ABSTRACT
BACKGROUND:
One anastomosis gastric bypass (OAGB) has gained prominence in the search for better results in bariatric surgery. However, its efficacy and safety compared to Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) remain ill-defined.
AIMS:
To compare the efficacy and safety of OAGB relative to RYGB and SG in the treatment of obesity.
METHODS:
We systematically searched PubMed, EMBASE, Cochrane Library, Lilacs, and Google Scholar databases for randomized controlled trials comparing OAGB with RYGB or SG in the surgical approach to obesity. We pooled outcomes for body mass index, percentage of excess weight loss, type-2 diabetes mellitus remission, complications, and gastroesophageal reflux disease. Statistical analyses were performed with R software (version 4.2.3).
RESULTS:
Data on 854 patients were extracted from 11 randomized controlled trials, of which 422 (49.4%) were submitted to OAGB with mean follow-up ranging from six months to five years. The meta-analysis revealed a significantly higher percentage of excess weight loss at 1-year follow-up and a significantly lower body mass index at 5-year follow-up in OAGB patients. Conversely, rates of type-2 diabetes mellitus remission, complications, and gastroesophageal reflux disease were not significantly different between groups. The overall quality of evidence was considered very low.
CONCLUSIONS:
Our results corroborate the comparable efficacy of OAGB in relation to RYGB and SG in the treatment of obesity, maintaining no significant differences in type-2 diabetes mellitus remission, complications, and gastroesophageal reflux disease rates.
HEADINGS: Gastric Bypass, Gastrectomy, Bariatric Surgery, Obesity
RESUMO
RACIONAL:
O bypass gástrico de uma anastomose (BGU tem ganhado destaque na busca por melhores resultados na cirurgia bariátrica. No entanto, a eficácia e segurança do BGUA, em relação ao bypass gástrico em Y de Roux (BGYR) e à gastrectomia vertical (GV), permanecem imprecisas.
OBJETIVOS:
Comparar a eficácia e segurança do BGUA em relação ao BGYR e GV no tratamento da obesidade.
MÉTODOS:
Realizamos uma busca sistemática nas bases de dados do PubMed, EMBASE, Cochrane, Lilacs e Google Scholar por ensaios clínicos randomizados que comparassem o BGUA ao BGYR ou GV na abordagem cirúrgica da obesidade. Agrupamos resultados para índice de massa corporal, porcentagem da perda de peso em excesso, remissão do diabetes mellitus tipo 2, complicações e taxas de doença do refluxo gastroesofágico. As análises estatísticas foram realizadas empregando o software R, versão 4.2.3.
RESULTADOS:
Dados de 854 pacientes foram extraídos de 11 ensaios clínicos randomizados, dos quais 422 (49,4%) foram submetidos ao BGUA, com seguimento médio variando de seis meses a cinco anos. Na análise combinada, o BGUA esteve associado a uma porcentagem da perda de peso em excesso significativamente maior no acompanhamento de um ano e a um índice de massa corporal significativamente menor no acompanhamento de cinco anos em pacientes submetidos ao BGUA. Por outro lado, as taxas de remissão da diabetes mellitus tipo 2, de complicações e da doença do refluxo gastroesofágico não foram significativamente diferentes entre os grupos. A qualidade geral das evidências foi considerada muito baixa.
CONCLUSÕES:
Nossos resultados corroboram a eficácia comparável do BGUA em relação ao BGYR e GV no tratamento da obesidade, mantendo diferenças não significativas nas taxas de remissão da diabetes mellitus tipo 2, complicações e doença do refluxo gastroesofágico.
DESCRITORES: Derivação Gástrica, Gastrectomia, Cirurgia Bariátrica, Obesidade
INTRODUCTION
Obesity is a growing condition worldwide, both in underdeveloped and developing countries 17 . Despite increasing global efforts to reduce the growth rate, a recent publication by the World Obesity Federation shows a projection that more than 50% of the world’s population will be overweight or obese by 2035 58 .
Although the existence of significant and promising new drugs in the approach to obesity disease 33 , bariatric and metabolic surgery remains the most effective and durable treatment 55 . Roux-en-Y gastric bypass (RYGB) surgery was the dominant procedure for many years 16,56,57 but has been surpassed by sleeve gastrectomy (SG) in recent years 6 .
Different surgical techniques have been gaining ground in the search for better results with fewer complications 44 . The one anastomosis gastric bypass (OAGB), first introduced by Dr. Rutledge in 1997, has been upheld by several publications with encouraging results 11,43,45 . OAGB is a “combined procedure” that incorporates both a “restrictive” and a “hypoabsorptive” component 41,51,56 . Demonstrating remarkable efficacy in mitigating obesity-related comorbidities, it also offers a good quality of life while maintaining a manageable complication rate 13,28,29,56 .
The escalating prevalence of OAGB in Europe and the Asia-Pacific has elevated its status to the third most commonly performed bariatric surgery, ranking behind SG and RYGB 4,5,13 .
Recent randomized controlled trials (RCTs) have compared OAGB with the main bariatric surgeries, SG and RYGB, showing promising results 15,21,31,42,44,50 . However, these studies included different populations with variations in body mass index (BMI) and surgical techniques, displaying divergent outcomes. Therefore, we performed a comprehensive systematic review and meta-analysis of all published RCTs, aiming at providing pooled effect estimates regarding the efficacy and safety of OAGB in the treatment of obesity, as compared with SG and RYGB.
METHODS
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, including design, implementation of steps, analysis, and description of results 39 .
Search strategy
The databases PubMed, Embase, Cochrane Library, Latin American and Caribbean Health Sciences Literature (Lilacs), and Google Scholar were systematically searched from inception to May 2023 with the following search strategy: (Bariatrics OR Bariatric Surgery OR Bariatric Surgical Procedures OR Bariatric Surgical Procedure OR Bariatric Surgeries OR Gastric Bypass) AND (One Anastomosis Gastric Bypass OR OAGB). Aiming at the inclusion of additional studies, the references of the included articles and systematic reviews of the literature were evaluated.
Inclusion criteria
Studies with the following criteria were included:
RCTs
Comparing OAGB with RYGB or SG and
Reporting at least one of the outcomes of interest.
Incomplete or unpublished trials, non-RCTs, and conference abstracts were excluded. There were no restrictions on language or publication date.
Data extraction
Two authors (L.C.T. and C.L.S.) independently extracted baseline characteristics and data outcomes following predefined search criteria. Disagreements were resolved by consensus between the two investigators and the senior author (W.M.B.). Data presented in the studies from the longest follow-up analysis with control group comparison were extracted for analyses. For data handling and conversion, the Cochrane Handbook for Systematic Reviews of Interventions guidelines were used 19 . The population of different publications from the same trial was only counted once.
Outcomes
The primary outcomes were the change in BMI (kg/m2) compared to the baseline value at six months, one year, and five years, and the percentage of excess weight loss (%EWL) at one year and five years. Accordingly, secondary outcomes were type-2 diabetes mellitus (T2DM) remission, complications, and gastroesophageal reflux disease (GERD) rates at the longest follow-up.
The definition of T2DM remission was heterogeneous among the included studies. Therefore, data of this endpoint was collected as equally as reported by each RCT 19,31,43,48,50 , precluding data manipulation.
Risk of bias and evidence quality
Two authors (T.O. and L.C.T.) independently assessed the risk of bias, and disagreements were resolved with the senior author (W.M.B.). The risk of bias assessment followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, with the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials (Rob-2) 52 . Each trial received a score of high, low, or some concerns risk of bias in five domains: randomization process; deviations from the intended interventions; missing outcomes; measurement of the outcome; and selection of reported results.
The evidence quality was assessed according to the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) guidelines 36 . Very low, low, moderate, or high-quality evidence grades were designed for the outcomes based on the risk of bias, inconsistency of results, imprecision, publication bias, and magnitude of treatment effects.
Statistical analysis
Endpoints were analyzed by weighted mean differences (WMDs) or risk ratios (RRs) with 95% confidence intervals (CIs) to compare treatment effects. Cochrane Q-test and I2 statistics were applied to assess heterogeneity; p<0.100 and I2>50% were considered significant 20 . DerSimonian and Laird random-effect models were used for all endpoints, including the surgery performed in the control group 14 . Statistical analyses were performed using R software, version 4.2.3 (R Core Team, 2021, Vienna, Austria).
RESULTS
Study selection and baseline characteristics
The systematic search yielded 4,828 studies. After removing duplicates and ineligible studies by title or abstract, 43 articles were fully reviewed for inclusion and exclusion criteria. Of these, 11 were included in this meta-analysis 15,21,24,30,31,44,48-50 . Data from one RCT was reported in three different publications 21,48,50 . A total of 854 patients were assessed, of whom 422 (49.4%) were submitted to OAGB. At baseline, the mean age ranged from 31 to 46 years, the mean body weight from 109.5 to 137.5 kg, and the mean BMI from 42.7 to 49.9 kg/m2. A flow diagram describing the selection process (inclusion and exclusion) is shown in Figure 1, and the basic characteristics of the included studies are summarized in Table 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of study screening and selection.
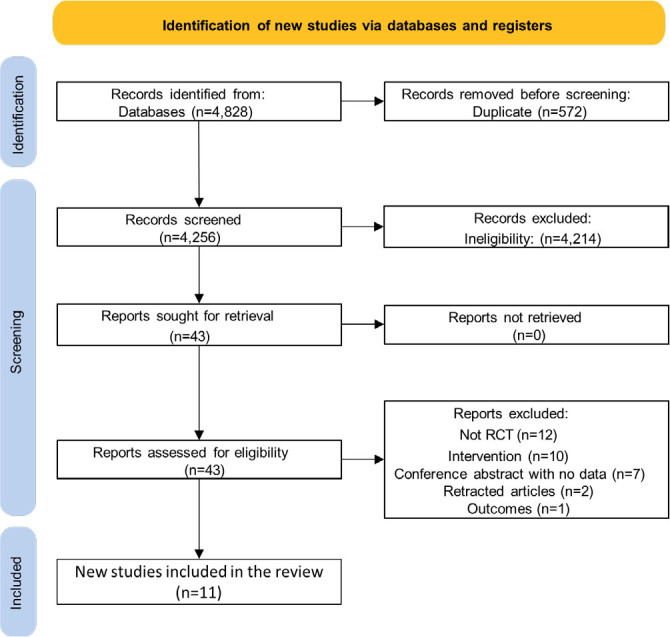
Table 1. Baseline characteristics of included studies.
| Author | Follow-up (years) | Intervention | Control group | Patients | Sample size IG/CG |
Initial BMI (kg/m2) | |
|---|---|---|---|---|---|---|---|
| IG | CG | ||||||
| Lee et al. 30 | 2 | MGB/OAGB | RYGB | Morbid obesity | 40/40 | 44.80±8.80 | 43.80±4.80 |
| Seetharamaiah et al. 49 | 1 | OAGB | LSG | Obesity | 101/100 | 44.32±7.88 | 44.57±7.16 |
| Shivakumar et al., 51 | 3 | OAGB | LSG | Obesity | 101/100 | 44.32±7.88 | 44.57±7.16 |
| Robert et al. 42 | 2 | OAGB | RYGB | Obesity | 129/124 | 43.80±6.10 | 43.90±5.10 |
| Kraljevic et al. 28 | 1 | LOAGB | LRYGB | Obesity | 40/40 | NA | NA |
| Eskandaros et al. 15 | 1 | LOAGB | LRYGB | GERD+obesity | 40/40 | 49.78±3.40 | 50.01±3.50 |
| Jain et al. 21 | 5 | OAGB | LSG | Obesity | 73/71 | 45.32±8.24 | 44.89±7.94 |
| Katayama et al. 24 | 6 months | OAGB | RYGB | Obesity | 10/10 | 43.20±3.70 | 43.10±3.90 |
| Level et al. 31 | 5 | OAGB | RYGB | Obesity | 9/24 | 42.90±5.50 | 42.60±5.90 |
| Musella et al. 38 | 1 | MGB/OAGB | SG | GERD | 28/30 | 48.50±8.90 | 47.50±7.30 |
| Singh et al. 50 | 4 | LOAGB | LRYGB | Obesity+T2DM | 25/24 | 47.00±6.70 | 44.70±4.90 |
IG: intervention group; CG: control group; BMI: body mass index; MGB: mini-gastric bypass; OAGB: one anastomosis gastric bypass; RYGB: Roux-en-Y gastric bypass; LSG: laparoscopic sleeve gastrectomy; LOAGB: laparoscopic one anastomosis gastric bypass; LRYGB: laparoscopic Roux-en-Y gastric bypass; GERD: gastroesophageal reflux disease; T2DM: type-2 diabetes mellitus; NA: not available; ± standard deviation; SG: sleeve gastrectomy.
Body mass index
No significant differences were observed between groups in BMI at 6-month follow-up (WMD 1.39 kg/m2; 95%CI -3.77; 0.99; p=0.250; I2: 61%; Figure 2A) and 1-year follow-up (WMD -0.69 kg/m2; 95%CI -1.55; 0.18; p=0.120; I2: 19%; Figure 2B). However, at 5-year follow-up, OAGB was associated with a significant decrease in BMI compared with control (WMD -1.78 kg/m2; 95%CI -2.85; -0.70; p=0.001; I2: 47%; Figure 2C).
Figure 2. Forest plots of pooled comparisons of body mass index (kg/m2) endpoints. Figure 2-A: at 6-month follow-up; Figure 2-B: at 1-year follow up; Figure 2-C: at 5- year follow-up.
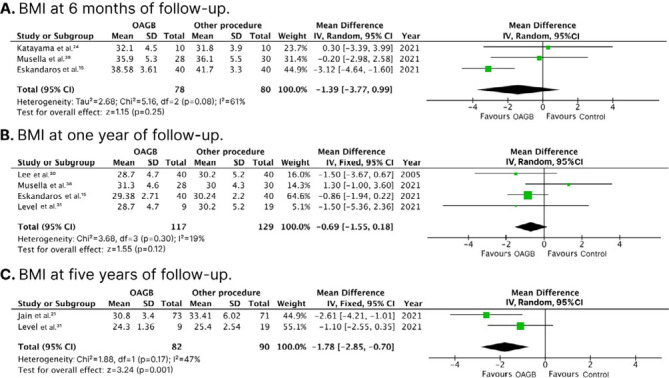
BMI: body mass index; CI: confidence interval; OAGB: one anastomosis gastric bypass; SD: standard deviation; df: degrees of freedom; p: p-value; I2: heterogeneity; z: standard normal distribution.
Percentage of excess weight loss
There was a significantly higher %EWL in the OAGB group at 1-year follow-up (WMD 6.92%; 95%CI 1.16; 12.69; p=0.020; I2: 74%; Figure 3A), compared with control. At 5-year follow-up, there was no significant difference between groups in %EWL (WMD 4.78%; 95%CI -2.68; 12.25; p=0.050; I2: 75%; Figure 3B).
Figure 3. Forest plots of pooled comparisons of the percentage excess weight loss endpoints. Figure 3-A: at 1-year follow-up; Figure 3-B: at 5-year follow-up.
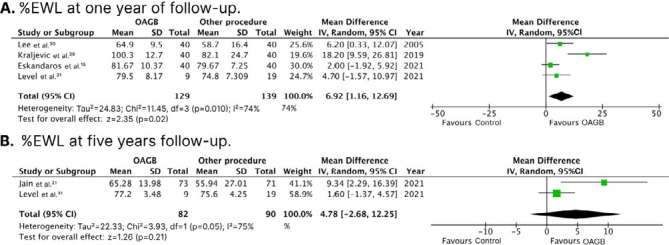
%EWL: percentage of excess weight loss; CI: confidence interval; OAGB: one anastomosis gastric bypass; SD: standard deviation; df: degrees of freedom; p: p-value; I2: heterogeneity; z: standard normal distribution.
Type-2 diabetes mellitus remission, complications, and gastroesophageal reflux disease rates
Patients submitted to OAGB showed similar rates of T2DM remission (RR 1.03; 95%CI 0.87; 1.22; p=0.720; I2: 0%; Figure 4A), complications (RR 0.72; 95%CI 0.38; 1.36; p=0.310; I2: 0%; Figure 4B), and GERD (RR 1.62; 95%CI 0.39; 6.77; p=0.510; I2: 17%; Figure 4C) in comparison with control.
Figure 4. Forest plots of pooled comparisons of type-2 diabetes mellitus remission (Figure 4-A), complication rates (Figure 4-B) and GERD (Figure 4-A) endpoints.
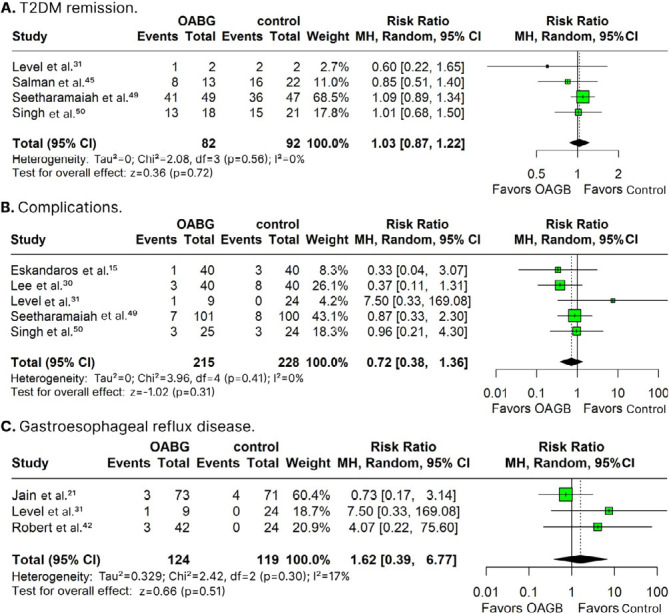
T2DM: type-2 diabetes mellitus; CI confidence interval; OAGB: one anastomosis gastric bypass; SD: standard deviation; df: degrees of freedom; p: p-value; I2: heterogeneity; z: standard normal distribution; MH: Mantel-Haenszel random-effects model.
Overall, among patients who underwent OAGB, there were three hemorrhages, two marginal ulcers, eight intraoperative complications, three early complications, and 22 late complications 16,17,18,23,29 . For those in the RYGB group, four intraoperative complications, five early complications, and 18 late complications were reported 30,31,42,50 . For patients who received SG, four hemorrhages and one anastomotic dehiscence were related 52 .
One study classified complications by the Clavien-Dindo score 42 . In the RYGB group, two cases (bowel obstruction and hemoperitoneum) were scored over grade 3 and required surgical management. In the OAGB group, one case (peritonitis) was scored over grade 3 and also required surgical treatment.
Risk of bias and evidence quality
Figure 5 outlines individual assessments of each RCT included in this meta-analysis. Due to the assignment of some concerns or high risk of bias in one or more domains of the Cochrane Collaboration’s tool, five studies were deemed at some concerns risk of bias and six studies at high risk.
Figure 5. Critical appraisal of randomized controlled trials according to the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials.
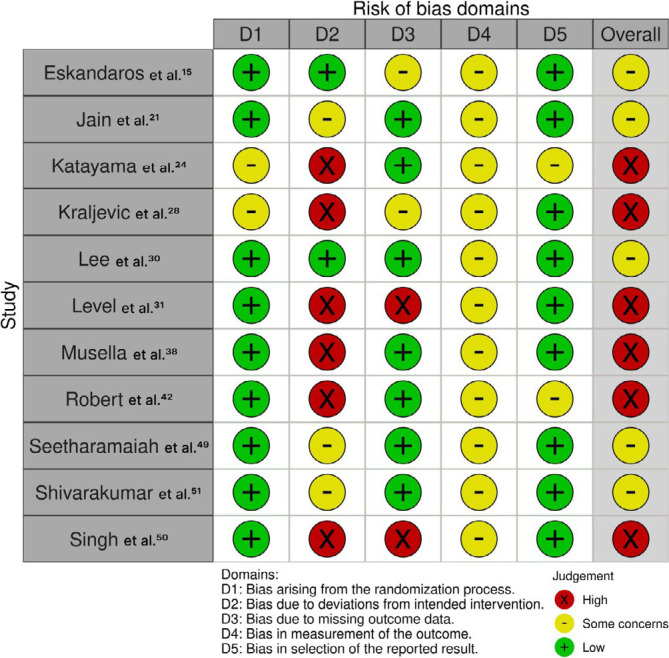
According to the GRADE assessment, BMI and %EWL outcomes were classified as very low-quality evidence (Table 2). The primary domains contributing to reduced evidence quality for these outcomes were inconsistency due to heterogeneity and imprecision, which resulted from a small number of RCTs assessing the outcome.
Table 2. Analysis of the quality of evidence (GRADE) in relation to the overall rate of occurrence of the assessed outcomes.
| Certainty assessment | Patients (n) | Certainty of evidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome follow-up | Studies (n) | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Others considerations | OAGB | Control | |
| BMI at 6-month | 3 | RCT | VS | Serious (I2>50) |
NS | VS | None | 78 | 80 | ⊕⭘⭕⭕ Very low |
| BMI at 1-year | 4 | RCT | VS | NS | NS | Serious | None | 117 | 129 | ⊕⭘⭕⭕ Very low |
| BMI at 5-year | 2 | RCT | VS | NS | NS | Serious | None | 82 | 90 | ⊕⭘⭕⭕ Very low |
| %EWL at 1-year | 4 | RCT | VS | Serious (I2>50) |
NS | NS | None | 129 | 139 | ⊕⭘⭕⭕ Very low |
| %EWL at 5-year | 2 | RCT | VS | Serious (I2>50) |
NS | VS | None | 82 | 90 | ⊕⭘⭕⭕ Very low |
BMI: body mass index; OAGB: one anastomosis gastric bypass; RCT: randomized controlled trial; VS: very serious; NS: not serious; EWL: excess weight loss.
DISCUSSION
In this systematic review and meta-analysis of eleven RCTs, comprising 854 patients, we compared OAGB to SG and RYGB for the treatment of obesity.
Our main findings were:
OAGB significantly decreased BMI at 5-year follow-up;
OAGB significantly improved %EWL at 1-year follow-up;
Non-significant differences were observed between OAGB and the control group regarding T2DM remission; and
OAGB and control groups exhibited comparable rates of complications and GERD at the longest follow-up.
For many years, RYGB was considered the gold standard procedure for obesity treatment 8,12 . However, other procedures have gained prominence in the pursuit of improved outcomes in bariatric surgery, with SG and OAGB being the most performed surgeries as an alternative to RYGB 25,54 . Meanwhile, despite OAGB being a less prevalent procedure, it has been associated with superior weight loss efficacy than the traditional RYGB approach due to the substantially longer biliopancreatic limb (BPL) 26,42,46 .
Many studies have been accomplished to enhance outcomes of bypass surgery by investigating limb lengths 9,10,22,40,41,47,53 . The length predominantly used in OAGB is 200 cm 35 . However, several studies advocate for a 150-cm BPL to minimize nutritional deficiencies while keeping an acceptable weight loss and comorbidities remission 1,9,27 . A recent meta-analysis comparing both 200 and 150 cm BPL in OAGB demonstrated that the 200 cm group achieved better weight loss outcomes and a comparable remission of comorbidities, at the expense of higher nutritional deficiency rates 6 . In our meta-analysis, the BPL in the included RCTs was mainly measured ranging from 180 to 220 cm.
Bariatric surgery is still the most efficacious and enduring intervention for severe obesity 5 . Nonetheless, 20 to 25% of patients experience weight regain after the surgical procedure, mainly due to a convergence of insufficient psychosocial counseling, high-calorie intake, and inadequate physical activity 1,3,7,18,23 . In this meta-analysis, OAGB was associated with a significantly higher %EWL and a significant decrease in BMI at 1- and 5-year follow-up, respectively. Accordingly, previous evidence comparing OAGB with RYGB exhibited increasing and significant %EWL values in individuals treated with OAGB after 1, 2, and 5 years of follow-up 34 . In light of these findings, it is essential to take into account the substantial long-term efficacy of OAGB, potentially contributing to decreased rates of weight regain after bariatric surgery.
Evidence-based knowledge has significantly expanded the management of obesity and associated comorbidities. Bariatric surgery has proven its efficacy for weight loss, expanding its action on T2DM remission 8,32 . In this study, the comparable effectiveness of OAGB with the control group showed a non-significant difference in T2DM remission. However, previous meta-analyses of RCTs comparing OAGB with RYGB found that OAGB delivered better remission rates for comorbidities 8 . Likewise, when compared with SG, previous meta-analyses have also supported the superiority of OAGB by increasing remission rates of T2DM 2,33 .
Other aspects should be considered when selecting the optimal bariatric surgical procedure, such as GERD 37,38 . Generally, OAGB is peformed with a wider gastric tube, leading to low intraluminal pressure and GERD 34 . In our pooled analysis, no significant difference in GERD rates was encountered between OAGB and the control group at follow-up. However, various publications indicated gastroesophageal reflux, typically bile reflux and its carcinogenic potential in the esophagus as prominent complications of OAGB 25 . Despite reports of these events in the literature being lower than expected, the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) 2018 task force recommended that these complications remain a theoretical risk 13,35 . Therefore, as a point of discussion among experts, a recent consensus on patient selection for OAGB indicated that this treatment should not be offered to patients with grade C or D esophagitis or Barrett’s metaplasia 26 .
Overall, complication rates of surgical procedures appeared to be comparable. Likewise, in another meta-analysis by Ali et. al., OAGB and SG were also associated with non-significant differences in complication rates 2 . Additionally, in a direct comparison between OAGB and RYGB, other studies also found low and comparable rates of complications 8,34 . Due to the long-stapled lines and gastrointestinal anastomoses, the main risks associated with bariatric procedures are linked to the possibility of leaks or hemorrhages. Mangoulitis et al., in a previous meta-analysis, examined these endpoints and found comparable incidences between OAGB and RYGB, supporting their safety 34 .
This study has limitations. First, there was moderate to high heterogeneity in some outcomes analyzed, such as the %EWL. However, this effect might be inherited from the small sample size and the small number of studies that reported this endpoint. Second, the absence of patient-level data regarding BMI and %EWL outcomes, precisely for participants with T2DM, precluded a potential subgroup analysis. Third, although this meta-analysis accomplished a comprehensive comparison of OAGB with RYGB and SG, the RCTs analyzed had high or some concerns at risk of bias assessment, limiting definite conclusions. Additional high-quality RCTs are expected to shed further light on the OAGB efficacy and safety.
CONCLUSIONS
Our meta-analysis of RCTs shows that OAGB surgery, compared with RYGB and SG, has significantly higher %EWL at 1-year follow-up and a significant decrease in BMI at 5-year follow-up, with a comparable rate of complications. Although bile reflux remains a theoretical risk after OAGB, our findings endorse OAGB as an effective and safe treatment for obese patients.
Financial source: None
Central Message
Obesity is a growing condition worldwide, both in underdeveloped and developing countries. Despite increasing global efforts to reduce the growth rate, a recent publication by the World Obesity Federation shows a projection that more than 50% of the world’s population will be overweight or obese by 2035. Although the existence of significant and promising new drugs in the approach to obesity disease, bariatric and metabolic surgery remains the most effective and durable treatment. Roux-en-Y gastric bypass surgery was the dominant procedure for many years but has been surpassed by sleeve gastrectomy in recent years.
Perspectives
One anastomosis gastric bypass, in comparison to Roux-en-Y gastric bypass and sleeve gastrectomy, was associated with a significantly higher percentage of excess weight loss at one year and a significantly lower body mass index at five years of follow-up, while maintaining non-significant differences in the rates of type-2 diabetes mellitus remission, complications, and gastroesophageal reflux disease. Our findings support the inclusion of one anastomosis gastric bypass in clinical practice as a potential surgical approach to obesity.
REFERENCES
- 1.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143–55. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali M, Wang Y, Ji J, Wang W, Wang D. One anastomosis gastric bypass versus sleeve gastrectomy for obesity: a systemic review and meta-analysis. J Gastrointest Surg. 2023;27(10):2226–44. doi: 10.1007/s11605-023-05782-x. [DOI] [PubMed] [Google Scholar]
- 3.Aliakbarian H, Bhutta HY, Heshmati K, Kunju SU, Sheu EG, Tavakkoli A. Pre-operative predictors of weight loss and weight regain following Roux-en-Y gastric bypass surgery: a prospective human study. Obes Surg. 2020;30(12):4852–9. doi: 10.1007/s11695-020-04877-7. [DOI] [PubMed] [Google Scholar]
- 4.Angrisani L, De Luca M, Formisano G, Santonicola A. Bariatric and metabolic surgery: indications complications and revisional procedures. Roma: Springer; 2017 [Google Scholar]
- 5.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric Surgery and Endoluminal Procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–89. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anvari M, Ghaferi A, Morton J, Shikora S. 8th global registry report. [Accessed: Mar. 03 2024]. Available at: https://www.ifso.com/pdf/8th-ifso-registry-report-2023.pdf .
- 7.Baig SJ, Priya P, Mahawar KK, Shah S. Indian Bariatric Surgery Outcome Reporting (IBSOR) Group. Weight regain after bariatric surgery-a multicentre study of 9617 patients from Indian Bariatric Surgery Outcome Reporting Group. Obes Surg. 2019;29(5):1583–92. doi: 10.1007/s11695-019-03734-6. [DOI] [PubMed] [Google Scholar]
- 8.Balamurugan G, Leo SJ, Sivagnanam ST, Balaji Prasad S, Ravindra C, Rengan V, et al. Comparison of efficacy and safety between Roux-en-Y Gastric Bypass (RYGB) vs One Anastomosis Gastric Bypass (OAGB) vs Single Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy (SADI-S): a systematic review of bariatric and metabolic surgery. Obes Surg. 2023;33(7):2194–209. doi: 10.1007/s11695-023-06602-6. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand T, Rives-Lange C, Jannot AS, Baratte C, Castelbajac F, Lu E, et al. 150-cm versus 200-cm biliopancreatic limb one-anastomosis gastric bypass: propensity score-matched analysis. Obes Surg. 2022;32(9):2839–45. doi: 10.1007/s11695-022-06203-9. [DOI] [PubMed] [Google Scholar]
- 10.Boyle M, Mahawar K. One anastomosis gastric bypass performed with a 150-cm biliopancreatic limb delivers weight loss outcomes similar to those with a 200-cm biliopancreatic limb at 18-24 months. Obes Surg. 2020;30(4):1258–64. doi: 10.1007/s11695-019-04359-5. [DOI] [PubMed] [Google Scholar]
- 11.Carbajo M, García-Caballero M, Toledano M, Osorio D, García-Lanza C, Carmona JA. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg. 2005;15(3):398–404. doi: 10.1381/0960892053576677. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-García EM, Frigolet ME, Canizales-Quinteros S, Gutiérrez-Aguilar R. Differential gene expression of subcutaneous adipose tissue among lean, obese, and after RYGB (different timepoints): systematic review and analysis. Nutrients. 2022;14(22):4925. doi: 10.3390/nu14224925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca M, Piatto G, Merola G, Himpens J, Chevallier JM, Carbajo MA, et al. IFSO update position statement on One Anastomosis Gastric Bypass (OAGB) Obes Surg. 2021;31(7):3251–78. doi: 10.1007/s11695-021-05413-x. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Eskandaros MS, Abbass A, Zaid MH, Darwish AA. Laparoscopic one anastomosis gastric bypass versus laparoscopic roux-en-y gastric bypass effects on pre-existing mild-to-moderate gastroesophageal reflux disease in patients with obesity: a randomized controlled study. Obes Surg. 2021;31(11):4673–81. doi: 10.1007/s11695-021-05667-5. [DOI] [PubMed] [Google Scholar]
- 16.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Health topics. [Accessed: Jan. 03, 2024]. Available at: https://www.who.int/health-topics .
- 18.Heinberg LJ, Bond DS, Carroll I, Crosby R, Fodor A, Fouladi F, et al. Identifying mechanisms that predict weight trajectory after bariatric surgery: rationale and design of the biobehavioral trial. Surg Obes Relat Dis. 2020;16(11):1816–26. doi: 10.1016/j.soard.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6. [Accessed: Feb. 28, 2024]. Available at: www.training.cochrane.org/handbook .
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain M, Tantia O, Goyal G, Chaudhuri T, Khanna S, Poddar A, et al. LSG vs MGB-OAGB: 5-year follow-up data and comparative outcome of the two procedures over long term-results of a randomised control trial. Obes Surg. 2021;31(3):1223–32. doi: 10.1007/s11695-020-05119-61. [DOI] [PubMed] [Google Scholar]
- 22.Jedamzik J, Eilenberg M, Felsenreich DM, Krebs M, Ranzenberger-Haider T, Langer FB, et al. Impact of limb length on nutritional status in one-anastomosis gastric bypass: 3-year results. Surg Obes Relat Dis. 2020;16(4):476–84. doi: 10.1016/j.soard.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Karmali S, Brar B, Shi X, Sharma AM, Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23(11):1922–33. doi: 10.1007/s11695-013-1070-4. [DOI] [PubMed] [Google Scholar]
- 24.Katayama RC, Arasaki CH, Herbella FAM, Neto RA, Lopes GJ., Filho One-anastomosis and roux-en-y gastric bypass promote similar weight loss, patient satisfaction, quality of life, inflammation grade, and cellular damage in the esophagus and gastric pouch in a short-term follow-up. J Obes Metab Syndr. 2021;30(4):396–402. doi: 10.7570/jomes21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keleidari B, Dehkordi MM, Shahraki MS, Ahmadi ZS, Heidari M, Hajian A, et al. Bile reflux after one anastomosis gastric bypass surgery: a review study. Ann Med Surg (Lond) 2021;64:102248. doi: 10.1016/j.amsu.2021.102248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kermansaravi M, Parmar C, Chiappetta S, Shahabi S, Abbass A, Abbas SI, et al. Patient selection in one anastomosis/mini gastric bypass-an expert modified delphi consensus. Obes Surg. 2022;32(8):2512–24. doi: 10.1007/s11695-022-06124-7. [DOI] [PubMed] [Google Scholar]
- 27.Komaei I, Sarra F, Lazzara C, Ammendola M, Memeo R, Sammarco G, et al. One anastomosis gastric bypass-mini gastric bypass with tailored biliopancreatic limb length formula relative to small bowel length: preliminary results. Obes Surg. 2019;29(9):3062–70. doi: 10.1007/s11695-019-04019-8. [DOI] [PubMed] [Google Scholar]
- 28.Kraljevic M, Ko¨stler T, Osto E, Taheri S, Lutz T, Zingg U, T, et al. Laparoscopic one anastomosis gastric bypass versus laparoscopic Roux-en-Y gastric bypass in the treatment of obesity: 1-year outcomes of the RCT. 24th IFSO World Congress. Obes Surg. 2019;29(Suppl 5):208. doi: 10.1007/s11695-019-04101-1. [DOI] [Google Scholar]
- 29.Lee WJ, Chong K, Lin YH, Wei JH, Chen SC. Laparoscopic sleeve gastrectomy versus single anastomosis (mini-) gastric bypass for the treatment of type 2 diabetes mellitus: 5-year results of a randomized trial and study of incretin effect. Obes Surg. 2014;24(9):1552–62. doi: 10.1007/s11695-014-1344-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005;242(1):20–8. doi: 10.1097/01.sla.0000167762.46568.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Level L, Rojas A, Piñango S, Avariano Y. One anastomosis gastric bypass vs. Roux-en-Y gastric bypass: a 5-year follow-up prospective randomized trial. Langenbecks Arch Surg. 2021;406(1):171–9. doi: 10.1007/s00423-020-01949-1. [DOI] [PubMed] [Google Scholar]
- 32.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–32. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 33.Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Sioka E, Zacharoulis D. One-anastomosis gastric bypass versus sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis. Obes Surg. 2017;27(9):2479–87. doi: 10.1007/s11695-017-2807-2. [DOI] [PubMed] [Google Scholar]
- 34.Magouliotis DE, Tasiopoulou VS, Tzovaras G. One anastomosis gastric bypass versus roux-en-y gastric bypass for morbid obesity: an updated meta-analysis. Obes Surg. 2019;29(9):2721–30. doi: 10.1007/s11695-019-04005-0. [DOI] [PubMed] [Google Scholar]
- 35.Mahawar KK, Himpens J, Shikora SA, Chevallier JM, Lakdawala M, De Luca M, et al. The first Consensus Statement on One Anastomosis/Mini Gastric Bypass (OAGB/MGB) using a modified Delphi approach. Obes Surg. 2018;28(2):303–12. doi: 10.1007/s11695-017-3070-2. [DOI] [PubMed] [Google Scholar]
- 36.Mercuri M, Gafni A. The evolution of GRADE (part 3): a framework built on science or faith? J Eval Clin Pract. 2018;24(5):1223–31. doi: 10.1111/jep.13016. [DOI] [PubMed] [Google Scholar]
- 37.Motola D, Zeini IM, Moon RC, Ghanem M, Teixeira AF, Jawad MA. Anti-reflux procedures after Roux-en-y gastric bypass. Arq Bras Cir Dig. 2022;34(3):e1614. doi: 10.1590/0102-672020210002e1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musella M, Vitiello A, Berardi G, Velotti N, Pesce M, Sarnelli G. Evaluation of reflux following sleeve gastrectomy and one anastomosis gastric bypass: 1-year results from a randomized open-label controlled trial. Surg Endosc. 2021;35(12):6777–85. doi: 10.1007/s00464-020-08182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omar I, Sam MA, Pegler ME, Pearson EJB, Boyle M, Mahawar K. Effect of one anastomosis gastric bypass on haematinics, vitamin d and parathyroid hormone levels: a comparison between 150 and 200 cm bilio-pancreatic limbs. Obes Surg. 2021;31(7):2954–61. doi: 10.1007/s11695-021-05281-5. [DOI] [PubMed] [Google Scholar]
- 41.Pizza F, Lucido FS, D’Antonio D, Tolone S, Gambardella C, Dell’Isola C, et al. Biliopancreatic limb length in one anastomosis gastric bypass: which is the best? Obes Surg. 2020;30(10):3685–94. doi: 10.1007/s11695-020-04687-x. [DOI] [PubMed] [Google Scholar]
- 42.Robert M, Espalieu P, Pelascini E, Caiazzo R, Sterkers A, Khamphommala L, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet. 2019;393(10178):1299–309. doi: 10.1016/S0140-6736(19)30475-1. [DOI] [PubMed] [Google Scholar]
- 43.Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11(3):276–80. doi: 10.1381/096089201321336584. [DOI] [PubMed] [Google Scholar]
- 44.Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15(9):1304–8. doi: 10.1381/096089205774512663. [DOI] [PubMed] [Google Scholar]
- 45.Salman MA, Abelsalam A, Nashed GA, Yacoub M, Abdalla A. Long biliopancreatic limb Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: a randomized controlled study. Obes Surg. 2023;33(7):1966–73. doi: 10.1007/s11695-023-06631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salman MA, Salman A, Assal MM, Elsherbiney M, Tourky M, Elewa A, et al. One Anastomosis Gastric Bypass (OAGB) with a 150-cm Biliopancreatic Limb (BPL) Versus a 200-cm BPL, a systematic review and meta-analysis. Obes Surg. 2023;33(6):1846–56. doi: 10.1007/s11695-023-06556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sam MA, Hussain A, Pegler ME, Pearson EJB, Omar I, Boyle M, et al. Effect of one anastomosis gastric bypass on liver function tests: a comparison between 150 cm and 200 cm biliopancreatic limbs. J Minim Access Surg. 2022;18(1):38–44. doi: 10.4103/jmas.JMAS_249_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandoval DA, Patti ME. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol. 2023;19(3):164–76. doi: 10.1038/s41574-022-00757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seetharamaiah S, Tantia O, Goyal G, Chaudhuri T, Khanna S, Singh JP, et al. LSG vs OAGB-1 year follow-up data-a randomized control trial. Obes Surg. 2017;27(4):948–54. doi: 10.1007/s11695-016-2403-x. [DOI] [PubMed] [Google Scholar]
- 50.Singh B, Saikaustubh Y, Singla V, Kumar A, Ahuja V, Gupta Y, et al. One Anastomosis Gastric Bypass (OAGB) vs Roux en Y Gastric Bypass (RYGB) for Remission of T2DM in patients with morbid obesity: a randomized controlled trial. Obes Surg. 2023;33(4):1218–27. doi: 10.1007/s11695-023-06515-4. [DOI] [PubMed] [Google Scholar]
- 51.Shivakumar S, Tantia O, Goyal G, Chaudhuri T, Khanna S, Ahuja A, et al. LSG vs MGB-OAGB-3 year follow-up data: a randomised control trial. Obes Surg. 2018;28(9):2820–8. doi: 10.1007/s11695-018-3255-3. [DOI] [PubMed] [Google Scholar]
- 52.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 53.Slagter N, Heide LJM, Jutte EH, Kaijser MA, Damen SL, van Beek AP, et al. Outcomes of the one anastomosis gastric bypass with various biliopancreatic limb lengths: a retrospective single-center cohort study. Obes Surg. 2021;31(10):4236–42. doi: 10.1007/s11695-021-05555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker ON, Szomstein S, Rosenthal RJ. Indications for sleeve gastrectomy as a primary procedure for weight loss in the morbidly obese. J Gastrointest Surg. 2008;12(4):662–7. doi: 10.1007/s11605-008-0480-4. [DOI] [PubMed] [Google Scholar]
- 55.Valezi AC, Campos ACL, Von Bahten LC. Brazilian multi-society position statement on emerging bariatric and metabolic surgical procedures. ABCD Arq Bras Cir Dig. 2023;36:e1759. doi: 10.1590/0102-672020230041e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Victorzon M. Single-anastomosis gastric bypass: better, faster, and safer? Scand J Surg. 2015;104(1):48–53. doi: 10.1177/1457496914564106. [DOI] [PubMed] [Google Scholar]
- 57.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4(4):353–7. doi: 10.1381/096089294765558331. [DOI] [PubMed] [Google Scholar]
- 58.World Obesity. Global Obesity Observatory. Obesity Atlas 2023. [Accessed: Feb. 28, 2024]. Available at: https://data.worldobesity.org/publications/?cat=19 .


