Summary
Background
Mucormycosis is a deadly invasive fungal infection recently included in the WHO priority pathogen list. Here we sought to describe epidemiological trends of mucormycosis in France, and to evaluate factors associated with mortality.
Methods
From 2012 to 2022, we implemented a nationwide prospective surveillance programme for mucormycosis in France, focusing on epidemiology, species, seasonal variations. Factors associated with 3-month mortality were studied by univariable and multivariable logistic regression.
Findings
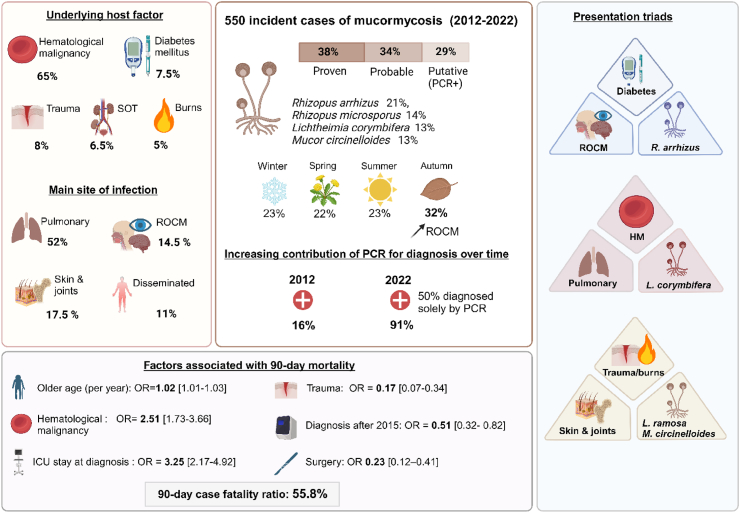
Among 550 cases of mucormycosis, the main underlying conditions were haematological malignancy (HM, 65.1%, 358/550), trauma (8%, 44/550), diabetes (7.5%, 41/550) and solid-organ transplants (6.5%, 36/550). Site of infection was pulmonary in 52.4% (288/550), rhinocerebral in 14.5% (80/550), and cutaneo-articular in 17.1% (94/550). Main species identified were Rhizopus arrhizus (21%, 67/316), Rhizopus microsporus (13.6%, 43/316), Lichtheimia corymbifera and Mucor circinelloides (13.3%, 42/316 each), Rhizomucor pusillus (12%, 38/316), and Lichtheimia ramosa (10.8%, 34/316). We found associations between underlying condition, site of infection, and infecting species, including a previously undescribed triad of trauma, cutaneo-articular localisations, and L. ramosa/M. circinelloides. Diagnostic contribution of Polymerase Chain Reaction (PCR) increased from 16% (4/25) in 2012 to 91% (61/67) in 2022, with more than 50% of diagnoses relying solely on PCR in 2022. We also found seasonal variations with relatively more cases in autumn. Ninety-day mortality was 55.8% (276/495). Independent prognostic factors were age, diagnosis in Intensive Care Unit (ICU), and HM while diagnosis after 2015 (i.e. large implementation of PCR) and surgery were associated with reduced mortality.
Interpretation
This study reveals major mucormycosis epidemiological changes in France, with a large predominance of HM patients, and a parallel between PCR multicentre implementation and improved prognosis. We also evidence new associations between species, localisations and risk factors, as well as seasonal variations.
Funding
Recurrent financial support from Santé Publique France and Institut Pasteur.
Keywords: Epidemiology, Mucormycosis, Zygomycosis, Mucorales, Fungal infection, Mucormycosis serum PCR
Research in context.
Evidence before this study
Mucormycosis is the second most frequent mold-related infection in Europe after Aspergillus and is the fungal infection associated with the highest mortality rate. Over the last decade, this disease has undergone significant changes in risk factors, diagnostics, and therapeutics, and its epidemiology varies greatly across the globe. We searched Pubmed (MEDLINE) using the terms “Mucormycosis” OR “Zygomycosis” OR “Mucorales infection” for studies published in English or in French from database inception to December 2023, excluding reviews and case reports. There is substantial literature concerning mucormycosis, but we focused on clinical trials and prospective and retrospective cohorts, worldwide but also specifically in European countries. There was a recent surge in mucormycosis-related publications following the Covid epidemic in India, which was not our target population. In France, the Retrozygo study was the largest cross-sectional study, with data only up to 2007. In Europe, large cohorts are scarce for this rare but deadly disease, and none have ever included more than 500 cases.
Added value of this study
Given all these reasons, we believed it critical to implement mucormycosis surveillance in our country. Over the last ten years, we therefore implemented a nationwide prospective surveillance in France at the National Reference Center for Invasive Mycoses and Antifungals, including polyphasic identification of all strains, epidemiology, seasonal variations, and factors associated with death.
With 550 cases, this prospective nationwide study is the largest of its kind ever reported in Europe, providing a unique perspective on mucormycosis over the last decade and revealing significant shifts in its epidemiology.
We report an increase in haematological malignancy (HM) patients and pulmonary localizations, alongside an increase in PCR use for diagnosis. One-third of the patients were in an intensive care unit. It is important to note that a quarter of patients had fungal coinfection. The distribution of fungal species also shifted by comparison with previous studies and in other geographical settings, and this was related to the underlying disease and localization. We showed associations between underlying conditions, site of infection, and infecting species, including previously undescribed triads of species, localization and underlying disease. Upon investigating the potential seasonal trends in the epidemiology of mucormycosis, more cases were observed during fall, especially those involving rhino-orbital presentations as well as specific species distributions.
Finally, our results highlight that surgery and diagnosis after PCR implementation were protective, whereas age, haematological malignancy and diagnosis while in the ICU was associated with increased mortality.
Implications of all the available evidence
These findings, which coincided with the widespread use of PCR as a diagnostic technique, demonstrated an epidemiological shift in the underlying causes, species involved, and geography of mucormycosis in France. The identification of seasonal variations and mortality factors opens new perspectives for research and management of the disease, especially in the context of climate change. We believe that our results are in favour of the generalization of PCR as a diagnostic tool, and for the necessity of early surgical treatment.
Introduction
Mucormycosis is an uncommon but severe emerging invasive fungal infection. Mucorales are ubiquitous in the environment, and were recently included in the WHO fungal priority pathogens list as a “high priority” concern. The airborne spores of several thermotolerant and thermophilic Mucorales species can be transmitted through inhalation or by deposition on injured skin, causing tissue infarction, necrosis, and dissemination through vascular invasion. The main genera involved in human mucormycosis are Rhizopus, Rhizomucor, Lichtheimia, Mucor, and to a lesser extent Cunninghamella, Saksenaea, Actinomucor and Apophysomyces,1, 2, 3, 4, 5 with geographical differences in their worldwide distribution.6 Predisposing conditions also differ according to geographical setting and healthcare systems: in Europe, mucormycosis mostly affects immunocompromised patients such as those with haematological malignancies (HM) or solid-organ transplants (SOT),1,7, 8, 9, 10, 11, 12 while diabetes mellitus is the main host factor underlying mucormycosis in India.13, 14, 15 Recently, COVID-19 has also emerged as a risk factor, with upwards of 40,000 cases of COVID-associated mucormycosis diagnosed in India, but also in France, and worldwide.16, 17, 18, 19, 20, 21
A rise in the incidence of mucormycosis has been reported in Europe and India,13,22, 23, 24 which may be attributed to an increasing population of at-risk individuals, better awareness, and the development of new diagnostic tools. In particular, Polymerase Chain Reaction (PCR)-based methods for the diagnosis of mucormycosis have been developed over the past decade and are now widely available in France.25, 26, 27, 28
The prognosis of mucormycosis remains poor, with mortality rates ranging from 47% to 57%, depending on the underlying condition, site of infection, and therapeutic management.1,7,8,29 The recommended first-line therapy includes a combination of liposomal amphotericin B and early extensive surgical treatment.25 New antifungal drugs such as isavuconazole have been developed,30 providing valid therapeutic alternatives.
Taking into account these various changes, it is likely that the epidemiology of mucormycosis has evolved over time. Underlying conditions, species distribution, diagnostic tools, and available treatments also highly vary according to country. Up-to-date data from different geographical regions, which has been called for by the WHO, are therefore crucial to increase the knowledge of this somewhat neglected disease.
In France, the National Reference Centre for Invasive Mycoses and Antifungals (NRCMA) implemented in 2012 a nationwide surveillance network called RESSIF (for RESeau de Surveillances des Infections Fongiques) that prospectively collects epidemiological and mycological data regarding all invasive fungal infections diagnosed in the volunteer participating centres through a standardised questionnaire and centralisation of strains. All isolates are morphologically and molecularly characterised.31 This network allowed us to have a unique prospective description of mucormycosis epidemiology over the past 11 years. We sought to describe epidemiological trends of mucormycosis in France from 2012 to 2022, including the current underlying diseases, their association with clinical presentations and the species involved, and seasonal variations. We also sought to evaluate factors associated with 3-month mortality.
Methods
We performed a cross-sectional study of mucormycosis nested within a prospective surveillance programme from January 1st, 2012, to December 31st, 2022. Data were collected prospectively through the RESSIF programme.31 The questionnaire included epidemiological, clinical, biological, and therapeutic data recorded anonymously through a secure database. Cases of mucormycosis were classified as proven, probable or putative. Proven and probable mucormycosis cases were defined according to the 2019 European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG)32 criteria, with the addition of diabetes, trauma and burns as risk factors for probable mucormycosis. The addition of these criteria is concordant with recent guidelines on diagnosis and management of mucormycosis from the European Confederation of Medical Mycology (ECMM) and the Mycoses Study Group Education & Research Consortium (MSG ERC).25 Probable cases were defined by the association of a host factor, clinical-radiological features concordant with the disease, and mycological evidence from culture and microscopic detection in sputum, BAL, bronchial brush, aspirate, rhino-cerebral or cutaneous non-sterile samples. Host factors included diabetes mellitus, trauma, burns, neutropenia <500 neutrophils/mm3 for >10 days, haematologic malignancy, receipt of an allogeneic stem cell transplant, receipt of solid organ transplant, corticotherapy at a dosage ≥0.3 mg/kg for ≥3 weeks within the prior 60 days, treatment with T-cell immunosuppressants or B-cell immunosuppressants during the prior 90 days, inherited severe immunodeficiency, and acute graft versus host disease. Neutropenia was defined by a neutrophil count below 500 cells/mm3 for at least 10 days at the time of diagnosis.
Putative cases were defined as those diagnosed with at least one PCR-positive serum or broncho-alveolar lavage (BAL) sample in patients with the above conditions and clinical presentation compatible with the diagnosis of mucormycosis. Coinfections with another fungal pathogen were recorded within 15 days of diagnosing mucormycosis and could affect either the same body site or another.
The date of diagnosis corresponded to the first microbiological evidence of mucormycosis. Survival at 90 days was calculated from the date of diagnosis.
Based on previous data7,12 and experts’ opinion regarding mucormycosis, six main underlying conditions (MUC) were considered, but only one was assigned to each patient in a hierarchical categorical variable according to the following hierarchy: 1- haematological malignancy (HM) (including all patients who underwent allogenic stem cell transplant even for non malignant haematological disorder).; 2- burns; 3- solid organ transplants (SOT); 4– severe skin injury thereafter called “trauma”; 5-diabetes; 6- others, in the absence of all the previous conditions.
Based on the anatomical sites, infection was categorized as localized “rhinocerebral”, “cutaneo-articular”, pulmonary (“lung”), or “others”, or disseminated (defined by the involvement of two or more non-contiguous sites except for lung and sinus).
Species were identified by culture only, and PCR testing was only reported if positive, with no information about the species. Up to 2017, all centres used the same in-house technique for PCR assay, developed by Besançon.27 From 2017 to 2022, most centres still used the in-house technique, and the others used a commercial kit (first Mucorgenius (Pathognostics), then Mycogenie (Ademtech)).
All fungal coinfections were diagnosed according to the definition from the 2019 European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG)32 criteria.
All isolates collected at the NRCMA were checked for purity and subsequently subcultured on malt extract agar (MEA) 2% (Oxoid, http://www.oxoid.com) and potato dextrose agar (PDA) (BD diagnostic systems https://www.bd.com) for three to 5 day at 30 °C to promote sporulation. Identification at the species level was based on macroscopic and microscopic criteria and confirmed by sequencing of the complete ITS1-5.8S-ITS2 region.33 Pairwise alignments were performed on the sequences against curated fungal reference databases available at the online MycoBank database (http://www.mycobank.org).
All cases were reviewed by 2 of the authors before inclusion, and only cases eligible through case definitions were included.
This study was approved by the Institut Pasteur Internal Review Board (2009–34/IRB) and by the Commission Nationale de l’Informatique et des Libertés according to French law.
For descriptive analyses, categorical variables were presented as counts and percentages. Associations were considered statistically significant for p values < 0.05. Univariable logistic regression was used to identify factors associated with all-cause mortality at day 90. All non-nested variables with p < 0.10 were subsequently included in a multivariable model and step-wise selection was used to determine the best parcimonious model. Patients for whom outcome data was missing were excluded from the main analysis. To ensure that missing data would not affect our results, we also conducted a sensitivity analysis after performing multiple imputations. Variables studied in that section were used as predictors, i.e. age, localisation, main underlying disease, cases before or after 2015, and intensive care unit (ICU) stay at diagnosis.
Variables related to medical or surgical treatment were studied in a sensitivity analysis with a landmark of one day to avoid immortal time bias. Survival analysis using Logrank test was also used.
To evaluate a proxy of mucormycosis burden at a national level, we estimated the incidence of mucormycoses as described in Bretagne et al.31 as number of events per hospitalisation days in the hospitals participating to surveillance.
All statistical analyses were performed using the R 4.0 software package (http://cran.r-project.org)? With the packages prettyR, ggplot2, binom, Epi, knitr, dplyr, survival, quetionr, Hmisc, ggpubr, lubridate, tidyr, survminer, viridis, gplots, ggsci, RColorBrewer and mice.
This study adheres to the STROBE checklist.
Role of the funding source
Institut Pasteur and Santé Publique France did not have any role in study design, collection, analysis, interpretation of the data, writing the report or decision to submit the paper for publication.
Results
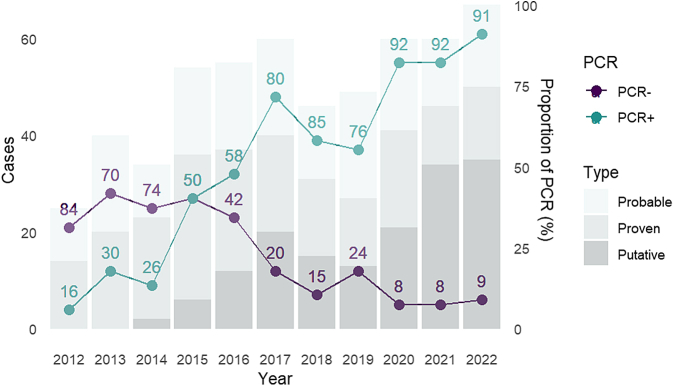
Overall, 550 cases were recorded, including 207 (37.6%) proven, 185 (33.6%) probable and 158 (28.7%) putative infections (Supplementary Table S1). Among these, 451 cases occurred after 2015, when Mucorales PCR was largely implemented in France (Fig. 1). Overall, despite limitations, we could estimate that the global incidence of mucormycosis in participating centres over the study period was 0.065/10 000 hospitalisation days (Supplementary Table S3).
Fig. 1.
Mucormycosis cases distribution (n = 550). Legend: Type: cases type, defined as Proven, Probable, or Putative (putative: cases diagnosed with at least one PCR-positive serum or broncho-alveolar lavage (BAL) sample (excluding proven or probable cases)). PCR +: cases with at least one PCR-positive sample; PCR −: cases without any PCR-positive sample.
Underlying conditions and localizations
Median age was 61 years (IQR 46–69, range, 1–90 years), with a majority of men (65.6%). As a hierarchical variable, the main underlying condition was HM (358/550, 65.1%), followed by trauma (n = 44, 8.0%), diabetes (n = 41, 7.5%), SOT (n = 36, 6.5%), burns (n = 29, 5.3%) and others (n = 42, 7.6%). HM mainly included acute leukaemia (222/355, 62.5%) and lymphoma (49/355, 13.8%). Most HM patients had recent neutropenia (258/355, 72.6%) and approximately a third had undergone haematopoietic stem-cell transplantation (HSCT) (112/355, 31.5%). Three patients in the HM hierarchical group underwent allogenic stem cell transplant for non malignant haematological disorders (i.e. sickle cell disease). Overall, 96/550 patients (17.5%) had diabetes, including 37/355 (10.4%) of HM and 12/36 (33.3%) of SOT patients. Corticosteroids (n = 122, 22.2%) and other immunosuppressive drugs (n = 333, 60.5%) were common (Table 1, Supplementary Table S1).
Table 1.
Patients characteristics, according to main underlying disease.
| All n = 550 | Haematological malignancy n = 358 (%) | Burns n = 29 (%) | Trauma n = 44 (%) | Solid Organ Transplant n = 36 (%) | Diabetes n = 41 (%) | Other n = 42 (%) | p (overall) | |
|---|---|---|---|---|---|---|---|---|
| Mean age (year) | 55.32 | 56.0 | 48.4 | 52.1 | 56.1 | 61.6 | 50.5 | 0.04 |
| Male | 361/550 (65.6) | 220/358 (61.5) | 20/29 (69.0) | 33/44 (75.0) | 27/36 (75.0) | 33/40 (80.8) | 28/42 (66.7) | 0.11 |
| ICU at diagnosis | 182/550 (33.4) | 79/358 (22.1) | 28/29 (96.6) | 15/44 (34.1) | 15/36 (41.7) | 16/41 (39.0) | 19/42 (45.2) | <0.001 |
| Acute leukaemia | 222/550 (40.4) | 222/358 (62.0) | – | – | – | – | – | – |
| Lymphoma | 49/550 (8.9) | 49/358 (13.7) | – | – | – | – | – | – |
| Case classification | ||||||||
| Proven | 207/550 (37.7) | 115/358 (32.1) | 11/29 (37.9) | 22/44 (50.0) | 17/36 (47.2) | 23/41 (56.1) | 19/42 (45.2) | |
| Probable | 185/550 (34.1) | 103/358 (28.7) | 14/29 (48.3) | 21/44 (47.7) | 18/36 (50.0) | 17/41 (41.5) | 12/42 (28.6) | 0.01 |
| Putative | 158/550 (28.1) | 140/358 (39.1) | 4/29 (13.8) | 1/44 (2.3) | 1/36 (2.8) | 1/41 (2.4) | 11/42 (26.2) | |
| Localisation | ||||||||
| Lung (localized) | 288/550 (52.4) | 229/358 (64.0) | 0 | 2/44 (4.5) | 22/36 (61.1) | 14/41 (34.1) | 21/42 (50.0) | <0.001 |
| Rhino-orbito-cerebral (localized) | 80/550 (14.5) | 44/358 (12.3) | 0 | 3/44 (6.8) | 7/36 (19.4) | 19/41 (46.4) | 7/42 (16.7) | |
| Cutaneo-articular (localized) | 94/550 (17.1) | 19/358 (5.3) | 26/29 (89.7) | 38/44 (86.4) | 3/36 (8.3) | 4/41 (9.8) | 4/42 (9.5) | |
| Other (localized) | 27/550 (4.9) | 17/358 (4.7) | 0 | 1/44 (2.3) | 3/36 (8.3) | 1/41 (2.5) | 5/42 (11.9) | |
| Disseminated | 61/550 (11.1) | 49/358 (13.7) | 3/29 (10.3) | 0 | 1/36 (2.7) | 3/41 (7.3) | 5/42 (11.9) | |
| Fungal species | ||||||||
| Rhizopus arrhizus | 67/316 (21.2) | 32/161 (19.9) | 0 | 2/42 (4.8) | 9/29 (31.0) | 18/31 (58.1) | 6/29 (20.7) | |
| Rhizopus microsporus | 43/316 (13.6) | 26/161 (16.1) | 1/24 (4.2) | 2/42 (4.8) | 6/29 (20.7) | 4/31 (12.9) | 4/29 (13.8) | <0.001 |
| Lichtheimia corymbifera | 42/316 (13.3) | 27//161 (16.7) | 3/24 (12.5) | 5/42 (11.9) | 3/29 (10.3) | 0 | 4/29 (13.8) | |
| Mucor circinelloides | 42/316 (13.3) | 5/161 (3.1) | 15/24 (62.5) | 17/42 (40.5) | 2/29 (6.9) | 0 | 3/29 (10.3) | |
| Rhizomucor pusillus | 38/316 (12.0) | 33/161 (20.5) | 0 | 1/42 (23.8) | 1/29 (3.4) | 0 | 3/29 (10.3) | |
| Lichtheimia ramosa | 34/316 (10.8) | 13/161 (8.1) | 5/24 (20.8) | 11/42 (26.2) | 1/29 (3.4) | 2/31 (6.4) | 2/29 (6.9) | |
| Others | 50/316 (15.8) | 25/161 (15.5) | 0 | 4/42 (9.5) | 7/29 (24.1) | 7/31 (22.6) | 7/29 (24.1) | |
| Fungal coinfection | 132/550 (24.0) | 80/358 (22.3) | 8/29 (27.6) | 20/44 (45.5) | 9/36 (25.0) | 5/41 (12.2) | 10/42 (23.8) | 0.01 |
| Positive PCRa | 379/550 (68.9) | 275/358 (76.8) | 19/29 (65.5) | 15/44 (34.1) | 17/36 (47.2) | 21/41 (51.2) | 32/42 (76.2) | |
| Positive PCR on serum | 278/550 (50.5) | 221/358 (61.7) | 19/29 (65.5) | 4/44 (9.1) | 8/36 (22.2) | 10/41 (24.4) | 16/42 (38.1) | <0.001 |
| Positive PCR on pulmonary samples | 131/550 (23.8) | 104/358 (29.1) | 0 | 0 | 8/36 (22.2) | 5/41 (12.2) | 14/42 (33.3) | <0.001 |
| Diagnosis after 2015 | 451/550 (82.0) | 299/358 (83.5) | 18/29 (62.1) | 37/44 (84.1) | 32/36 (88.9) | 29/41 (70.7) | 36/42 (85.7) | 0.051 |
| Surgical treatment | 166/441 (37.6) | 58/276 (21.0) | 27/29 (93.1) | 40/41 (97.6) | 11/29 (37.9) | 19/34 (55.9) | 11/32 (34.4) | <0.001 |
| First-line therapy | ||||||||
| Liposomal amphotericin B (all) | 413/550 (75.1) | 267/358 (74.6) | 24/29 (82.8) | 39/44 (88.6) | 26/36 (72.2) | 32/41 (78.0) | 27/42 (64.2) | |
| Liposomal amphotericin B (monotherapy) | 318/550 (57.8) | 193/358 (53.9) | 19/29 (65.5) | 35/44 (79.5) | 21/36 (58.3) | 27/41 (65.9) | 23/42 (54.8) | <0.01 |
| Isavuconazole (monotherapy) | 36/550 (6.5) | 26/358 (7.3) | 0 | 0 | 2/36 (5.6) | 3/41 (7.3) | 5/42 (11.9) | |
| Posaconazole (monotherapy) | 11/550 (2.0) | 8/358 (2.2) | 0 | 1/44 (2.3) | 1/36 (2.8) | 1/41 (2.5) | 0 | |
| Death before day 90 | 276/495 (55.8) | 203/317 (64.0) | 10/27 (37.0) | 8/43 (18.6) | 19/34 (55.9) | 16/37 (43.2) | 20/37 (54.1) | <0.001 |
ICU: Intensive Care Unit. Denominator represents the number of patients for whom the information was available.
All samples included. Denominators for PCR data are 550 despite the fact that not all patients had PCR screening.
Approximately a third of patients were in an intensive care unit (ICU) at diagnosis, more frequently for those with burns and SOT recipients (28/29, 96.6%, and 41.7%, 15/36, respectively, p < 0.001) (Table 1), and for cutaneo-articular localisations (p < 0.0001) (Table 2). Forty percent of patients (223/550) were receiving prophylactic, preemptive or curative antifungal drugs at diagnosis [fluconazole (n = 86), posaconazole (n = 48), isavuconazole (n = 13), voriconazole (n = 13), caspofungin (n = 52) or micafungin (n = 5)], mostly HM patients.
Table 2.
Patients characteristics, according to localisation.
| All n = 550 | Lung N = 288 (%) | Rhino cerebral N = 80 (%) | Cutaneo articular N = 94 (%) | Other localization N = 27 (%) | Disseminated N = 61 (%) | p (overall) | |
|---|---|---|---|---|---|---|---|
| Median age (year) | 55.32 | 62 | 62.5 | 54 | 51 | 60 | <0.0001 |
| Male | 361/550 (65.6) | 179/288 (62.2) | 54/80 (67.5) | 68/94 (72.3) | 15/27 (55.6) | 45/61 (73.8) | 0.15 |
| ICU at diagnosis | 182/550 (33.4) | 92/288 (31.9) | 17 (21.3) | 42/94 (44.7) | 12/27 (44.4) | 19/61 (31.1) | <0.0001 |
| Case classification | |||||||
| Proven | 207/550 (37.7) | 43/288 (14.9) | 61/80 (76.3) | 41/94 (43.6) | 21/27 (77.8) | 41/61 (67.2) | <0.0001 |
| Probable | 185/550 (34.1) | 110/288 (38.2) | 13/80 (16.3) | 47/94 (50.0) | 0 | 15/61 (24.6) | |
| Putative | 158/550 (28.1) | 135/288 (46.9) | 6/80 (7.5) | 6/94 (6.4) | 6/27 (22.2) | 5/61 (8.2) | |
| Main underlying disease | |||||||
| Haematological malignancy | 358/550 (65.1) | 229/288 (79.5) | 44/80 (55) | 19/94 (20.2) | 17/27 (63.0) | 49/61 (80.3) | |
| Burn | 29/550 (5.3) | 0 | 0 | 26/94 (27.7) | 0 | 3/61 (4.9) | <0.0001 |
| Solid organ transplant | 36/550 (6.5) | 22/288 (7.6) | 7/80 (8.75) | 3/94 (3.2) | 3/27 (11.1) | 1/61 (1.6) | |
| Trauma | 44/550 (8.0) | 2/288 (0.7) | 3/80 (3.75) | 38/94 (40.4) | 1/27 (3.7) | 0 | |
| Diabetes | 41/550 (7.5) | 14/288 (4.9) | 19/80 (23.75) | 4/94 (4.2) | 1/27 (3.7) | 3/61 (4.9) | |
| Other | 42/550 (7.6) | 21/288 (7.3) | 7/80 (8.75) | 4/94 (4.2) | 5/27 (18.5) | 5/61 (8.2) | |
| Neutropenia | 171/288 (59.4) | 28/80 (35.0) | 15/94 (16.0) | 14/27 (51.9) | 38/61 (62.3) | <0.0001 | |
| Acute leukaemia | 222/550 (40.4) | 149/288 (51.7) | 23/80 (28.8) | 10/94 (10.6) | 10/27 (37.1) | 30/61 (49.2) | <0.0001 |
| Lymphoma | 49/550 (8.9) | 34/288 (11.8) | 3/80 (3.75) | 2/94 (2.1) | 3/27 (11.1) | 7/61 (11.5) | <0.0001 |
| Fungal species | |||||||
| Rhizopus arrhizus | 67/316 (21.2) | 25/117 (21.4) | 30/53 (56.6) | 7/83 (8.4) | 2/17 (11.7) | 3/46 (6.5) | |
| Rhizopus microsporus | 43/316 (13.6) | 25/117 (21.4) | 7/53 (13.2) | 4/53 (4.8) | 3/17 (17.6) | 4/46 (8.7) | <0.0001 |
| Lichtheimia corymbifera | 42/316 (13.3) | 16/117 (13.7) | 7/53 (13.2) | 12/53 (14.5) | 1/17 (5.9) | 6/46 (13.0) | |
| Mucor circinelloides | 42/316 (13.3) | 4/117 (3.4) | 0 | 31/53 (37.3) | 2/17 (11.8) | 5/46 (10.9) | |
| Rhizomucor pusillus | 38/316 (12.0) | 19/117 (16.2) | 2/53 (3.8) | 0 | 0 | 17/46 (37.0) | |
| Lichtheimia ramosa | 34/316 (10.8) | 5/117 (4.3) | 3/53 (5.7) | 20/53 (24.1) | 3/17 (17.6) | 3/46 (6.5) | |
| Others | 50/316 (15.8) | 23/117 (19.7) | 4/53 (7.5) | 9/53 (10.8) | 6/17 (35.3) | 8/46 (17.4) | |
| Fungal coinfection | 132/550 (23.7) | 83/288 (28.8) | 11/80 (13.75) | 28/94 (29.8) | 2/27 (7.4) | 8/61 (13.1) | <0.001 |
| PCR positivea | 379/550 (68.9) | 223/288 (77.4) | 47/80 (58.8) | 46/94 (48.9) | 18/27 (66.7) | 45/61 (73.8) | <0.0001 |
| PCR on serum | 278/550 (50.5) | 168/288 (58.3) | 26/80 (32.5) | 31/94 (33.0) | 14/27 (51.9) | 39/61 (63.9) | <0.0001 |
| PCR on pulmonary sample | 131/550 (23.8) | 114/288 (39.6) | 1/80 (1.23) | 0 | 0 | 16/61 (26.2) | <0.0001 |
| Diagnosis after 2015 | 451/550 (82.0) | 253/288 (87.8) | 61/80 (76.3) | 73/94 (77.7) | 20/27 (74) | 44/61 (72.1) | 0.005 |
| Surgical treatment | 166/441 (37.6) | 19/213 (8.9) | 45/62 (72.6) | 73/84 (86.9) | 12/23 (52.2) | 17/59 (28.8) | <0.0001 |
| Death before day 90 | 276/495 (55.8) | 155/248 (62.5) | 41/74 (55.4) | 26/92 (28.3) | 16/27 (59.3) | 38/54 (70.4) | <0.0001 |
ICU: Intensive Care Unit. Denominator represents the number of patients for whom the information was available (not stated when it was available for all patients).
All samples included. Denominators for PCR data are 550 despite the fact that not all patients had PCR screening.
Sites of infection included localised lung (n = 288, 52.4%), rhinocerebral (n = 80, 14.5%), cutaneo-articular (n = 94, 17.1%), other body site (n = 27, 4.9%), or disseminated (n = 61, 11.1%) infections. A concurrent fungal coinfection was diagnosed in 132 (24.0%) cases, mostly aspergillosis (98/132, 74.2%) and fusariosis (13/132, 9.8%). Coinfections were more frequent in pulmonary (83/288, 28.8%) and cutaneo-articular localisations (28/94, 29.8%), than in other sites of infection (p < 0.001). Thirty seven patients had viral coinfection including 20 SARS-CoV2 coinfections and 64 patients had bacterial coinfections.
Diagnosis
While 314 cases were culture-positive (57.1%), 379/550 patients (68.9%) had a positive PCR result, including 278/550 patients (50.5%) with a positive serum PCR test and 131/550 (23.8%) with a positive PCR result from a respiratory sample. The proportion of diagnosed cases with a positive PCR result increased over time, from 16.0% (4/25) in 2012 to 91.0% (61/67) in 2022, with a massive surge in 2015; in parallel so did the proportion of putative cases, with more than 50% of diagnoses in 2022 relying solely on PCR (Fig. 1). PCR was most often positive in HM patients (275/358, 76.8%) and in pulmonary infections (233/288, 77.4%). Among cases diagnosed solely with PCR (putative cases), 88.6% (140/158) had HM and 138 were diagnosed after 2015.
Species distribution
Six main species were identified among the 316 cases with a positive culture: Rhizopus arrhizus (n = 67, 21.2%), Rhizopus microsporus (n = 43, 13.6%) Lichtheimia corymbifera and Mucor circinelloides, (n = 42, 13.3% each), Rhizomucor pusillus (n = 38, 12.0%) and Lichtheimia ramosa (n = 34, 10.8%). Other species such as Cunninghamella bertholletiae, Mucor indicus, Mucor velutinosus, Mucor irregularis, M. hiemalis, Rhizomucor miehei, Lichtheimia ornata, Actinomucor elegans, Saksenaea vasiformis, Saksenaea erythrospora, Syncephalastrum racemosum were recovered in 50 cases. Patient main characteristics according to Mucorales species are presented in Table 3 and Fig. 2.
Table 3.
Patients characteristics, according to species (N = 316).
| Rhizopus arrhizus N = 67 | Rhizopus microsporus N = 43 | Lichtheimia corymbifera N = 42 | Lichtheimia ramosa N = 34 | Mucor circinelloides N = 42 | Rhizomucor pusillus N = 38 | Otherb N = 50 | p (overall) | |
|---|---|---|---|---|---|---|---|---|
| Mean age (year) | 57.3 | 60.7 | 50.7 | 51.4 | 46.9 | 58.0 | 51.6 | 0.01 |
| Male | 41/67 (61.2) | 29/43 (67.4) | 29/42 (69.0) | 26/34 (76.5) | 27/42 (64.3) | 28/38 (73.7) | 37/50 (74.0) | 0.64 |
| Main underlying disease | <0.0001 | |||||||
| Haematological malignancy | 32/67 (47.8) | 26/43 (60.5) | 27/42 (64.3) | 13/34 (38.2) | 5/42 (11.9) | 33/38 (86.8) | 25/50 (50.0) | |
| Burn | 0 | 1/43 (2.3) | 3/42 (7.1) | 5/34 (14.7) | 15/42 (35.7) | 0 | 0 | |
| Trauma | 2/67 (3.0) | 2/43 (4.7) | 5/42 (11.9) | 11/34 (32.4) | 17/42 (40.5) | 1/38 (2.6) | 4/50 (8.0) | |
| Solid organ transplant | 9/67 (13.4) | 6/43 (14.0) | 3/42 (7.1) | 1/34 (2.9) | 2/42 (4.8) | 1/38 (2.6) | 7/50 (14.0) | |
| Diabetes | 18/67 (26.9) | 4/43 (9.3) | 0 | 2/34 (5.9) | 0 | 0 | 7/50 (14.0) | |
| Other | 6/67 (9.0) | 4/43 (9.3) | 4/42 (9.5) | 2/34 (5.9) | 3/42 (7.1) | 3/38 (7.9) | 7/50 (14.0) | |
| Neutropenia | 16/67 (23.9) | 18/43 (41.9) | 22/42 (52.4) | 12/34 (35.3) | 3/42 (7.1) | 25/38 (65.8) | 17/50 (34.0) | <0.0001 |
| Localisation | <0.0001 | |||||||
| Lung (localized) | 25/67 (37.3) | 25/43 (58.1) | 16/42 (38.1) | 5/34 (14.7) | 4/42 (9.5) | 19/38 (50.0) | 23/50 (46.0) | |
| Rhino-cerebral (localized) | 30/67 (44.8) | 7/43 (16.3) | 7/42 (16.7) | 3/34 (8.8) | 0 | 2/38 (5.3) | 4/50 (8.0) | |
| Cutaneo-articular (localized) | 7/67 (10.4) | 4/43 (9.3) | 12/42 (28.6) | 20/34 (58.8) | 31/42 (73.8) | 0 | 9/50 (18.0) | |
| Other (localized) | 2/67 (3.0) | 3/43 (7.0) | 1/42 (2.4) | 3/34 (8.8) | 2/42 (4.8) | 0 | 6/50 (12.0) | |
| Disseminated | 3/67 (4.5) | 4/43 (9.3) | 6/42 (9.3) | 3/34 (8.8) | 5/42 (11.9) | 17/38 (44.7) | 8/50 (16.0) | |
| PCR positivea | 36/67 (53.7) | 23/43 (53.5) | 25/42 (59.5) | 11/34 (32.3) | 21/42 (50.0) | 20/38 (52.6) | 26/50 (52.0) | 0.37 |
| PCR on serum | 20/67 (29.9) | 18/43 (41.9) | 20/42 (47.6) | 7/34 (20.6) | 15/42 (35.7) | 16/38 (42.1) | 17/50 (34.0) | 0.19 |
| Surgical treatment | 28/54 (51.9) | 12/31 (38.7) | 14/34 (41.2) | 20/28 (71.4) | 34/39 (87.2) | 6/33 (18.2) | 15/34 (34.1) | <0.0001 |
| Death before day 90 | 34/64 (53.1) | 33/43 (76.7) | 28/40 (70.0) | 12/32 (37.5) | 12/37 (32.4) | 29/36 (80.6) | 28/45 (62.2) | <0.0001 |
Denominator represents the number of patients for whom the information was available (not stated when it was available for all patients).
PCR on serum samples. Denominators for PCR data are all patients despite not all had a PCR screening.
Other species included Cunninghamella bertholletiae (n = 8), Rhizomucor miehei (n = 7), Mucor indicus (n = 5), Rhizopus arrhizus var. delemar (n = 4), Saksenaea vasiformis (n = 3), Lichtheimia ornata, Mucor velutinosus and Actinomucor elegans (n = 2 each), Mucor hiemalis, Mucor irregularis, Rhizopus stolonifer var stolonifer, Saksenaea erythrospora, and Syncephalastrum racemosum (n = 1 each). A few isolates were not available for identification to the species level (3 Lichtheimia sp. (n = 2), Cunnninghamella sp. (n = 2), Rhizomucor sp. (n = 2), Rhizopus sp. (n = 3), and 1 Mucor sp. (n = 3)).
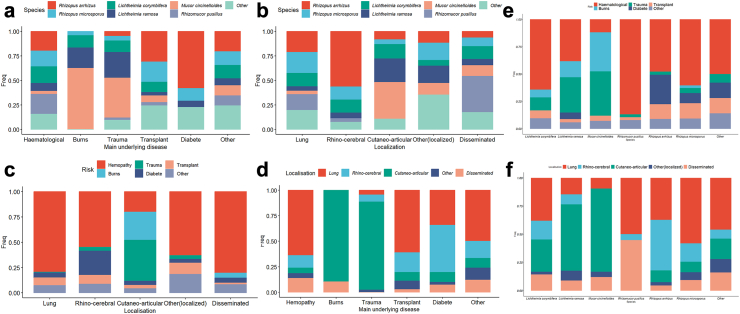
Fig. 2.
Interplays between species, localization and main underlying disease. a Proportion of species involved according to main underlying disease; b Proportion of species involved according to localization; c Proportion of main underlying disease according to localization; d Proportion of localization according to main underlying disease e. Proportion of main underlying disease according to species; f Proportion of localization according to species.
Characteristics according to main underlying condition
The site of infection was mainly pulmonary in HM (229/358, 64.0%) and in SOT patients (22/36, 61.1%), cutaneo-articular in those with trauma (38/44, 86.4%) and burns (26/29, 89.7%), and rhinocerebral in those with diabetes (19/41, 46.4%) (Fig. 2). The fungal species identified also differed according to main underlying condition (p < 0.01) (Fig. 2 and Table 1, Table 3). R. arrhizus was mostly recovered in patients with HM (32/67, 47.8%), and diabetes (18/67, 26.9%), and was the most common species in patients with diabetes (18/31, 58.1%, p < 0,01) and in SOT patients (9/29, 31%). L. corymbifera, R. pusillus and R. microsporus were predominantly found in patients with HM [27/42 (64.3%), 33/38 (86.8%) and 26/43 (60.5%), respectively] and neutropenia was more frequent in patients with R. pusillus (25/38, 65.8%) and L. corymbifera (22/42, 52.4%) infections than with other species (p < 0.001). L. ramosa and M. circinelloides were the main species recovered in patients with trauma (11/42, 26.2% and 17/42, 40.5% respectively) and burns (5/24, 20.8% and 15/24, 62.5% respectively).
Characteristics according to infection site
HM was the main underlying condition in pulmonary (229/288, 79.5%), rhinocerebral (44/80, 55%), and disseminated cases of mucormycosis (49/61, 80.3%), while injury through trauma (n = 38) and burns (n = 26) accounted for the majority (64/94, 68.1%) of cutaneo-articular localisations (Fig. 2). Overall, diabetes was more prevalent (37/80, 46.25%) in patients with rhinocerebral infections than in other localisations (59/470, 12.5%, p < 0.0001). Fungal species also differed according to localisation (Fig. 2). While R. arrhizus was the main species in rhinocerebral infections (30/53, 56.6%), L. ramosa and M. circinelloides were predominant in cutaneo-articular localisations (20/53, 24.1% and 31/53, 37.3% respectively) and R. pusillus in disseminated mucormycosis (17/46, 37%).
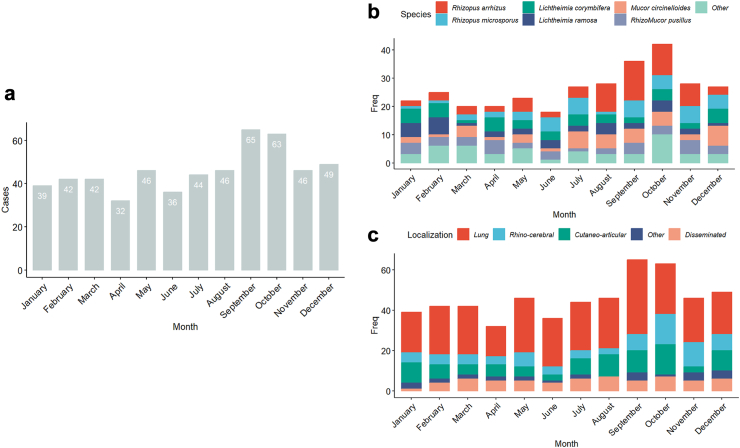
Seasonal variations
Overall, 174/550 (31.6%) cases were diagnosed in autumn, significantly more frequently than in spring (120/550, 21.8%), summer (126/550, 22.9%) or winter (130/550, 23.9%) (p < 0,01) (Fig. 3). When analysing seasonal variations according to species recovered by culture, R. arrhizus, R. microsporus and M. circinelloides were more frequently recovered during summer and autumn than in other seasons, while cases due to L. ramosa and L. corymbifera were more frequent during winter than in other seasons (Fig. 3). Rhinocerebral localisations were disproportionally more often diagnosed in autumn compared with other forms of disease [35/80 (43.75%) versus 139/470 (29.5%)].
Fig. 3.
Seasonality of mucormycosis diagnosis. a—Number of cases per month; b—species plotted by month c—localization plotted by month.
Therapeutic management
First-line therapy included liposomal amphotericin B in most cases (413/550, 75.1%) (Table 1). Antifungal dosage was not available for all cases, but centres followed the ECMM/MSG guidelines25; specifically for liposomal amphotericin B, almost all individuals received over 5 mg/kg/day IV.
Fifty-one patients did not receive antifungal therapy (26 due to post-mortem diagnosis, eight had died within 24 h and seven more within 72 h).
Surgical treatment was performed for 37.6% (166/441) of the patients for whom the information was available. It was more common in patients with trauma (40/41, 97.6%) and burns (27/29, 93.1%) than in HM (58/276, 21.0%) patients (Table 2). Excluding HM, most patients underwent surgery (108/165, 65.5%), particularly for those with rhinocerebral (45/62, 72.6%) and cutaneo-articular (73/84, 86.9%) localisations (versus patients with other localisations, p < 0.0001) (Table 3).
Outcome and prognostic factors
Median survival time was 50 days after diagnosis and the 90-day case-fatality ratio was 55.8% (276/495) (Table 1). The main analysis was performed using 495 complete cases.
In univariable analysis (Table 4), the crude odds of death at day 90 increased with older age (OR = 1.02 [1.01–1.03] per year, p < 0.0001), and ICU stay at diagnosis (OR = 3.25 [2.17–4.92], p < 0.0001). HM (OR = 2.51 [1.73–3.66], p < 0.0001), HSCT (OR = 1.66 [1.07–2.63], p = 0.027), and neutropenia (OR = 2.11 [1.47–3.03], p < 0.0001) were associated with increased mortality, while corticosteroids were not (OR = 1.52 (0.99–2.34), p = 0.058); but corticosteroids, neutropenia and HSCT were nested within the HM variable. Conversely, the odds of death decreased with trauma (OR = 0.17 [0.07–0.34], p < 0.0001). Diagnosis after 2015, as a proxy for qPCR use, was associated with reduced mortality (65/95 (68.4%) versus 189/400 (47.3%), OR = 0.51 [0.32–0.82], p = 0.006) (Fig. 4), but a positive PCR test was not (OR = 1.13 [0.77–1.65], p = 0.09). Fungal coinfection had no impact on 90-day mortality (OR 1.39 [0.95–2.05], p = 0.09). Diagnosis after 2020, as a proxy for the COVID-19 period, had no impact either. There was no difference in mortality among putative cases versus proven/probable cases (OR 1.05 [0.70–1.57], p = 0.82).
Table 4.
Factors related to outcome on univariable and multivariable analysis (N = 495).a
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Male | 0.95 (0.65–1.38) | 0.78 | ||
| Age | 1.02 (1.01–1.03) | <0.0001 | 1.02 (1.01–1.03) | 0.0009 |
| Intensive Care Unit at diagnosis | 3.25 (2.17–4.92) | <0.0001 | 8.88 (4.96–16.8) | <0.0001 |
| Haematological malignancy | 2.51 (1.73–3.66) | <0.0001 | ||
| Solid organ transplant | 1.26 (0.66–2.47) | 0.48 | ||
| Diabetes | 1.07 (0.67–1.70) | 0.76 | ||
| Burns | 0.45 (0.19–0.98) | 0.048 | ||
| Trauma | 0.17 (0.07–0.34) | <0.0001 | ||
| Haematopoietic stem cell transplantation | 1.66 (1.07–2.63) | 0.027 | ||
| Neutropenia | 2.11 (1.47–3.03) | <0.0001 | ||
| Corticosteroids | 1.52 (0.99–2.34) | 0.058 | ||
| Main underlying disease | <0.0001 | |||
| Hemopathy | 1 | 1 | ||
| Burn | 0.33 (0.14–0.74) | 0.007 | 0.09 (0.02–0.30) | 0.0001 |
| Solid organ transplant | 0.71 (0.35–1.48) | 0.35 | 0.34 (0.14–0.80) | 0.01 |
| Trauma | 0.13 (0.05–0.27) | <0.0001 | 0.10 (0.03–0.29) | <0.0001 |
| Diabete | 0.43 (0.21–0.85) | 0.02 | 0.17 (0.07–0.42) | 0.0001 |
| Other | 0.66 (0.33–1.34) | 0.24 | 0.30 (0.12–0.71) | 0.007 |
| Localisation | 0.004 | |||
| Disseminated | 1 | 1 | ||
| Lung (localized) | 0.70 (0.36–1.31) | 0.27 | 0.65 (0.31–1.33) | 0.25 |
| Rhino-cerebral (localized) | 0.52 (0.24–1.08) | 0.08 | 0.75 (0.31–1.74) | 0.50 |
| Cutaneo-articular (localized) | 0.17 (0.08–0.34) | <0.0001 | 0.34 (0.13–0.9) | 0.03 |
| Other (localized) | 0.61 (0.23–1.62) | 0.32 | 0.66 (0.22–1.97) | 0.44 |
| Speciesb | <0.0001 | |||
| Rhizopus arrhizus | 1 | |||
| Rhizopus microsporus | 2.91 (1.26–7.14) | 0.02 | ||
| Lichtheimia corymbifera | 2.06 (0.91–4.86) | 0.09 | ||
| Mucor circinelloides | 0.42 (0.18–0.97) | 0.04 | ||
| Rhizomucor pusillus | 3.66 (1.46–10.17) | 0.01 | ||
| Lichtheimia ramosa | 0.53 (0.22–1.25) | 0.15 | ||
| Others | 1.45 (0.67–3.19) | 0.35 | ||
| Diagnosis after 2015 | 0.51 (0.32–0.82) | 0.006 | 0.42 (0.23–0.72) | <0.0001 |
| Diagnosis after 2020 | 0.72 (0.49–1.06) | 0.10 | ||
| PCR positive | 1.13 (0.77–1.65) | 0.52 | ||
| Putative cases | 1.05 (0.70–1.57) | 0.82 | ||
| Fungal coinfection | 1.22 (0.80–1.87) | 0.35 | ||
Results highlighted with [bold] were statistically significant.
Data for survival at day 90 were available in 495 cases only.
Analysis was performed only on complete cases. Missing data for species were 108.
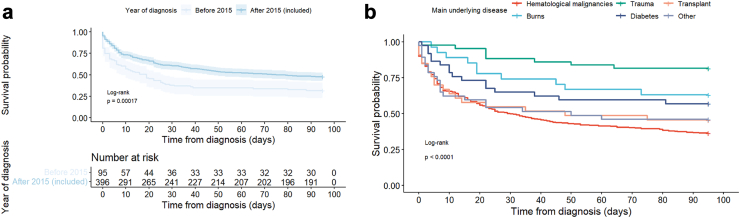
Fig. 4.
90 Day survival. a–according before and after 2015, b–according to main underlying disease.
Survival probability differed significantly according to main underlying condition studied via the hierarchical variable (p < 0.0001), from 18.6% (8/43) in trauma to 64.0% (203/317) in HM patients (Table 1, Fig. 4). It was significantly different according to the site of infection, the prognosis being worse for disseminated (38/54, 70.4%) compared to localised infections (238/441, 54.0%, p = 0.004) (Table 2), and according to the identified species (p < 0.0001), the prognosis being worse with infections caused by R. pusillus and R. microsporus than with R. arrhizus, and better with M. circinelloides (Table 3, Table 4).
In a multivariable analysis including statistically significant factors (Table 4), age, diagnosis in ICU, and HM compared to all others underlying condition, remained significantly associated with higher mortality, while diagnosis after 2015 and cutaneo-articular localization compared to disseminated were associated with lower mortality. These results were robust to missing outcome, as the same trends were observed on the imputed datasets.
The sensitivity analysis with a landmark of one day was performed on 433 cases and showed that surgery was associated with reduced mortality in univariable (OR 0.23 [0.12–0.41], p < 0.0001) and multivariable analysis (Supplementary Table S2). Diagnosis in an ICU and HM were associated with an increased risk of death, whilst age and site of infection were not. There was no significant impact of either polyene treatment, posaconazole, isavuconazole, or combination therapy on survival. However, most patients (n = 413) received treatment with a polyene.
Discussion
This prospective nationwide survey is the largest ever reported in Europe to collect epidemiological and mycological data on a national scale over a 11-year period. These data allow us to describe the major characteristics of mucormycosis in France (Fig. 5), and highlights factors impacting these features, to encourage similar reports from other countries.
Fig. 5.
Graphical abstract.
The study population here is limited to the patients cared for in the 29 participating RESSIF centres,31 covering a substantial part of France (15/18 regions). We previously estimated by capture-recapture method (RetroZygo study) that the database preceeding RESSIF recorded approximately 51% of mucormycosis cases in France,34 preventing us from accurately estimating the true incidence of mucormycosis in this study.
However, the burden of mucormycosis over the study period was evaluated using a method similar to that described by Bretagne et al.31; while it yields the observed incidence of these infections at the local and regional scales, it should be interpreted with caution. First, in each participating centre, the denominator of that incidence rate corresponds to reported hospitalisation-days as provided by the French annual statistical report for healthcare centres. However, since the duration of hospitalisation of each mucormycosis episode was not available, we could only derive incidence as a number of events per hospitalisation-days. Second, incidence reported at the centre level is highly dependent of that centre’s specificity. Aggregation of results at the regional (and national) scale helped mitigate this heterogeneity.
These data nevertheless allow us to describe the major characteristics of mucormycosis in France, and highlight factors impacting these features, to encourage similar reports from other countries.
Here, more than 60% of patients had HM, which is higher than previously reported in France (50%) or in Europe (44%) (10,11). Better prognosis of patients with HM and more aggressive therapies,35 especially in patients with acute leukaemia, could have altered the population at risk. However, the most likely explanation for this increase is the surge in PCR-based diagnoses, enabling diagnosis of mucormycosis in patients for whom invasive sampling methods are otherwise too intrusive. Indeed, the proportion of HM patients was stable at 55.4% when excluding putative cases. By contrast, the proportion of patients with diabetes as the main underlying condition (7.5%) was lower than in the Retrozygo (23%) or Skiada et al. (17%) studies.1,7 Diabetes was recorded in 17.5% of all patients, while the prevalence of treated diabetes in France was estimated at 4.6% in 2012.36 This confirms that uncontrolled diabetes alone or in addition to other conditions is a major risk factor for mucormycosis. However, these French data contrast with results from India, where diabetes remains by far the most prevalent risk factor for mucormycosis (44 to >70%15,37).
The availability of PCR-based methods for diagnosing mucormycosis has altered the epidemiological picture of mucormycosis in France compared to our previous report.7 Introduced in French routine practice in 2015,26, 27, 28 it was used as the only diagnostic tool for more than half of cases in 2022, while cases diagnosed as proven or putative remained stable through the years. A significant decrease in the overall mortality rate was observed after 2015, especially for HM patients (from 78.9% to 60.7%). It is likely that PCR testing on a readily accessible sample allowed for earlier diagnosis and prompted earlier therapeutic intervention, thereby improving outcome. In a previous prospective study of 232 patients, a positive serum PCR result could be observed a median of four days before positive mycological or histological sampling, which supports our hypothesis.28 One could argue that outcome could have been improved by false positive from PCR testing rather than earlier diagnosis. However, this seems unlikely, as Mucorales PCR has previously demonstrated an excellent sensitivity and specificity of 85.2% and 89.8% respectively in the Modimucor study.28
The lack of association between a positive PCR and reduced mortality could be explained by the implementation of PCR in the follow-up of a diagnosis previously made by culture. Indeed, our study only reported PCR-positive cases, and not the number of cases that were tested by PCR, nor the number of PCR tests done by patients or the timing of positive PCR in relation to other means of diagnosis, which could have been a proxy for delayed diagnosis. Another potential impact of changes in laboratory practices was the increase in pulmonary infections (52.4% versus 28% in Retrozygo and 30% in Skiada et al.1,7), at the expense of rhinocerebral localisations (14.5%), while other forms of the disease remained stable. Indeed, 85.4% of putative cases were pulmonary infections, almost all in HM patients. Therefore, our data support that wide implementation of PCR use in France as an early diagnostic tool may have resulted in reduced mortality, an increase in diagnoses of pulmonary infections, and of cases among patients with HM.
In terms of species distribution, R. arrhizus (21.2%) remains the most common species responsible for mucormycosis in France, but it was not as predominant as in Retrozygo (32%)7 or as in India (80.8%).15
This could be linked to the decline of diabetes as a risk factor in France, where R. arrhizus predominates, which was not compensated by the proportion of HM affected by R. arrhizus. Notably, species of the genus Lichtheimia accounted for nearly 25% of all species, which is higher than previously reported,1 as did M. circinelloides (13.3%). Although Lichtheimia species are common in Europe, they are relatively rare worldwide (7–13%1, 2, 3, 4). R. microsporus, which represented 17% of isolates in Retrozygo, is on the decline (13.6%).
These results were not influenced by PCR testing, as all species were identified by culture only, and PCR testing was only reported if positive, with no information about the species.
Although susceptibility testing was performed for all available strains, these results are very dense and will be provided in a subsequent publication.
While prior studies1,3,7,15,38 have pointed out the association between HM and pulmonary localisations, and between diabetes and rhinocerebral infections, we were able to further explore the relationship between underlying condition, site of infection, and fungal species involved. Here, severe skin injuries (burns or trauma), cutaneo-articular localisations and two species, L. ramosa and M. circinelloides, appeared linked together, constituting a first undescribed “triad”. In the review of posttraumatic mucormycosis (excluding burns) from the RetroZygo study and a literature review,9 the species involved were clearly influenced by the geographical setting. Thus, in the Asia–Pacific region, non-Rhizopus species predominated, with frequent isolation of Apophysomyces and Saksenaea species, in contrast with our data.8,39 Interestingly, the two species L. ramosa and L. corymbifera did not share the same underlying conditions or body localisations: L. corymbifera was mostly found in HM patients (64.3%) and in pulmonary localisations (38.1%), whereas L. ramosa was less frequently associated with HM patients (38.2%) than with severe skin injuries (47.1%), and cutaneo-articular localisations (58.8%). Considering that Schwartze and colleagues have reported similar virulence factors in their study,40 our findings suggest as-of-yet unknown subtle differences in the host/fungi interplay.
A second triad, previously underlined in India,2,15 is the association between diabetes, R. arrhizus, and rhinocerebral localisations, even though R. arrhizus more often affected HM patients (47.8%) than patients with diabetes (26.9%). An elegant study recently published by Ibrahim’s group found a mechanistic explanation for this triad, with specific binding of the Rhizopus protein CotH3 to the nasal epithelial cells GRP78, the expression of both proteins being increased by high glucose levels and during ketoacidosis,10,41,42 and showed that another fungal protein recognizes an integrin on alveolar epithelial cell subsequently leading to host cell invasion through the lungs.
Seasonal variations in the occurrence of mucormycosis have previously been reported,43, 44, 45 notably in Japan and Iran, and were here observed as well, especially for R. arrhizus, R. microsporus and M. circinelloides (Fig. 3). With the exception of M. circinelloides which was associated with an outbreak in a burn care unit,46 there was no clustering of cases that could easily explain these seasonal variations, nor were seasonal changes in predisposing conditions noted. However, the peak occurrence during autumn makes sense in France. Indeed, optimal temperature and rainfalls in autumn presumably favour growth and sporulation for micromycetes and macromycetes. The reason why it would lead to infection by some species and not others remains to be determined. Surveillance programs therefore remain of the utmost importance in the context of global warming and increasing climate change.
Treatment of mucormycosis included antifungal and surgical treatments. To properly study their influence on mortality, we implemented a landmark of 1 day in our subsidiary analysis, eliminating all patients who died before they could be treated. Surgical treatment was associated with a decrease in mortality consistent with previous studies,1,15,38,47,48 but it should be noted that nearly 20% of cases had missing data for this variable. Evaluating the impact of surgical treatment also presents some limitations: while it can usually be performed in cutaneous mucormycosis, pulmonary lobectomy and pneumonectomy are highly invasive surgeries that may be challenging for patients to endure.
Mortality at day 90 was 55.8%, similar to the previously reported 56% mortality in the French Retrozygo study. However it was higher than in most prior European studies: 31.3% in Spain, 47% in Europe.1,7,24
One of our study’s strengths was to explore the associations between mortality and many variables such as main underlying condition, localisation, and fungal species, which few prior studies in Western countries were powered enough to do.1,7 There were many cases without culture identification, so Mucorales species could not be evaluated in multivariable analysis.
We confirmed the influence of older age, HM, and dissemination on mortality.1,7,8,48 Diagnosis while in ICU was also confirmed as a risk factor for death, as previously reported.8,49 Conversely, surgical treatment and diagnosis after 2015 were associated with lower mortality, highlighting the importance of PCR testing and early surgical treatment to improve patient outcome. Species identification might also become a key to personalised care, as the understanding of the interplay between host factors and species virulence evolves.
In conclusion, we observed an increased proportion of HM patients and of pulmonary localisations among patients with mucormycosis in this 11-year study, coinciding with a wider use of PCR as an early diagnostic tool, thus helping paint a more accurate picture of the current epidemiology of mucormycosis in France. Fungal species distribution also changed, which was linked to localisation and underlying condition. Seasonal specificities were also observed, together with the variation of identified species; this underlies the major importance of monitoring the incidence, clinical presentation, species distribution, and outcome of mucormycosis in the evolving context of climate change and global warming. Increased mortality was associated with older age, HM, and diagnosis while in ICU, but prognosis could be improved by surgery and maybe early detection by PCR. These data must encourage us to continue research and surveillance for this severe disease.
Contributors
LG, KS and FL had full access to all the study data, and take responsibility for the integrity of the data. All authors had final responsibility for the decision to submit for publication. Study concept and design: LG, DC, OL, DGH, FL. Acquisition of data: all authors. Drafting of the first version of the manuscript: LG, DGH, FL. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: LG, DC, JD, TO. Study supervision: FL.
Data sharing statement
We support data sharing of the deidentified participant data in accordance a with FAIR principles. Request for access should be directed beginning 3 months and ending 1 year after publication to Fanny Lanternier (fanny.lanternier@pasteur.fr). These proposals will be reviewed and approved on the basis of scientific merit and in accordance with the principles of Institut Pasteur Ethics Charter (https://www.pasteur.fr/sites/default/files/rubrique_linstitut_pasteur/nos_engagements/ethique/ethics_charter_pasteur-2022.pdf).
The request concerns the deidentified participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendix), and software code. Data will be available at the CNRMA in Pasteur Institute, Paris, France. To gain access, data requesters will need to sign a data access agreement and use the secure data sharing tools provide by Institut Pasteur.
Declaration of interests
SC reports travel grants for congresses for Gilead and Pfizer. GD reports being a speaker for Gilead. LM reports personal travel grants for Gilead and Pfizer. FL reports being a speaker for MSD and F2G board. AA reports educational lecture and travel grant from Gilead. Other authors declare no competing interests.
Acknowledgements
We would like to thank Professor Françoise Dromer for her tremendous input in the conceptualization of this study, and in particular for the expansion of the RESSIF surveillance network and her continuous investment in its management over ten years.
We are thankful for the technical help of Emilie Fruquiere and Damien Hoinard (Institut Pasteur) for the characterization of the isolates received at the NRCMA.
This work was supported by recurrent financial support from Santé Publique France and Institut Pasteur.
The French Mycoses Study Group is composed of the following individuals who actively participated in the data collection. They are listed in alphabetical order of a city in France and for each center, in alphabetical order of the last names:
In Angers, Marc Pihet; in Limoges, Marie-Fleur Durieux; in Nîmes, Milène Sasso; in Paris, in Hôpital Cochin, André Paugam; in Institut Gustave Roussy, Elisabeth Chachaty; in Hôpital Lariboisiere, Stéphane Bretagne; in Hôpital Robert Debré, Patricia Mariani; in Centre Hospitalier de Versailles, Odile Eloy; in Rennes, Jean-Pierre Gangneux, Hélène Guegan.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101010.
Appendix ASupplementary data
References
- 1.Skiada A., Pagano L., Groll A., et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European confederation of medical Mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 2.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019;5(1):26. doi: 10.3390/jof5010026. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6462913/ [cited 2020 Jun 15];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong W., Keighley C., Wolfe R., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Badali H., Cañete-Gibas C., McCarthy D., et al. Epidemiology and antifungal susceptibilities of mucoralean fungi in clinical samples from the United States. J Clin Microbiol. 2021;59(9) doi: 10.1128/JCM.01230-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Hermoso D., Alanio A., Lortholary O., Dromer F. Manual of clinical microbiology. John Wiley & Sons, Ltd; 2015. Agents of systemic and subcutaneous mucormycosis and entomophthoromycosis; pp. 2087–2108.https://onlinelibrary.wiley.com/doi/abs/10.1128/9781555817381.ch121 [cited 2023 Nov 17];Available from: [Google Scholar]
- 6.Serris A., Danion F., Lanternier F. Disease entities in mucormycosis. J Fungi. 2019;5(1):23. doi: 10.3390/jof5010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanternier F., Dannaoui E., Morizot G., et al. A global analysis of mucormycosis in France: the RetroZygo study (2005–2007) Clin Infect Dis. 2012;54(suppl_1):S35–S43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy K.J., Daveson K., Slavin M.A., et al. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infection. 2016;22(9):775–781. doi: 10.1016/j.cmi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Lelievre L., Garcia-Hermoso D., Abdoul H., et al. Posttraumatic mucormycosis. Medicine (Baltimore) 2014;93(24):395–404. doi: 10.1097/MD.0000000000000221. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4602436/ [cited 2019 Mar 20]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rammaert B., Lanternier F., Poirée S., Kania R., Lortholary O. Diabetes and mucormycosis: a complex interplay. Diabetes Metab. 2012;38(3):193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Jestin M., Azoulay E., Pène F., et al. Poor outcome associated with mucormycosis in critically ill hematological patients: results of a multicenter study. Ann Intensive Care. 2021;11(1):31. doi: 10.1186/s13613-021-00818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitar D., Lortholary O., Le Strat Y., et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis. 2014;20(7):1149–1155. doi: 10.3201/eid2007.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti A., Das A., Mandal J., et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44(4):335–342. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- 14.Prakash H., Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):523. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel A., Kaur H., Xess I., et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7):944.e9–944.e15. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Selarka L., Sharma S., Saini D., et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses. 2021 doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudramurthy S.M., Hoenigl M., Meis J.F., et al. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses. 2021;64(9):1028–1037. doi: 10.1111/myc.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzmán-Castro S., Chora-Hernandez L.D., Trujillo-Alonso G., et al. COVID-19–associated mucormycosis, diabetes and steroid therapy: experience in a single centre in Western Mexico. Mycoses. 2022;65(1):65–70. doi: 10.1111/myc.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthu V., Rudramurthy S.M., Chakrabarti A., Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186(6):739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danion F., Letscher-Bru V., Guitard J., et al. Coronavirus disease 2019-associated mucormycosis in France: a rare but deadly complication. Open Forum Infect Dis. 2021;9(2) doi: 10.1093/ofid/ofab566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoenigl M., Seidel D., Carvalho A., et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3(7):e543–e552. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitar D., Van Cauteren D., Lanternier F., et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15(9):1395–1401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash H., Ghosh A.K., Rudramurthy S.M., et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):395–402. doi: 10.1093/mmy/myy060. [DOI] [PubMed] [Google Scholar]
- 24.Parra Fariñas R., Alonso-Sardón M., Velasco-Tirado V., et al. Increasing Incidence of mucormycosis in Spanish inpatients from 1997 to 2018. Mycoses. 2022;65(3):344–353. doi: 10.1111/myc.13418. [DOI] [PubMed] [Google Scholar]
- 25.Cornely O.A., Alastruey-Izquierdo A., Arenz D., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical Mycology in cooperation with the Mycoses study group education and research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millon L., Herbrecht R., Grenouillet F., et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF) Clin Microbiol Infection. 2016;22(9):810.e1–810.e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Millon L., Larosa F., Lepiller Q., et al. Quantitative Polymerase Chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis. 2013;56(10):e95–e101. doi: 10.1093/cid/cit094. [DOI] [PubMed] [Google Scholar]
- 28.Millon L., Caillot D., Berceanu A., et al. Evaluation of serum Mucorales Polymerase Chain reaction (PCR) for the diagnosis of mucormycoses: the MODIMUCOR prospective trial. Clin Infect Dis. 2022;75(5):777–785. doi: 10.1093/cid/ciab1066. [DOI] [PubMed] [Google Scholar]
- 29.Muthu V., Agarwal R., Dhooria S., et al. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(4):538–549. doi: 10.1016/j.cmi.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 31.Bretagne S., Sitbon K., Desnos-Ollivier M., et al. Active surveillance program to increase awareness on invasive fungal diseases: the French RESSIF network (2012 to 2018) mBio. 2022;13(3):e00920–e00922. doi: 10.1128/mbio.00920-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly J.P., Chen S.C., Kauffman C.A., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Hermoso D., Hoinard D., Gantier J.C., Grenouillet F., Dromer F., Dannaoui E. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. Ramosa. J Clin Microbiol. 2009;47(12):3862–3870. doi: 10.1128/JCM.02094-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitar D., Morizot G., Van Cauteren D., et al. Estimating the burden of mucormycosis infections in France (2005–2007) through a capture-recapture method on laboratory and administrative data. Rev Epidémiol Santé Publique. 2012;60(5):383–387. doi: 10.1016/j.respe.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Keykhaei M., Masinaei M., Mohammadi E., et al. A global, regional, and national survey on burden and Quality of Care Index (QCI) of hematologic malignancies; global burden of disease systematic analysis 1990–2017. Exp Hematol Oncol. 2021;10:11. doi: 10.1186/s40164-021-00198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno L.M., Fosse-Edorh S., Fagot-Campagna A., Denis P., Mandereau Bruno L. Prévalence du diabète traité pharmacologiquement et disparités territoriales en France en 2012. N° thématique. Journée mondiale du diabète, 14 novembre 2014. Bull Épidémiol Hebdomadaire. 2014;30:493–499. [Google Scholar]
- 37.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roden M.M., Zaoutis T.E., Buchanan W.L., et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 39.Slavin M.A., Chakrabarti A. Opportunistic fungal infections in the Asia-Pacific region. Med Mycol. 2012;50(1):18–25. doi: 10.3109/13693786.2011.602989. [DOI] [PubMed] [Google Scholar]
- 40.Schwartze V.U., Hoffmann K., Nyilasi I., et al. Lichtheimia species exhibit differences in virulence potential. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoshbayan A., Didehdar M., Chegini Z., Taheri F., Shariati A. A closer look at pathogenesis of cerebral mucormycosis in diabetic condition: a mini review. J Basic Microbiol. 2021;61(3):212–218. doi: 10.1002/jobm.202000692. [DOI] [PubMed] [Google Scholar]
- 42.Alqarihi A., Gebremariam T., Gu Y., et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. mBio. 2020;11(3) doi: 10.1128/mBio.01087-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivagnanam S., Sengupta D.J., Hoogestraat D., et al. Seasonal clustering of sinopulmonary mucormycosis in patients with hematologic malignancies at a large comprehensive cancer center. Antimicrob Resist Infect Control. 2017;6:123. doi: 10.1186/s13756-017-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirzaie A.Z., Jahangiri A., Sadeghipour A., Shayanfar N. Seven years of experience with zygomycosis in Iran: a seasonal disease. Braz J Infect Dis. 2011;15(5):504. [PubMed] [Google Scholar]
- 45.Al-Ajam M.R., Bizri A.R., Mokhbat J., Weedon J., Lutwick L. Mucormycosis in the eastern mediterranean: a seasonal disease. Epidemiol Infect. 2006;134(2):341–346. doi: 10.1017/S0950268805004930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Hermoso D., Criscuolo A., Lee S.C., et al. Outbreak of invasive wound mucormycosis in a burn unit due to multiple strains of mucor circinelloides f. circinelloides resolved by whole-genome sequencing. mBio. 2018;9(2) doi: 10.1128/mBio.00573-18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5915733/ [cited 2019 Mar 20]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claustre J., Larcher R., Jouve T., et al. Mucormycosis in intensive care unit: surgery is a major prognostic factor in patients with hematological malignancy. Ann Intensive Care. 2020;10(1):74. doi: 10.1186/s13613-020-00673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagano L., Valentini C.G., Posteraro B., et al. Zygomycosis in Italy: a survey of FIMUA-ECMM (federazione italiana di micopatologia umana ed animale and European confederation of medical Mycology) J Chemother. 2009;21(3):322–329. doi: 10.1179/joc.2009.21.3.322. [DOI] [PubMed] [Google Scholar]
- 49.Patel A., Agarwal R., Rudramurthy S.M., et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27(9):2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.