Abstract
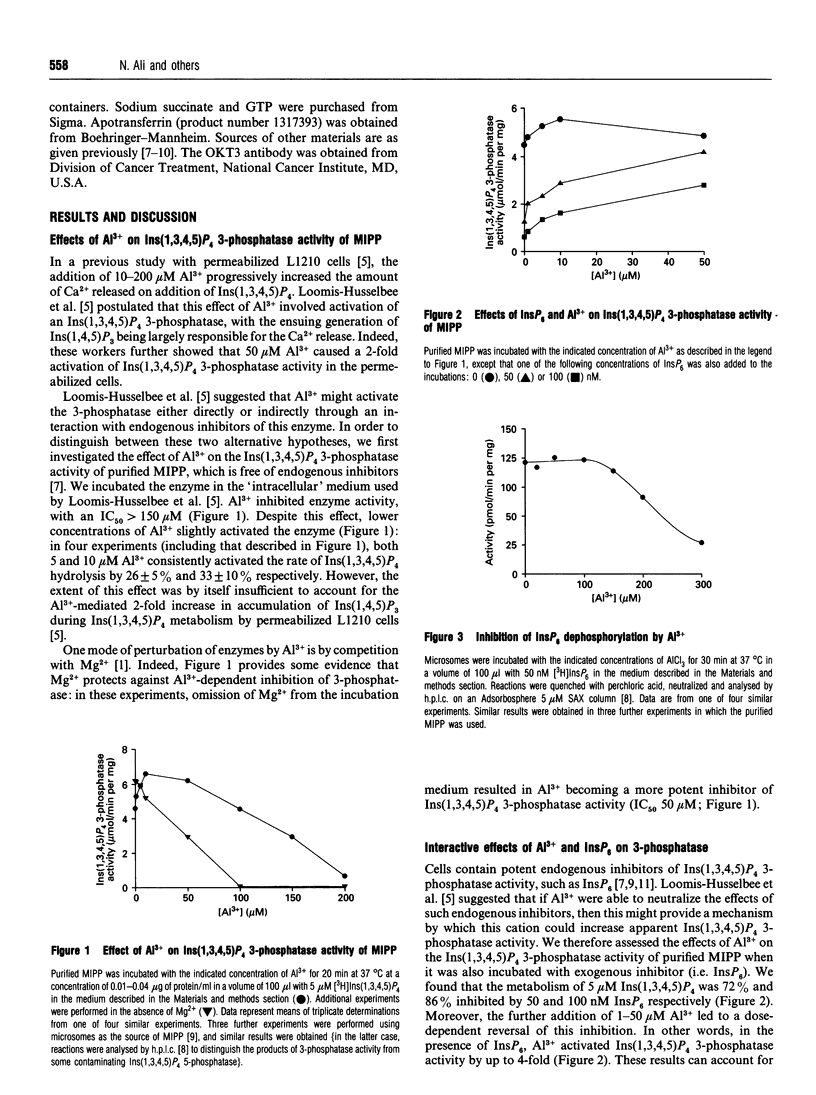
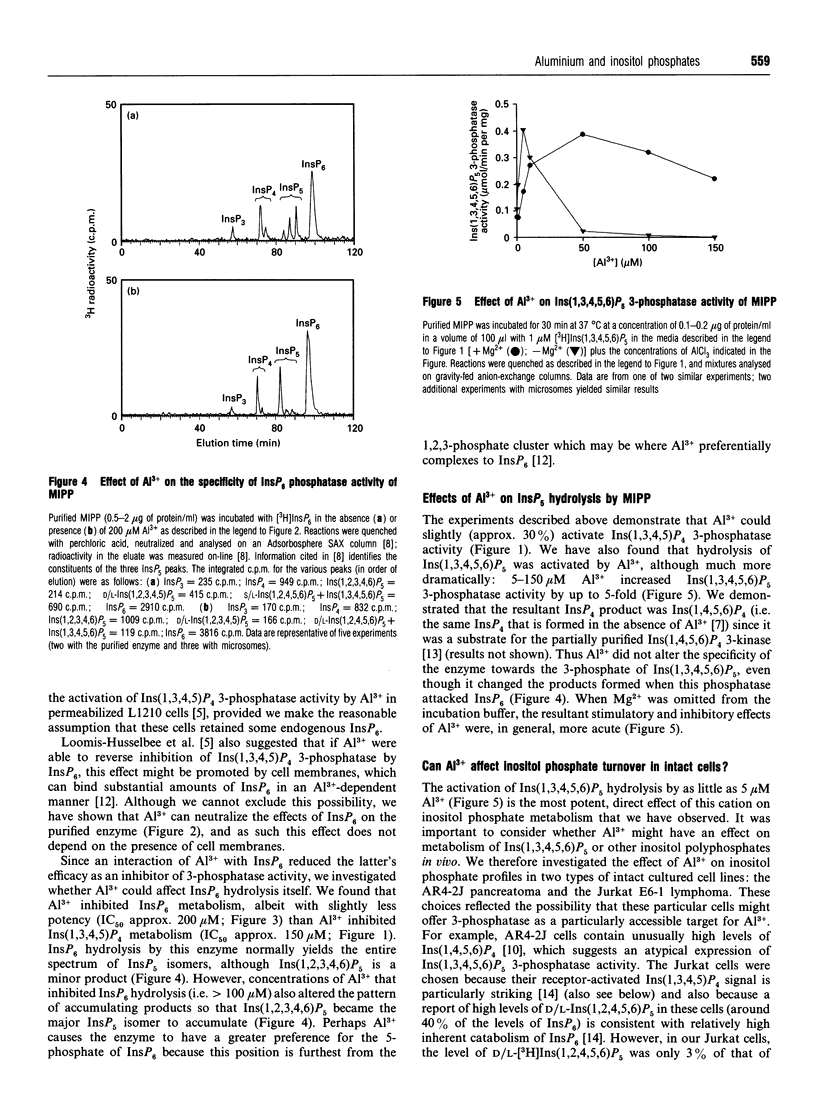
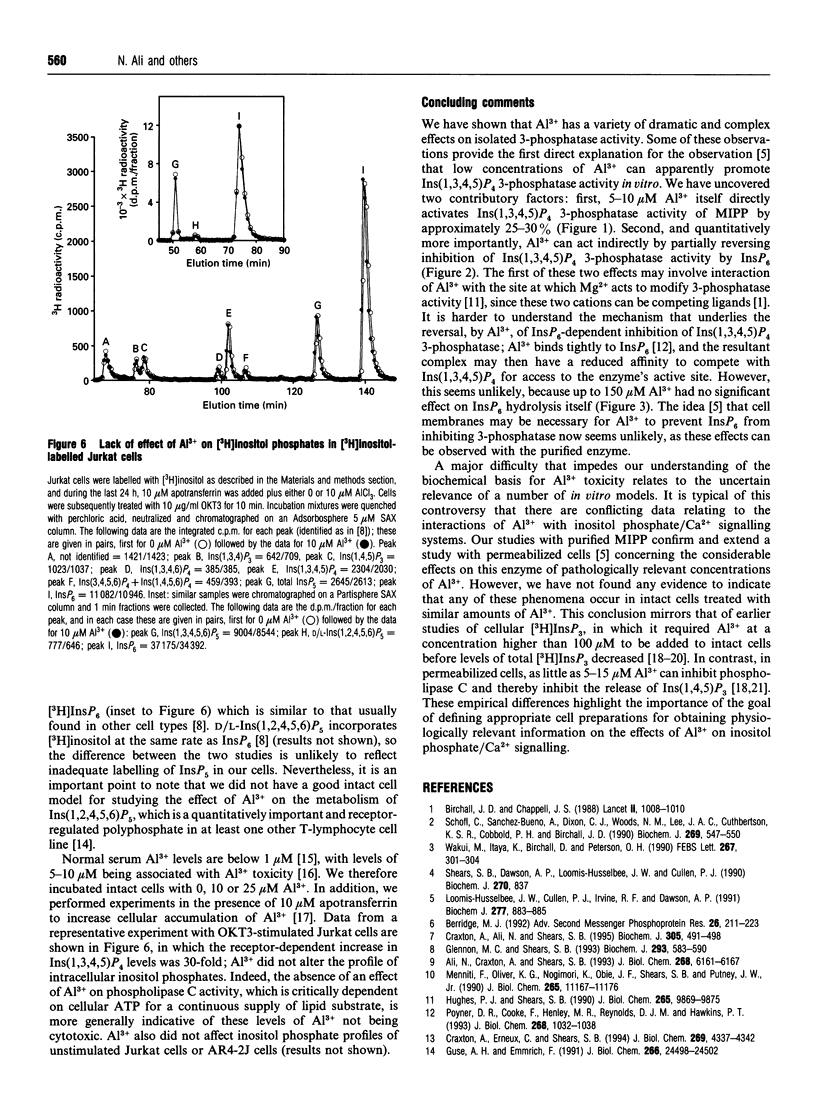
There is speculation that some of the toxic effects of Al3+ may originate from it perturbing inositol phosphate/Ca2+ signalling. For example, in permeabilized L1210 mouse lymphoma cells, 10-50 microM Al3+ activated Ins(1,3,4,5)P4-dependent Ca2+ mobilization and Ins(1,3,4,5)P4 3-phosphatase activity [Loomis-Husselbee, Cullen, Irvine and Dawson (1991) Biochem. J. 277, 883-885]. Ins(1,3,4,5)P4 3-phosphatase activity is performed by a multiple inositol polyphosphate phosphatase (MIPP) that also attacks Ins(1,3,4,5,6)P5 and InsP6 [Craxton, Ali and Shears (1995) Biochem. J. 305, 491-498]: 5-50 microM Al3+ increased MIPP activity towards both Ins(1,3,4,5)P4 (by 30%) and Ins(1,3,4,5,6)P5 (by up to 500%), without affecting metabolism of InsP6. Higher concentrations of Al3+ inhibited metabolism of all three substrates, and in the case of InsP6, Al3+ altered the pattern of accumulating products. When 1-50 microM Al3+ was present, InsP6 became a less effective inhibitor of Ins(1,3,4,5)P4 3-phosphatase activity; this effect did not depend on the presence of cellular membranes, contrary to a previous proposal. The latter phenomenon largely explains how, in a cell-free system where Ins(1,3,4,5)P4 3-phosphatase is inhibited by endogenous InsP6, the addition of Al3+ can apparently increase the enzyme activity. However, there was no effect of either 10 or 25 microM Al3+ (in either the presence or absence of apotransferrin) on inositol phosphate profiles in either Jurkat E6-1 lymphoma cells or AR4-2J pancreatoma cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Craxton A., Shears S. B. Hepatic Ins(1,3,4,5)P4 3-phosphatase is compartmentalized inside endoplasmic reticulum. J Biol Chem. 1993 Mar 25;268(9):6161–6167. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium oscillations. Adv Second Messenger Phosphoprotein Res. 1992;26:211–223. [PubMed] [Google Scholar]
- Birchall J. D., Chappell J. S. Aluminum, chemical physiology, and Alzheimer's disease. Lancet. 1988 Oct 29;2(8618):1008–1010. doi: 10.1016/s0140-6736(88)90754-4. [DOI] [PubMed] [Google Scholar]
- Craxton A., Ali N., Shears S. B. Comparison of the activities of a multiple inositol polyphosphate phosphatase obtained from several sources: a search for heterogeneity in this enzyme. Biochem J. 1995 Jan 15;305(Pt 2):491–498. doi: 10.1042/bj3050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton A., Erneux C., Shears S. B. Inositol 1,4,5,6-tetrakisphosphate is phosphorylated in rat liver by a 3-kinase that is distinct from inositol 1,4,5-trisphosphate 3-kinase. J Biol Chem. 1994 Feb 11;269(6):4337–4342. [PubMed] [Google Scholar]
- Glennon M. C., Shears S. B. Turnover of inositol pentakisphosphates, inositol hexakisphosphate and diphosphoinositol polyphosphates in primary cultured hepatocytes. Biochem J. 1993 Jul 15;293(Pt 2):583–590. doi: 10.1042/bj2930583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse A. H., Emmrich F. T-cell receptor-mediated metabolism of inositol polyphosphates in Jurkat T-lymphocytes. Identification of a D-myo-inositol 1,2,3,4,6-pentakisphosphate-2-phosphomonoesterase activity, a D-myo-inositol 1,3,4,5,6-pentakisphosphate-1/3-phosphatase activity and a D/L-myo-inositol 1,2,4,5,6-pentakisphosphate-1/3-kinase activity. J Biol Chem. 1991 Dec 25;266(36):24498–24502. [PubMed] [Google Scholar]
- Hughes P. J., Shears S. B. Inositol 1,3,4,5,6-pentakisphosphate and inositol hexakisphosphate inhibit inositol-1,3,4,5-tetrakisphosphate 3-phosphatase in rat parotid glands. J Biol Chem. 1990 Jun 15;265(17):9869–9875. [PubMed] [Google Scholar]
- Loomis-Husselbee J. W., Cullen P. J., Irvine R. F., Dawson A. P. Electroporation can cause artefacts due to solubilization of cations from the electrode plates. Aluminum ions enhance conversion of inositol 1,3,4,5-tetrakisphosphate into inositol 1,4,5-trisphosphate in electroporated L1210 cells. Biochem J. 1991 Aug 1;277(Pt 3):883–885. doi: 10.1042/bj2770883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Nogimori K., Obie J. F., Shears S. B., Putney J. W., Jr Origins of myo-inositol tetrakisphosphates in agonist-stimulated rat pancreatoma cells. Stimulation by bombesin of myo-inositol 1,3,4,5,6-pentakisphosphate breakdown to myo-inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 1990 Jul 5;265(19):11167–11176. [PubMed] [Google Scholar]
- Poyner D. R., Cooke F., Hanley M. R., Reynolds D. J., Hawkins P. T. Characterization of metal ion-induced [3H]inositol hexakisphosphate binding to rat cerebellar membranes. J Biol Chem. 1993 Jan 15;268(2):1032–1038. [PubMed] [Google Scholar]
- Schöfl C., Sanchez-Bueno A., Dixon C. J., Woods N. M., Lee J. A., Cuthbertson K. S., Cobbold P. H., Birchall J. D. Aluminium perturbs oscillatory phosphoinositide-mediated calcium signalling in hormone-stimulated hepatocytes. Biochem J. 1990 Jul 15;269(2):547–550. doi: 10.1042/bj2690547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer T. J., Mundy W. R., Tilson H. A. Aluminum decreases muscarinic, adrenergic, and metabotropic receptor-stimulated phosphoinositide hydrolysis in hippocampal and cortical slices from rat brain. Brain Res. 1993 Nov 26;629(1):133–140. doi: 10.1016/0006-8993(93)90491-5. [DOI] [PubMed] [Google Scholar]
- Shafer T. J., Nostrandt A. C., Tilson H. A., Mundy W. R. Mechanisms underlying AlCl3 inhibition of agonist-stimulated inositol phosphate accumulation. Role of calcium, G-proteins, phospholipase C and protein kinase C. Biochem Pharmacol. 1994 Apr 20;47(8):1417–1425. doi: 10.1016/0006-2952(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Dawson A. P., Loomis-Husselbee J. W., Cullen P. J. The perturbation, by aluminium, of receptor-generated calcium transients in hepatocytes is not due to effects of Ins(1,4,5)P3-stimulated Ca2+ release or Ins(1,4,5)P3 metabolism by the 5-phosphatase and 3-kinase. Biochem J. 1990 Sep 15;270(3):837–837. doi: 10.1042/bj2700837a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Chou K., Haug A. Aluminium impacts elements of the phosphoinositide signalling pathway in neuroblastoma cells. Mol Cell Biochem. 1993 Apr 21;121(2):109–118. doi: 10.1007/BF00925969. [DOI] [PubMed] [Google Scholar]
- Shi B., Haug A. Aluminum uptake by neuroblastoma cells. J Neurochem. 1990 Aug;55(2):551–558. doi: 10.1111/j.1471-4159.1990.tb04169.x. [DOI] [PubMed] [Google Scholar]
- Wakui M., Itaya K., Birchall D., Petersen O. H. Intracellular aluminium inhibits acetylcholine- and caffeine-evoked Ca2+ mobilization. FEBS Lett. 1990 Jul 16;267(2):301–304. doi: 10.1016/0014-5793(90)80949-j. [DOI] [PubMed] [Google Scholar]
- Wood P. C., Wojcikiewicz R. J., Burgess J., Castleden C. M., Nahorski S. R. Aluminium inhibits muscarinic agonist-induced inositol 1,4,5-trisphosphate production and calcium mobilization in permeabilized SH-SY5Y human neuroblastoma cells. J Neurochem. 1994 Jun;62(6):2219–2223. doi: 10.1046/j.1471-4159.1994.62062219.x. [DOI] [PubMed] [Google Scholar]


