Abstract
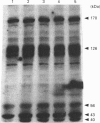
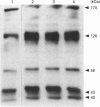
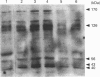
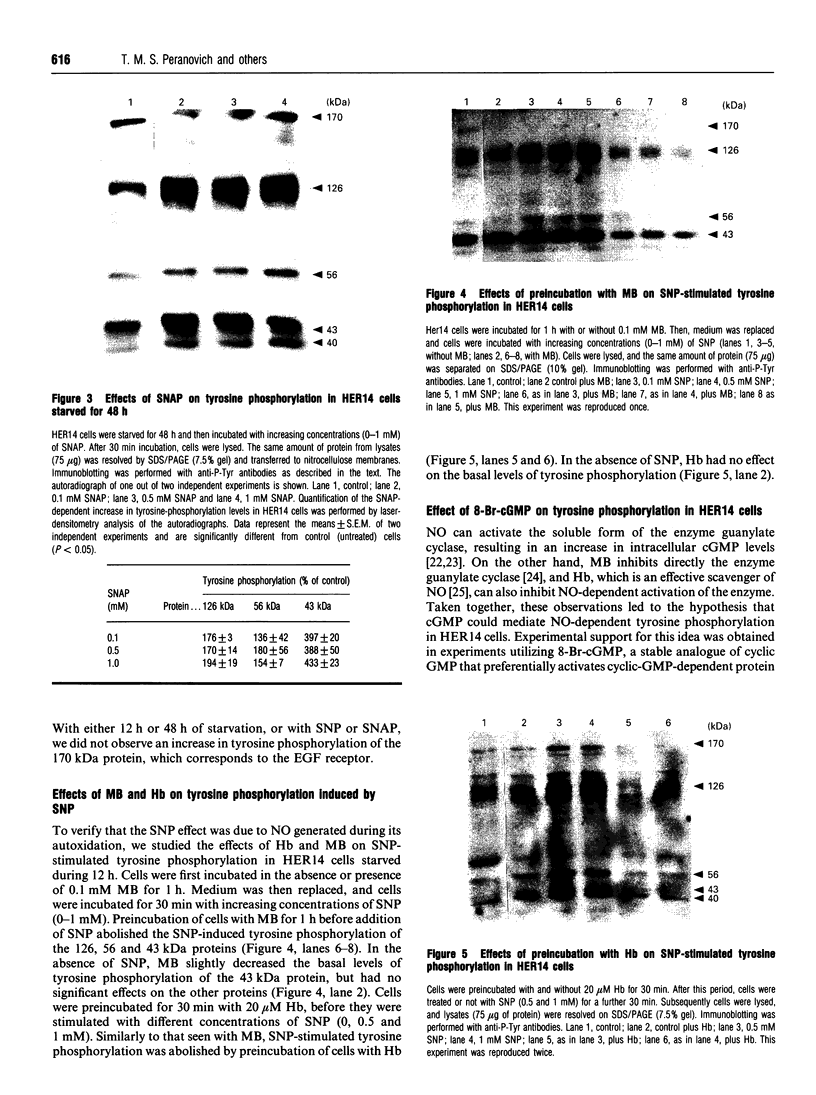
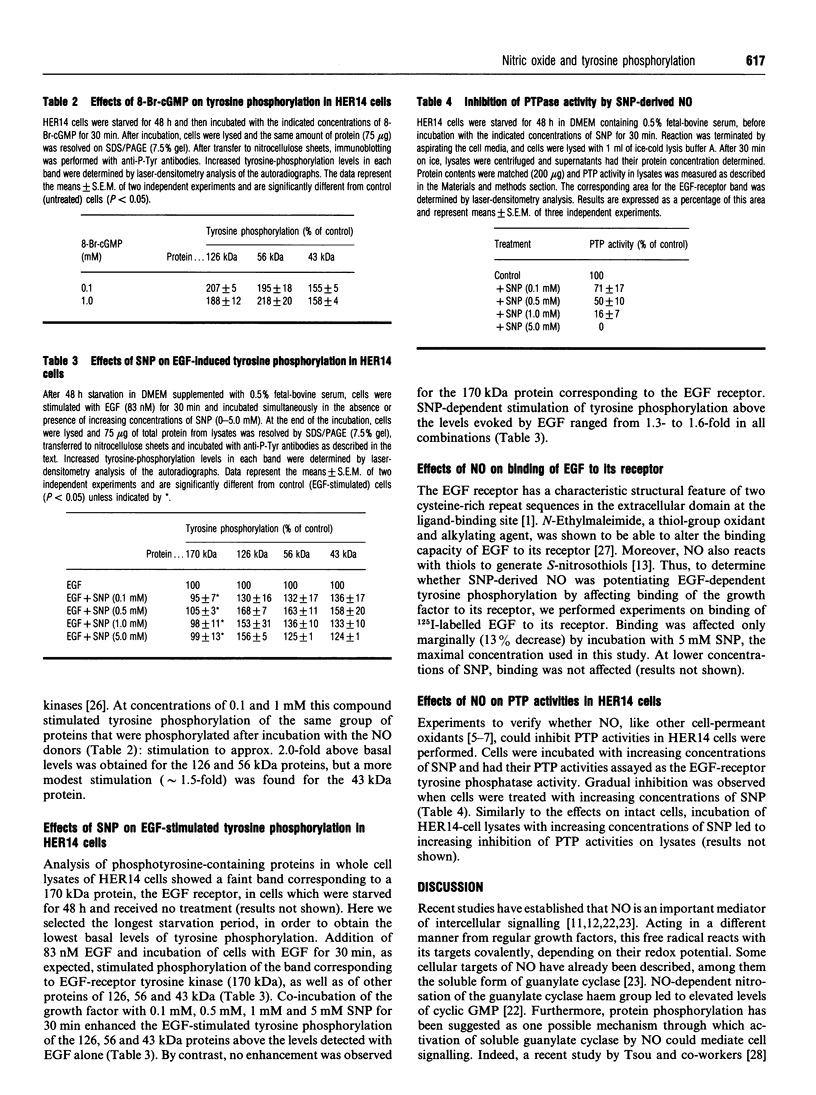
In the present study, utilizing anti-phosphotyrosine monoclonal antibodies, sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine (SNAP) as sources of NO and murine fibroblasts expressing the human epidermal growth factor (EGF) receptor (HER14 cells), we showed that tyrosine phosphorylation of a set of proteins (126, 56 and 43 kDa) was stimulated when cells were incubated with either SNP or SNAP and abolished by Methylene Blue and oxyhaemoglobin. Inhibition by Methylene Blue suggested an involvement of cyclic GMP in the process, which was evidenced by the effects of 8-bromo cyclic GMP. This analogue of cyclic GMP stimulated tyrosine phosphorylation of the same set of proteins phosphorylated after incubation with the NO source. Tyrosine phosphorylation of the same set of proteins was stimulated when cells were incubated simultaneously with SNP and EGF, showing that NO also potentiates EGF-evoked tyrosine kinase activity in HER14 cells. However, stimulation of the autophosphorylation of the EGF receptor, above the levels obtained for EGF alone, was not observed under those conditions. Additionally, we investigated the effects of NO on EGF-receptor tyrosine phosphatase activities in HER14 cells. Increasing concentrations of NO correlate with a gradual inhibition of these activities in HER14 cells, either in intact cells or in cell lysates. Taken together, these observations suggest that NO modulates tyrosine phosphorylation in HER14 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauskin A. R., Alkalay I., Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991 Aug 23;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pike L. J., Grant G. A., Krebs E. G., Glaser L. Interaction of epidermal growth factor-dependent protein kinase with endogenous membrane proteins and soluble peptide substrate. J Biol Chem. 1983 Mar 10;258(5):2945–2950. [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J., Nolte C., Butt E., Sage S. O., Walter U. Role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of agonist-evoked calcium elevation in human platelets. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffetz D., Bushkin I., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990 Feb 15;265(5):2896–2902. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Kanner S. B., Kavanagh T. J., Grossmann A., Hu S. L., Bolen J. B., Rabinovitch P. S., Ledbetter J. A. Sulfhydryl oxidation down-regulates T-cell signaling and inhibits tyrosine phosphorylation of phospholipase C gamma 1. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):300–304. doi: 10.1073/pnas.89.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Maiese K., Boniece I., DeMeo D., Wagner J. A. Peptide growth factors protect against ischemia in culture by preventing nitric oxide toxicity. J Neurosci. 1993 Jul;13(7):3034–3040. doi: 10.1523/JNEUROSCI.13-07-03034.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Monteiro H. P., Ivaschenko Y., Fischer R., Stern A. Ascorbic acid inhibits protein tyrosine phosphatases in NIH 3T3 cells expressing human epidermal growth factor receptors. Int J Biochem. 1993 Dec;25(12):1859–1864. doi: 10.1016/0020-711x(88)90317-5. [DOI] [PubMed] [Google Scholar]
- Monteiro H. P., Ivaschenko Y., Fischer R., Stern A. Inhibition of protein tyrosine phosphatase activity by diamide is reversed by epidermal growth factor in fibroblasts. FEBS Lett. 1991 Dec 16;295(1-3):146–148. doi: 10.1016/0014-5793(91)81405-w. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Pillay T. S., Makgoba M. W. Enhancement of epidermal growth factor (EGF) and insulin-stimulated tyrosine phosphorylation of endogenous substrates by sodium selenate. FEBS Lett. 1992 Aug 10;308(1):38–42. doi: 10.1016/0014-5793(92)81045-n. [DOI] [PubMed] [Google Scholar]
- Riggs A. Preparation of blood hemoglobins of vertebrates. Methods Enzymol. 1981;76:5–29. doi: 10.1016/0076-6879(81)76111-1. [DOI] [PubMed] [Google Scholar]
- Shargill N. S., Tatoyan A., el-Refai M. F., Pleta M., Chan T. M. Impaired insulin receptor phosphorylation in skeletal muscle membranes of db/db mice: the use of a novel skeletal muscle plasma membrane preparation to compare insulin binding and stimulation of receptor phosphorylation. Biochem Biophys Res Commun. 1986 May 29;137(1):286–294. doi: 10.1016/0006-291x(86)91208-8. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K., Snyder G. L., Greengard P. Nitric oxide/cGMP pathway stimulates phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, in the substantia nigra. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3462–3465. doi: 10.1073/pnas.90.8.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K. M., Dixon J. E. Protein tyrosine phosphatases. Annu Rev Biochem. 1993;62:101–120. doi: 10.1146/annurev.bi.62.070193.000533. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Garbers D. L. Receptor guanylyl cyclases. J Clin Invest. 1992 Aug;90(2):299–305. doi: 10.1172/JCI115862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]