Abstract
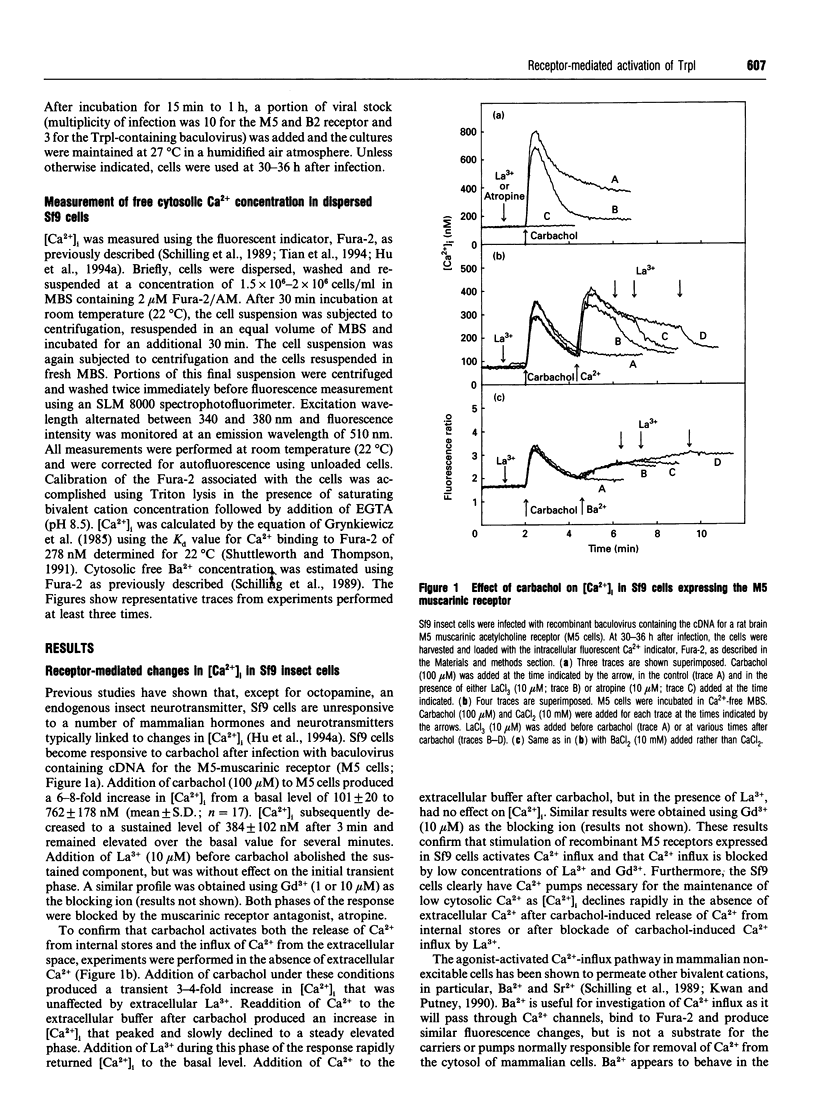
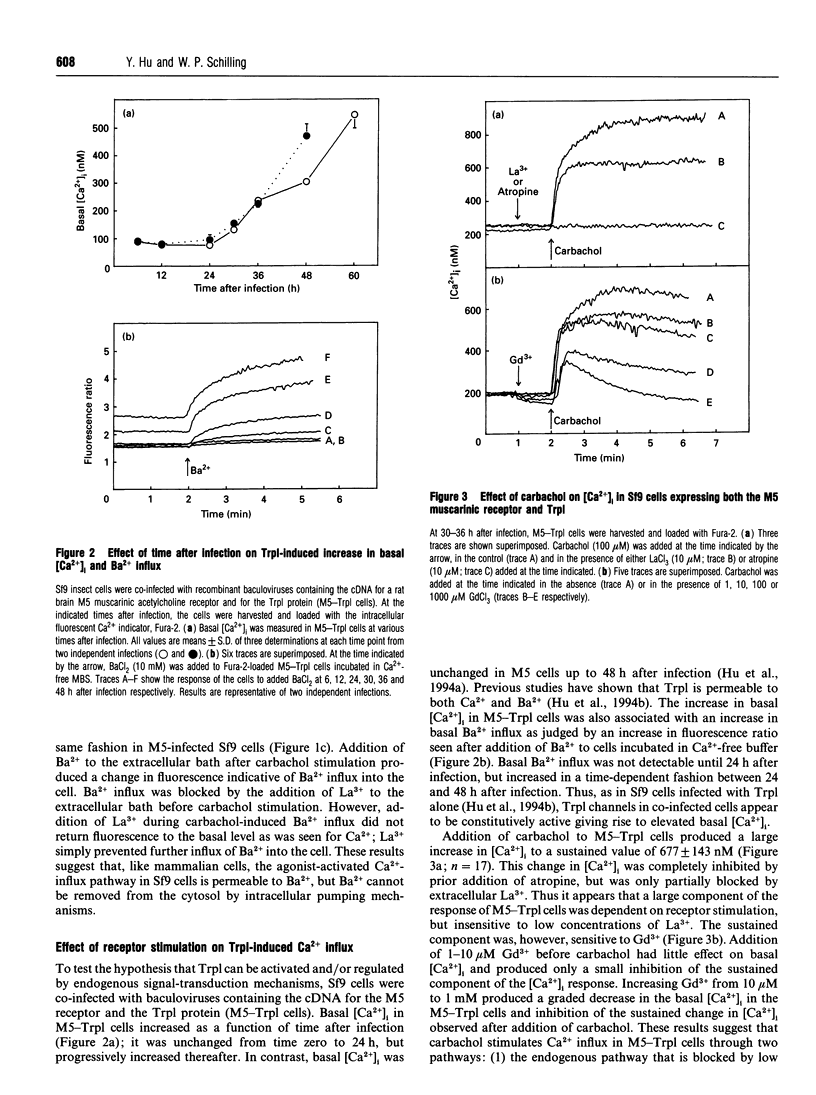
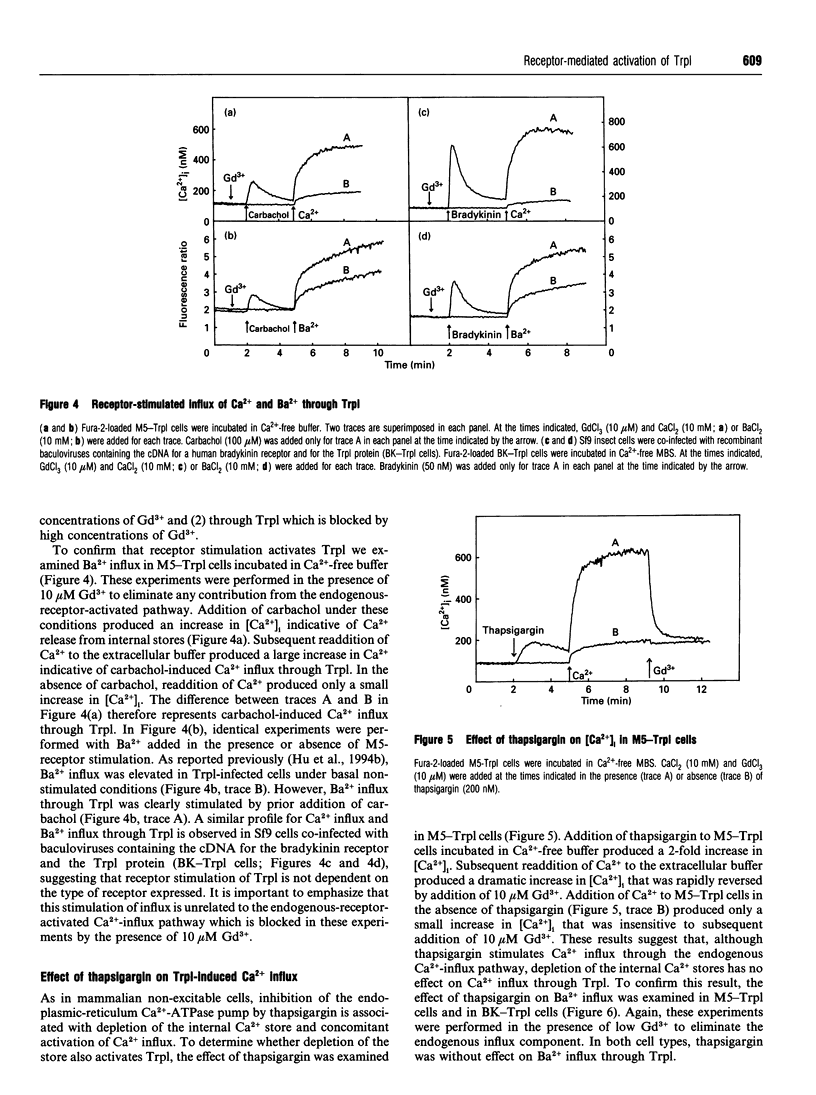
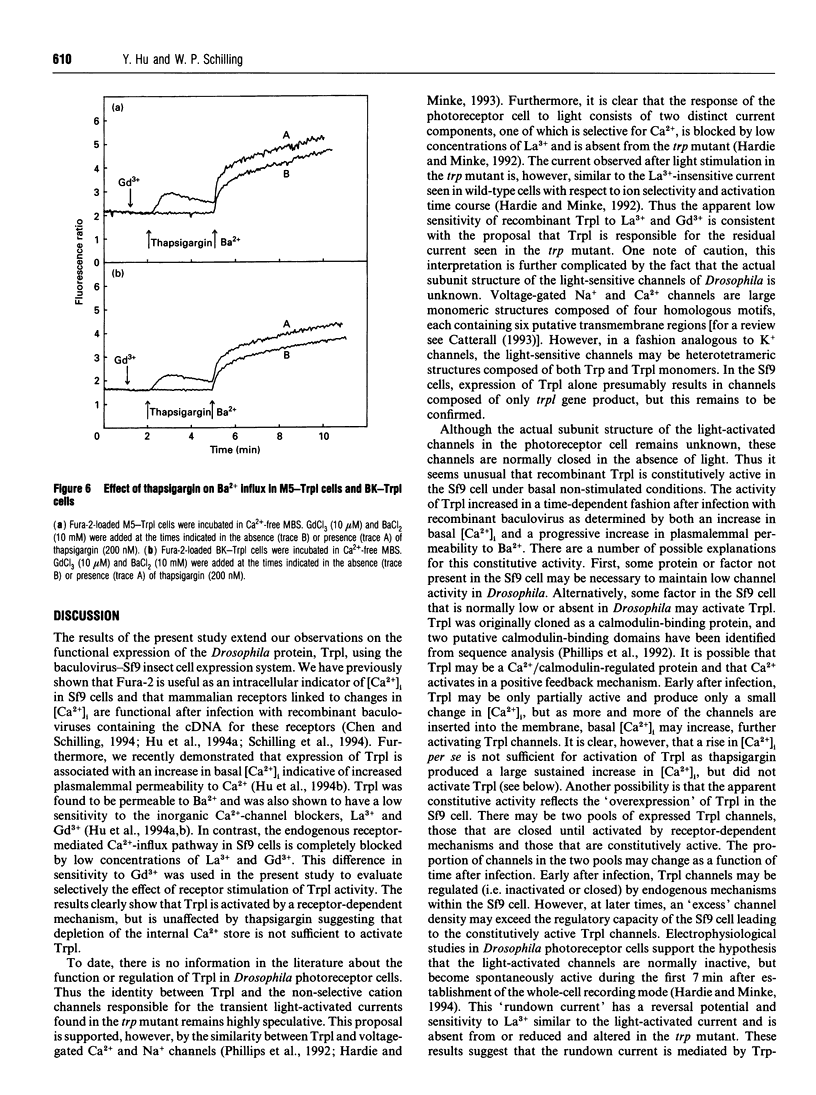
The Drosophila proteins, Trp and Trpl, are suggested to be cation channels responsible for depolarization of the receptor potential associated with stimulation of insect photoreceptor cells by light. Consistent with this hypothesis, we recently showed that recombinant Trpl forms Ca(2+)- and Ba(2+)-permeable non-selective cation channels when expressed in Sf9 cells using the baculovirus expression vector. As Trpl may be activated in the photoreceptor cell after stimulation of phospholipase C, we hypothesized that a similar regulation of recombinant Trpl may be observed in the Sf9 cell after activation of heterologous membrane receptors linked to Ca(2+)-signal-transduction pathways. To test this hypothesis, Ca2+ signalling was examined in Fura-2-loaded Sf9 cells infected with baculovirus containing cDNA for the M5 muscarinic receptor alone (M5 cells) or in cells co-infected with both M5 and Trpl-containing baculoviruses (M5-Trpl cells). Addition of carbachol (100 microM) to M5 cells produced an increase in cytosolic free Ca2+ concentration ([Ca2+]i) (mean +/- S.D.; n = 17) from 101 +/- 20 to 762 +/- 178 nM which declined to a sustained elevated level of 384 +/- 102 nM after 3 min. The sustained component was eliminated by removal of extracellular Ca2+ or by addition of La3+ or Gd3+ (10 microM). In M5-Trpl cells, basal [Ca2+]i increased as a function of time after infection. To evaluate the contribution of Ca2+ influx to the overall profile observed, Ba2+, a Ca2+ surrogate that is not a substrate for the Ca2+ pump, was used. The increase in basal [Ca2+]i seen in M5-Trpl cells was associated with an increase in basal Ba2+ influx. Addition of carbachol to M5-Trpl cells at 30-36 h after infection produced a large increase in [Ca2+]i to a sustained value of 677 +/- 143 nM. This change in [Ca2+]i was (1) blocked by atropine, (2) attenuated in the absence of extracellular Ca2+, and (3) relatively insensitive to La3+, but blocked by Gd3+ in the 0.1-1 mM range. In the presence of 10 microM Gd3+ to block the endogenous-receptor-mediated Ca(2+)-influx in M5-Trpl cells. In sharp contrast increase in Ba2+ influx in M5-Trpl cells. In sharp contrast, neither Ca2+ nor Ba2+ influx through Trpl was affected by thapsigargin, a selective inhibitor of the endoplasmic reticulum Ca(2+)-ATPase pump.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catterall W. A. Structure and function of voltage-gated ion channels. Trends Neurosci. 1993 Dec;16(12):500–506. doi: 10.1016/0166-2236(93)90193-p. [DOI] [PubMed] [Google Scholar]
- Cosens D. J., Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969 Oct 18;224(5216):285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Fafournoux P., Ghysdael J., Sardet C., Pouysségur J. Functional expression of the human growth factor activatable Na+/H+ antiporter (NHE-1) in baculovirus-infected cells. Biochemistry. 1991 Oct 1;30(39):9510–9515. doi: 10.1021/bi00103a018. [DOI] [PubMed] [Google Scholar]
- Foder B., Scharff O., Thastrup O. Ca2+ transients and Mn2+ entry in human neutrophils induced by thapsigargin. Cell Calcium. 1989 Oct;10(7):477–490. doi: 10.1016/0143-4160(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hardie R. C., Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993 Sep;16(9):371–376. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- Hardie R. C., Minke B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994 Mar;103(3):389–407. doi: 10.1085/jgp.103.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R. C., Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992 Apr;8(4):643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993 Jun;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hu Y., Vaca L., Zhu X., Birnbaumer L., Kunze D. L., Schilling W. P. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochem Biophys Res Commun. 1994 Jun 15;201(2):1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- Klaiber K., Williams N., Roberts T. M., Papazian D. M., Jan L. Y., Miller C. Functional expression of Shaker K+ channels in a baculovirus-infected insect cell line. Neuron. 1990 Aug;5(2):221–226. doi: 10.1016/0896-6273(90)90311-3. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Kwan C. Y., Takemura H., Obie J. F., Thastrup O., Putney J. W., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990 Jun;258(6 Pt 1):C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Li Z., Smith C. D., Smolley J. R., Bridge J. H., Frank J. S., Philipson K. D. Expression of the cardiac Na(+)-Ca2+ exchanger in insect cells using a baculovirus vector. J Biol Chem. 1992 Apr 15;267(11):7828–7833. [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Montell C., Rubin G. M. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989 Apr;2(4):1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Phillips A. M., Bull A., Kelly L. E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992 Apr;8(4):631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol phosphates and calcium entry. Adv Second Messenger Phosphoprotein Res. 1992;26:143–160. [PubMed] [Google Scholar]
- Schilling W. P., Cabello O. A., Rajan L. Depletion of the inositol 1,4,5-trisphosphate-sensitive intracellular Ca2+ store in vascular endothelial cells activates the agonist-sensitive Ca(2+)-influx pathway. Biochem J. 1992 Jun 1;284(Pt 2):521–530. doi: 10.1042/bj2840521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Shuttleworth T. J., Thompson J. L. Effect of temperature on receptor-activated changes in [Ca2+]i and their determination using fluorescent probes. J Biol Chem. 1991 Jan 25;266(3):1410–1414. [PubMed] [Google Scholar]
- Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990 May;253(2):688–697. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Hu Y., Schilling W. P., Lindsay D. A., Eiden J., Estes M. K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994 Jan;68(1):251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L., Kunze D. L. Depletion and refilling of intracellular Ca2+ stores induce oscillations of Ca2+ current. Am J Physiol. 1993 Apr;264(4 Pt 2):H1319–H1322. doi: 10.1152/ajpheart.1993.264.4.H1319. [DOI] [PubMed] [Google Scholar]
- Wong F., Schaefer E. L., Roop B. C., LaMendola J. N., Johnson-Seaton D., Shao D. Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron. 1989 Jul;3(1):81–94. doi: 10.1016/0896-6273(89)90117-7. [DOI] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


