Abstract
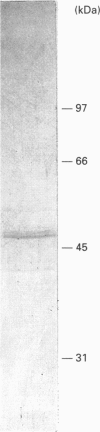
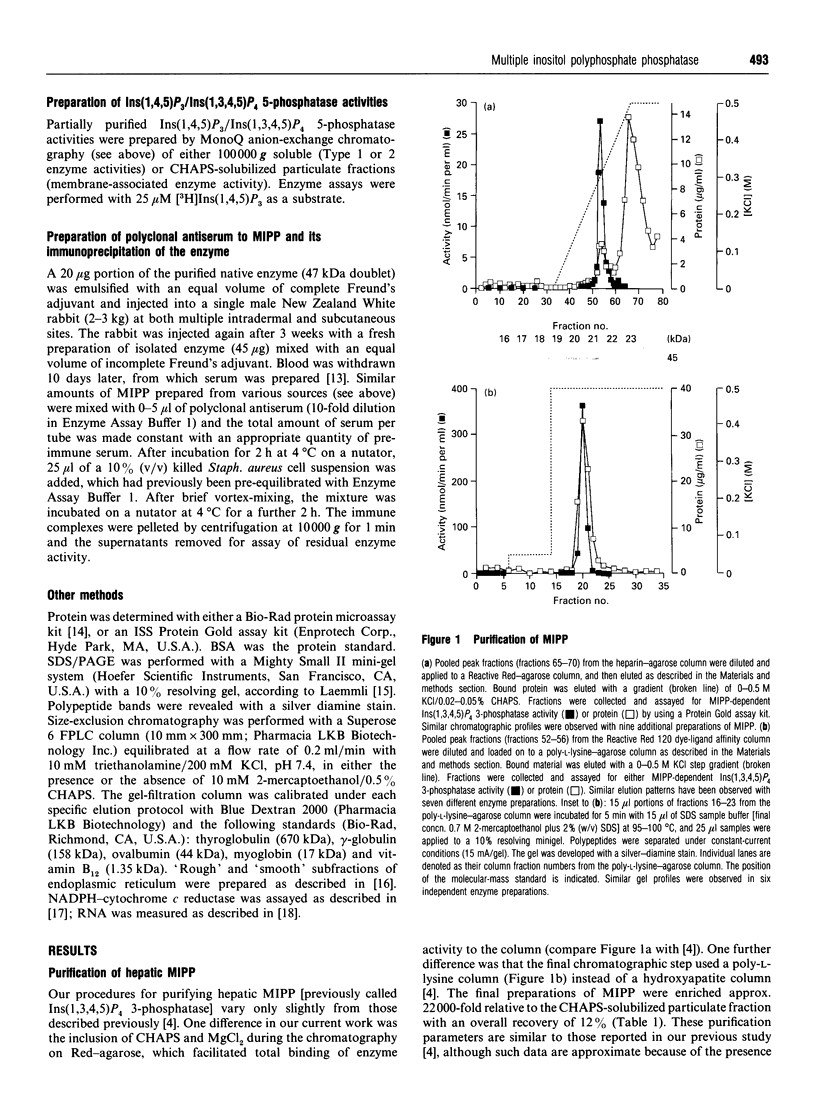
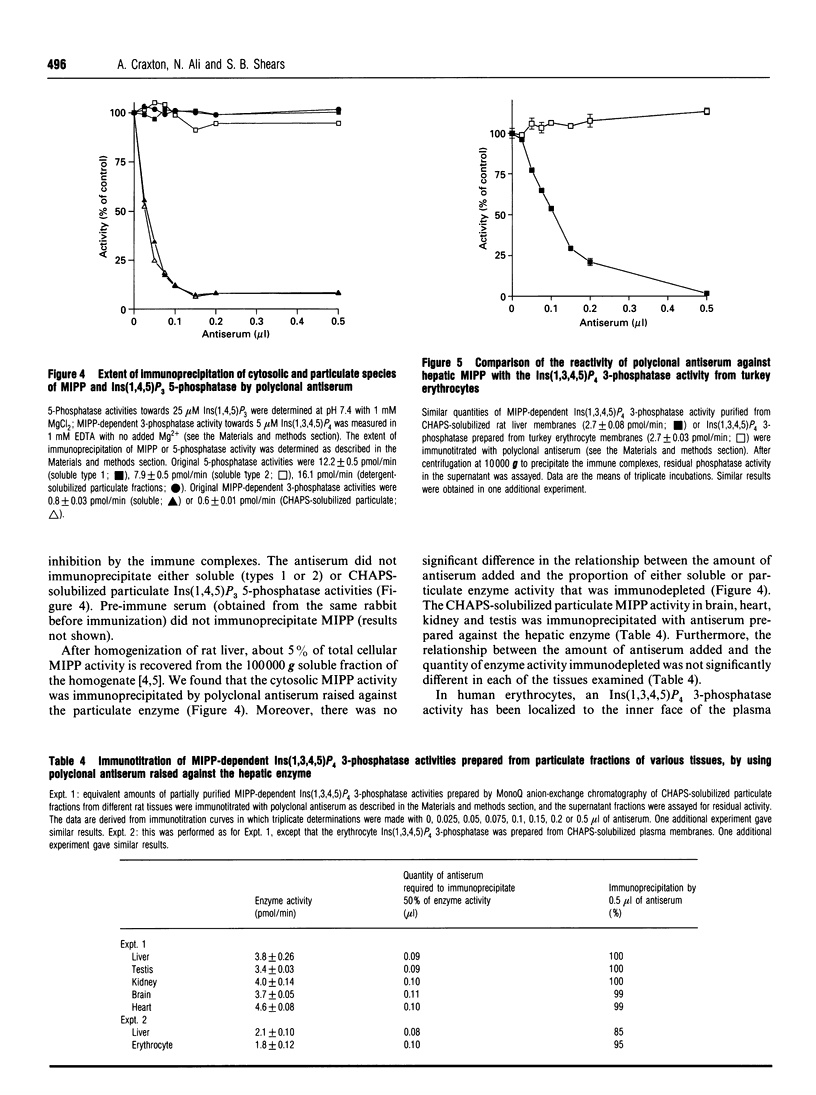
A multiple inositol polyphosphate phosphatase (formerly known as inositol 1,3,4,5-tetrakisphosphate 3-phosphatase) was purified approx. 22,000-fold from rat liver. The final preparation migrated on SDS/PAGE as a doublet with a mean apparent molecular mass of 47 kDa. Upon size-exclusion chromatography, the enzyme was eluted with an apparent molecular mass of 36 kDa. This enzyme was approximately evenly distributed between the 'rough' and 'smooth' subfractions of endoplasmic reticulum. There was a 20-fold range of specific activities of this phosphatase in CHAPS-solubilized particulate fractions prepared from the following rat tissues: liver, heart, kidney, testis and brain. However, each of these extracts contained different amounts of endogenous inhibitors of enzyme activity. After removal of these inhibitors by MonoQ anion-exchange chromatography, there was only a 2.5-fold range of specific activities; kidney contained the most and brain contained the least. We prepared and characterized polyclonal antiserum to the hepatic phosphatase, which immunoprecipitated 85-100% of both particulate and soluble phosphatase activities. The antiserum also immunoprecipitated, with equivalent efficacy, CHAPS-solubilized phosphatase activities from heart, kidney, testis, brain and erythrocytes (all prepared from rat). Our data strengthen the case that the function of the mammalian phosphatase is unrelated to the metabolism of Ca(2+)-mobilizing cellular signals. The CHAPS-solubilized phosphatase from turkey erythrocytes was not immunoprecipitated by the polyclonal antiserum, and is therefore an isoform that is structurally distinct, and possibly functionally unique.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Craxton A., Shears S. B. Hepatic Ins(1,3,4,5)P4 3-phosphatase is compartmentalized inside endoplasmic reticulum. J Biol Chem. 1993 Mar 25;268(9):6161–6167. [PubMed] [Google Scholar]
- Beckers C. J., Keller D. S., Balch W. E. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987 Aug 14;50(4):523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Garcia T., Craxton A., Kirk C. J., Michell R. H. A salt-activated inositol 1,3,4,5-tetrakisphosphate 3-phosphatase at the inner surface of the human erythrocyte membrane. Proc Biol Sci. 1991 Apr 22;244(1309):63–68. doi: 10.1098/rspb.1991.0052. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Heacock A. M., Seguin E. B., Agranoff B. W. Developmental and regional studies of the metabolism of inositol 1,4,5-trisphosphate in rat brain. J Neurochem. 1990 Apr;54(4):1405–1411. doi: 10.1111/j.1471-4159.1990.tb01976.x. [DOI] [PubMed] [Google Scholar]
- Hodgson M. E., Shears S. B. Rat liver contains a potent endogenous inhibitor of inositol 1,3,4,5-tetrakisphosphate 3-phosphatase. Biochem J. 1990 May 1;267(3):831–834. doi: 10.1042/bj2670831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P., Lowe C. R., Sherwood R. F. Metal ion-promoted binding of proteins to immobilized triazine dye affinity adsorbents. Biochim Biophys Acta. 1982 Jan 4;700(1):90–100. doi: 10.1016/0167-4838(82)90296-5. [DOI] [PubMed] [Google Scholar]
- Höer A., Höer D., Oberdisse E. Properties of a soluble inositol 1,3,4,5-tetrakisphosphate 3-phosphatase from porcine brain. Biochem J. 1990 Sep 15;270(3):715–719. doi: 10.1042/bj2700715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Majerus P. W. Properties of inositol polyphosphate 1-phosphatase. J Biol Chem. 1988 Oct 5;263(28):14559–14565. [PubMed] [Google Scholar]
- Irvine R. F. Is inositol tetrakisphosphate the second messenger that controls Ca2+ entry into cells? Adv Second Messenger Phosphoprotein Res. 1992;26:161–185. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Milani D., Volpe P., Pozzan T. D-myo-inositol 1,4,5-trisphosphate phosphatase in skeletal muscle. Biochem J. 1988 Sep 1;254(2):525–529. doi: 10.1042/bj2540525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin F., Tay S., Simpkins H. A comparative study of the molecular structures of the plasma membranes and the smooth and the rough endoplasmic-reticulum membranes from rat liver. Biochem J. 1972 Sep;129(3):781–788. doi: 10.1042/bj1290781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. J., Downes C. P., Harden T. K., Michell R. H. Turkey erythrocytes possess a membrane-associated inositol 1,4,5-trisphosphate 3-kinase that is activated by Ca2+ in the presence of calmodulin. Biochem J. 1987 Dec 1;248(2):489–493. doi: 10.1042/bj2480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nogimori K., Hughes P. J., Glennon M. C., Hodgson M. E., Putney J. W., Jr, Shears S. B. Purification of an inositol (1,3,4,5)-tetrakisphosphate 3-phosphatase activity from rat liver and the evaluation of its substrate specificity. J Biol Chem. 1991 Sep 5;266(25):16499–16506. [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol phosphates and calcium entry. Adv Second Messenger Phosphoprotein Res. 1992;26:143–160. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of inositol phosphates. Adv Second Messenger Phosphoprotein Res. 1992;26:63–92. [PubMed] [Google Scholar]
- Sillence D. J., Downes C. P. Subcellular distribution of agonist-stimulated phosphatidylinositol synthesis in 1321 N1 astrocytoma cells. Biochem J. 1993 Mar 1;290(Pt 2):381–387. doi: 10.1042/bj2900381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992 Oct;3(10):1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Downes C. P. Product-precursor relationships amongst inositol polyphosphates. Incorporation of [32P]Pi into myo-inositol 1,3,4,6-tetrakisphosphate, myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol 3,4,5,6-tetrakisphosphate and myo-inositol 1,3,4,5,6-pentakisphosphate in intact avian erythrocytes. Biochem J. 1990 Jan 15;265(2):435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E. A., Smith A. I., Wallace C. A., White L. B. Evidence for a lack of inositol--(1,4,5)trisphosphate kinase activity in norepinephrine-perfused rat hearts. Biochem Biophys Res Commun. 1987 Oct 14;148(1):68–77. doi: 10.1016/0006-291x(87)91077-1. [DOI] [PubMed] [Google Scholar]