous carcinomas. Curr Oncol. 2021;28(4):2868–2880. doi: 10.3390/curroncol28040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenaerts L., Brison N., Maggen C., et al. Comprehensive genome-wide analysis of routine non-invasive test data allows cancer prediction: a single-center retrospective analysis of over 85,000 pregnancies. eClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dow E., Freimund A., Smith K., et al. Cancer diagnoses following abnormal noninvasive prenatal testing: a case series, literature review, and proposed management model. JCO Precision Oncology. 2021;(5):1001–1012. doi: 10.1200/PO.20.00429. [DOI] [PubMed] [Google Scholar]
- 19.Dharajiya N.G., Grosu D.S., Farkas D.H., et al. Incidental detection of maternal neoplasia in noninvasive prenatal testing. Clin Chem. 2018;64(2):329–335. doi: 10.1373/clinchem.2017.277517. [DOI] [PubMed] [Google Scholar]
- 20.Ji X., Chen F., Zhou Y., et al. Copy number variation profile in noninvasive prenatal testing (NIPT) can identify co-existing maternal malignancies: case reports and a literature review. Taiwan J Obstet Gynecol. 2018;57(6):871–877. doi: 10.1016/j.tjog.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Sun K., Jiang P., Chan K.C., et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci U S A. 2015;112(40):E5503–E5512. doi: 10.1073/pnas.1508736112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberhofer A., Bronkhorst A.J., Uhlig C., Ungerer V., Holdenrieder S. Tracing the origin of cell-free DNA molecules through tissue-specific epigenetic signatures. Diagnostics. 2022;12(8) doi: 10.3390/diagnostics12081834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenaerts L., Che H., Brison N., et al. Breast cancer detection and treatment monitoring using a noninvasive prenatal testing platform: utility in pregnant and nonpregnant populations. Clin Chem. 2020;66(11):1414–1423. doi: 10.1093/clinchem/hvaa196. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Herrero E., Serna-Blasco R., Robado de Lope L., González-Rumayor V., Romero A., Provencio M. Circulating tumor DNA as a cancer biomarker: an overview of biological features and factors that may impact on ctDNA analysis. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.943253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettegowda C., Sausen M., Leary R.J., et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224) doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momen N.C., Arendt L.H., Ernst A., et al. Pregnancy-associated cancers and birth outcomes in children: a Danish and Swedish population-based register study. BMJ Open. 2018;8(12) doi: 10.1136/bmjopen-2018-022946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Haan J., Verheecke M., Van Calsteren K., et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 28.Maggen C., Wolters V., Cardonick E., et al. Pregnancy and cancer: the INCIP project. Curr Oncol Rep. 2020;22(2):17. doi: 10.1007/s11912-020-0862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S.A., Liu M.C., Aleshin A. Practical recommendations for using ctDNA in clinical decision making. Nature. 2023;619(7969):259–268. doi: 10.1038/s41586-023-06225-y. [DOI] [PubMed] [Google Scholar]
- 30.Hasenleithner S.O., Speicher M.R. A clinician's handbook for using ctDNA throughout the patient journey. Mol Cancer. 2022;21(1):81. doi: 10.1186/s12943-022-01551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.