ABSTRACT
We have previously reported two single-agent phase I trials, evaluating the dose or schedule, of a DNA vaccine (pTVG-HP) encoding prostatic acid phosphatase (PAP) administered with GM-CSF as the adjuvant. These were in patients with PSA-recurrent, radiographically non-metastatic, prostate cancer (PCa). We report here the long-term safety and overall survival of these patients. Specifically, 22 patients with non-metastatic, castration-sensitive PCa (nmCSPC) were treated with pTVG-HP, 100–1500 µg, administered over 12 weeks and followed for 15 y. 17 patients with non-metastatic castration-resistant PCa (nmCRPC) were treated with 100 µg pTVG-HP with different schedules of administration over 1 y and followed for 5 y. No adverse events were detected in long-term follow-up from either trial that were deemed possibly related to vaccination. Patients with nmCSPC had a median overall survival of 12.3 y, with 5/22 (23%) alive at 15 y. 8/22 (36%) died due to prostate cancer with a median survival of 11.0 y, and 9/22 (41%) died of other causes. Patients with nmCRPC had a median overall survival of 4.5 y, with 8/17 (47%) alive at 5 y. The presence of T-cells specific for the PAP target antigen was detectable in 6/10 (60%) individuals with nmCSPC, and 3/5 (60%) individuals with nmCRPC, many years after immunization. The detection of immune responses to the vaccine target years after immunization suggests durable immunity can be elicited in patients using a DNA vaccine encoding a tumor-associated antigen.
Trial Registration: NCT00582140 and NCT00849121
KEYWORDS: DNA vaccine, prostate cancer, prostatic acid phosphatase, long-term follow up, survival
Introduction
Surgery and/or radiation therapy are used to treat localized prostate cancer. Notwithstanding, approximately one third of patients will have recurrence after these primary therapies.1 This is first detected as a rise in serum prostate-specific antigen (PSA), which historically was described as M0 (non-metastatic, castration-sensitive; nmCSPC) prostate cancer, as there was no radiographic evidence of metastases seen by conventional CT or bone scan imaging. The median time to the development of radiographically detectable metastases is 8 y for patients with this stage of disease.2 Androgen deprivation can be used in this setting; however, many patients are keen to avoid the treatment-related adverse effects of androgen deprivation and elect to wait until the disease is radiographically detectable. Patients who elect early androgen deprivation for nmCSPC eventually develop castration-resistant disease with subsequent rise in serum PSA. If repeat imaging is negative, this is termed non-metastatic castration-resistant prostate cancer (nmCRPC). In this stage of disease, the median time to the development of radiographic metastases is 30 months, and the median time to death is 37.6 months.3,4 For both nmCSPC and nmCRPC, it has been reported that the rate of PSA rise is associated with time to metastases and overall survival.3,5
The setting of PSA-recurrent prostate cancer (nmCSPC and nmCRPC) has been considered ideal for evaluating anti-cancer vaccines as it has been believed that these treatments might slow tumor growth or have a delayed onset of action, unlike traditional cytotoxic therapies.6 Moreover, non-hormonal therapies that modulate the rate of rise of PSA have been demonstrated to affect the time to metastasis, at least in patients with nmCSPC.7 Thus, the impact of these treatments on the rate of PSA rise might be used as a biomarker for their effect on disease progression and overall survival. Several different types of vaccines have been evaluated in PSA-recurrent prostate cancer, as we have previously reviewed.8 These have included early phase clinical trials using carbohydrate vaccines, peptide vaccines, poxviral vaccines, and plasmid DNA vaccines, each of which have demonstrated positive effects by slowing the rate of PSA rise.9–12 The largest vaccine trial conducted in patients with nmCSPC was a randomized phase II trial using a plasmid DNA vaccine encoding prostatic acid phosphatase (PAP, pTVG-HP). By encoding this prostate tissue-specific antigen, the goal of using this plasmid DNA vaccine was to elicit PAP-specific CD8 T cells with the capacity of lysing prostate tumor cells expressing PAP.13,14 That trial randomized 99 patients with rapidly rising PSA and nmCSPC to receive vaccine and GM-CSF as the vaccine adjuvant versus GM-CSF alone.15 While immune responses to PAP were elicited with treatment, that trial failed to demonstrate a difference in time to metastatic disease progression.15 As a result, more recent trials in this population have used vaccines in combination with other immune-modulating therapies, including the immune checkpoint blockade.16,17 A small trial conducted in patients with nmCSPC using pTVG-HP in combination with nivolumab demonstrated more marked decreases in the rate of PSA rise.16 Further studies with this combination approach are underway.
We have previously reported the results from two phase 1 trials using the pTVG-HP DNA vaccine delivered intradermally as a monotherapy with GM-CSF given as an adjuvant A dose-escalation phase 1 trial was first conducted in patients with nmCSPC (NCT00582140). Because this was an early first-in-human trial administering recombinant DNA to patients, FDA requested 15 y of follow up to evaluate for possible late complications. In addition, we conducted a phase I trial evaluating different schedules of administration with a fixed vaccine dose in patients with nmCRPC (NCT00849121). For that trial, patients were followed for 5 y for late complications. Blood was collected where feasible from multiple subjects several years after completing these trials and used to evaluate for immune response to the PAP target antigen. We report here the long-term safety data, overall survival, and long-term immune response data from these two trials.
Materials and methods
Study agent and regulatory information
pTVG-HP (a.k.a. MVI-816, Madison Vaccines, Inc., Madison, WI) is a plasmid DNA encoding the full-length human PAP cDNA.14 The trial protocols were reviewed and approved by all local and federal regulatory entities. All patients gave written informed IRB-approved consent for participation in the original trials. Where feasible, patients who remained in long-term follow-up also subsequently gave separate written informed IRB-approved consent for collection of blood for immune monitoring.
Patient populations and treatment
For the nmCSPC trial (NCT00582140), eligible subjects were male patients with a histological diagnosis of adenocarcinoma of the prostate and biochemical (serum PSA) recurrence after definitive surgery and/or radiation therapy, provided there was no evidence of the suspected lymph node, bone, or visceral metastatic disease on bone scan or CT scan prior to study entry. This was conducted as a standard “3 + 3” dose escalation trial with three planned dose cohorts of 100 µg, 500 µg, or 1500 µg administered 6 times at 2-week intervals. Three patients were enrolled at each dose level, and in the absence of significant adverse events, an additional 13 patients were enrolled in the highest 1500 µg dose cohort to more fully assess safety and immunogenicity. Thus, 22 patients were treated in this trial between 2005 and 2007. The original inclusion criteria required that patients have a Karnofsky performance score of >70 and normal bone marrow, liver, and renal function blood tests. Patients were excluded if they had been treated with immunosuppressive therapy, including chemotherapy, corticosteroids, or extensive radiation therapy, within six months of study entry, or were on concurrent medications with possible anti-cancer effects. There was no requirement for a pre-treatment PSA doubling time to be within a specific range; however, PSA values were required to be >2 ng/mL by two measurements at least two weeks apart. A minimum of 4 pre-treatment PSA values collected over 2–10 months prior to treatment were available and used to determine pre-treatment PSA doubling times.12,18 As shown in Figure 1, these individuals received pTVG-HP six times at biweekly intervals over a period of 12 weeks. Vaccinations were at doses of 100 µg (3 patients), 500 µg (3 patients), or 1500 µg (16 patients) and were delivered intradermally with 250 µg recombinant human GM-CSF (Sargramostim, Berlex Oncology).
Figure 1.
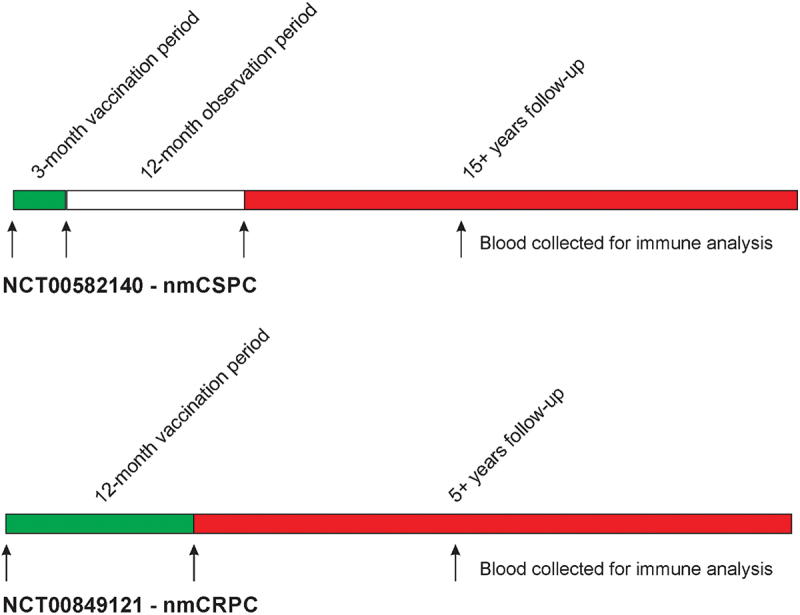
Schemas. Shown are the original trial schemas and long-term follow-up. In the nmCSPC trial, 6 vaccinations were delivered every 2 weeks over a 3-month vaccination course, and patients were then monitored for immune responses over an additional 12 months.12 In the nmCRPC trial, 6 vaccinations were delivered every 2 weeks over a 3-month vaccination course, and then, patients received either fixed booster immunizations every 3 months for 1 y total, or received vaccinations continuing every 2 weeks until evidence of immune response, with the schedule of immunization effectively being determined by immune monitoring.19 Cryopreserved blood samples for immune analysis were available pre-treatment, at the end of the treatment period, and at later time points during long-term follow-up.
For the nmCRPC trial (NCT00849121), eligible subjects were male patients with a histological diagnosis of prostate adenocarcinoma of the prostate and PSA recurrence following castration (surgical or ongoing luteinizing hormone-releasing hormone agonist therapy), with documentation of castrate levels of serum testosterone (<50 ng/mL) prior to treatment, provided there was no evidence of suspected lymph node, bone, or visceral metastatic disease on bone scans or CT scans. Patients were treated with 100 µg pTVG-HP six times at 2-week intervals and then either received booster immunizations every 3 months or continued with vaccination every 2 weeks until there was evidence of immune response to the vaccine target antigen identified by ELISPOT immune monitoring.19 30 patients were planned for enrollment to detect a difference of at least 50% in the immune response rates between the two arms with at least 80% power at the 2-sided 0.05 significance level. The trial was conducted between 2009 and 2012, and stopped early after 17 patients due to slow accrual.19 All patients had to have been previously treated with a first-generation anti-androgen (e.g., flutamide or bicalutamide), but with rising PSA on treatment and persistent rise in PSA after withdrawal. Patients were required to have an Eastern Cooperative Oncology Group performance score of <2 and normal bone marrow, liver, and renal function blood tests. Patients were excluded if they had been treated with immunosuppressive therapy (chemotherapy, corticosteroids, or extensive radiation therapy) within six months of study entry, or were on concurrent medications with possible anti-cancer effects. Patients were further excluded if they had a history of HIV, hepatitis B, or hepatitis C infection, or if they had received a prior anti-cancer vaccine. While a specific pre-treatment PSA doubling time was not required, patients were required to have at least four serum PSA values from the same clinical laboratory, over a 3- to 6-month period of time and immediately prior to entry, to calculate a pre-treatment PSA DT. The final PSA was required to be >2.0 ng/mL. As shown in Figure 1, patients received vaccinations with pTVG-HP (100 µg) and concurrent GM-CSF adjuvant (250 µg) intradermally 6 times at two-week intervals, and then either quarterly or with individualized schedules as determined by immune monitoring, for up to 1 y.
Long-term follow up procedures
22 patients were treated in NCT00582140 and followed for 1 y with immune monitoring. These patients were then contacted annually in person or by telephone for 15 y (Figure 1). The information collected included the date of contact, current medications, hospitalizations, current stage and treatment for prostate cancer, most recent serum PSA level, new important medical diagnoses (including cancer, autoimmune disorders, hematologic disorders, or neurologic disorders), and date of death if the patient was deceased. 17 patients were treated in NCT00849121 over the course of 12 months. These patients were then contacted annually in person or by telephone for 5 y with the same information collected (Figure 1). Possible attribution of late events to the vaccine treatment were all reviewed by an independent physician study monitor.
Immunological response evaluation
Peripheral blood samples were collected from individual subjects as per the original study (immediately pre-treatment, post-treatment and 1 y after treatment) and at various times years after treatment. T-cell immune response to the PAP target antigen was analyzed using IFNγ ELISPOT with cryopreserved peripheral blood mononuclear cells (PBMCs) as previously described.15,16 Test antigens included media alone (negative control), a pool of peptides derived from viral antigens (CEF, positive control), a pool of 15-mer peptides spanning the amino acid sequence of PAP, or phytohemagglutinin (PHA, positive control).15,20 Cryopreserved samples from different timepoints from an individual subject were all assessed at the same time in 4-well replicates. Immune response to PAP was assessed as the number of IFNγ spot-forming units (sfu) following PAP stimulation, subtracting the contribution of sfu from media alone. Comparisons of test antigens to media alone controls were conducted using a paired t-test. An immune response was defined as a significant difference at the two-sided 0.05 significance level, after applying the Benjamini–Hochberg False Discovery Rate (FDR) adjustment procedure.
Statistical analysis
Categorical clinical outcomes were summarized in terms of frequencies and percentages. Time to event outcomes, including the time to death and time to development of metastases, were analyzed using the Kaplan–Meier method and summarized in terms of medians and corresponding two-sided 95% confidence intervals (CIs). Time to death was defined as the date from the start of the pTVG-HP treatment (day 1) to the time of documented death. Survival times were censored for patients alive at the end of the follow-up period. Time to the development of metastases was defined as the date from the start of the pTVG-HP treatment (day 1) to the earliest date of documented metastases, or the last date of follow-up if no development of metastases was documented (censored). Durations of treatment periods were summarized in terms of medians. The pre-treatment PSA doubling time was calculated for each patient as the logarithm of 2 divided by the slope of a linear regression of the log(PSA) over time (months). Clinical outcomes and treatment periods were summarized for each patient in graphical format using swimmer plots. Statistical analyses were conducted using SAS software (SAS Institute, Cary NC) version 9.4 and R software version 4.2.2 (https://www.R-project.org/).
Results
Long-term outcomes
22 patients with nmCSPC treated in NCT00582140 were followed for 15 y, although one patient declined further follow up after 11 y. At the end of this period, 5/22 (23%) patients were alive. 8/22 (36%) died due to prostate cancer, and 9/22 (41%) died from other causes. 16 (73%) patients developed metastases, and the median time to the development of metastases was 8.2 (95% CI: 4.1–11.5) y. The median time to death due to any cause was 12.3 (95% CI: 11.2–15.9) y. 12 patients began androgen deprivation therapy due to rising PSA, with a median time to the start of androgen deprivation of 2.7 y. For these patients, the median time to the development of metastases was 6.4 y. Overall, the median time from the development of metastases to death was 5.7 y. The median time on androgen deprivation before beginning another therapy was 6.4 y, and the median time from any therapy following androgen deprivation to death was 3.1 y. These clinical courses are summarized in Figure 2, ordered by the individual subject’s pre-treatment PSA doubling time. From this, it can be observed that most patients who died of prostate cancer were those with rapid PSA doubling times prior to original treatment.
Figure 2.
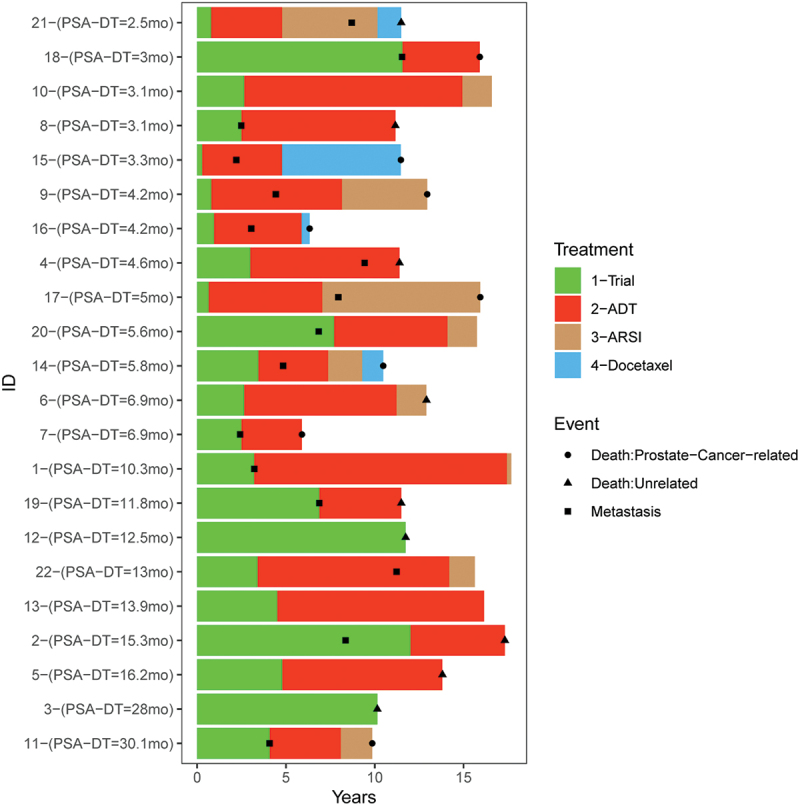
Long-term follow up of patients with nmCSPC (NCT00582140). Patients were followed for 15 y for significant medical events. Shown are the times at which patients developed documented metastatic disease (■), started androgen deprivation therapy (red), started a second generation AR targeted therapy (brown), or started docetaxel (blue). The time of death due to prostate cancer (●) or other cause (▲) is also shown. Subjects are ordered with respect to the pre-treatment PSA doubling time.
In terms of long-term adverse events, one patient was diagnosed with non-small cell lung cancer 3 y after treatment and Waldenstrom macroglobulinemia 8 y after treatment. One subject was diagnosed with squamous cell cancer of mediastinal lymph nodes 6 y after treatment, and another was diagnosed with melanoma 11 y after treatment. One patient developed autoimmune hepatitis 4 y after treatment. Two patients experienced transient ischemic attacks 9 and 13 y after treatment, and another patient experienced a cerebral vascular accident (CVA) 3 y after treatment. There were otherwise no other malignancies, autoimmune events, or other major medical events, and none of these events were deemed possibly related to treatment.
17 patients with nmCRPC treated in NCT00849121 were followed for 5 y after discontinuing treatment. 8/17 (47%) were alive at 5 y, and the median time to death was 4.5 (95% CI: 2.4–6.4) y. The median time to the development of metastases was 1.4 (95% CI: 0.9–2.6) y, and the median time to next treatment was 2.1 y. 14/17 (82%) patients received subsequent treatment with a second-generation AR pathway signaling inhibitor (ARSI), 12 of which were at or after the time of detection of metastatic disease. The median duration of ARSI therapy was 1.7 y. 8/17 (47%) patients received subsequent taxane chemotherapy with a median duration of 0.8 y until next therapy or death. 16/17 (94%) patients died in longer term follow-up. 14/16 deaths were due to prostate cancer while 2/16 deaths were due to other causes. The median time from the development of metastases to death was 2.4 y, and the median time from any subsequent therapy to death was 2.5 y. These clinical courses are summarized in Figure 3, ordered by the individual subject’s pre-treatment PSA doubling time. In terms of adverse events, one patient developed esophageal cancer 5 y after treatment. One patient experienced myocardial infarction and CVA within 1 y of treatment, and another developed bronchial edema of unknown etiology 1 y after treatment. There were otherwise no malignancies, autoimmune events, or other major medical events in the 5 y of follow up. None of these events were deemed possibly related to treatment.
Figure 3.
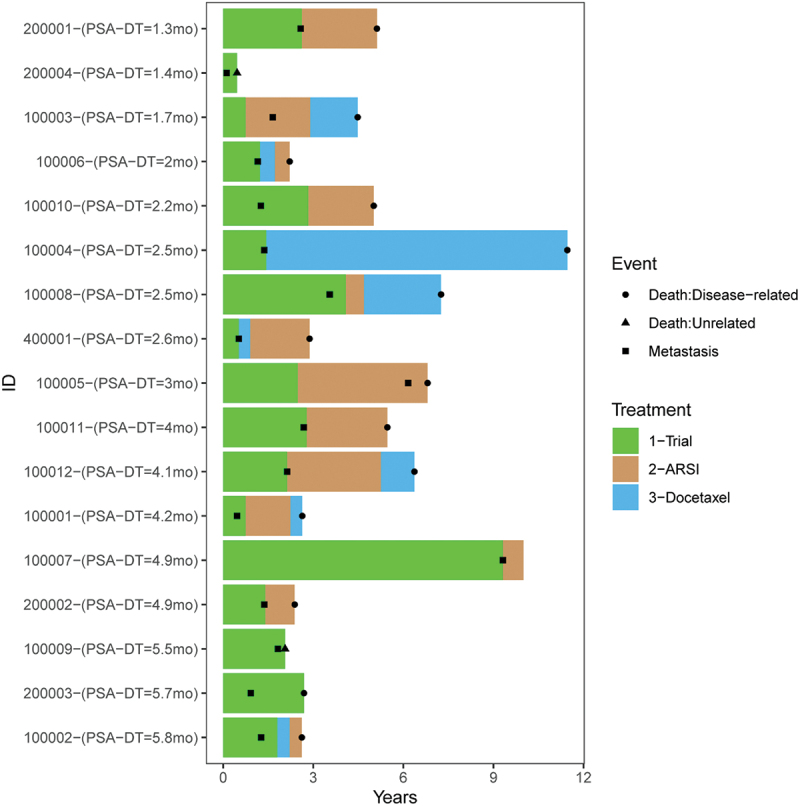
Long-term follow up of patients with nmCRPC (NCT00849121). Patients were followed for 5 y for significant medical events. Shown are the times at which patients developed documented metastatic disease (■), started ARSI treatment (brown), or started docetaxel (blue). Time of death due to prostate cancer (●) or other cause (▲) is also shown. Subjects are ordered with respect to the pre-treatment PSA doubling time.
Immunological response
PBMC samples were available from 10/22 patients at various time points several years after original treatment for nmCSPC. Similarly, PBMCs were available from 5/17 patients at various times at least one year after original treatment for nmCRPC. ELISPOT was used to evaluate T-cell immunity to the PAP vaccine target antigen at the time of original treatment and at these long-term time points. As shown in Figure 4 (and individual patients in Supplemental Figure S1), PAP-specific IFNγ-secreting T cells were detected in 2/10 (20%) patients pre-treatment, but in 6/10 (60%) of patients with nmCSPC 3–15 y after original treatment and in 3/5 (60%) of patients with nmCRPC 1–5 y after original treatment. Four patients with nmCSPC (patients 1, 9, 18, and 22) had no PAP-specific immune response pre-treatment and significant responses to PAP at every post-treatment time point over many years (Supplemental Figure S1).
Figure 4.
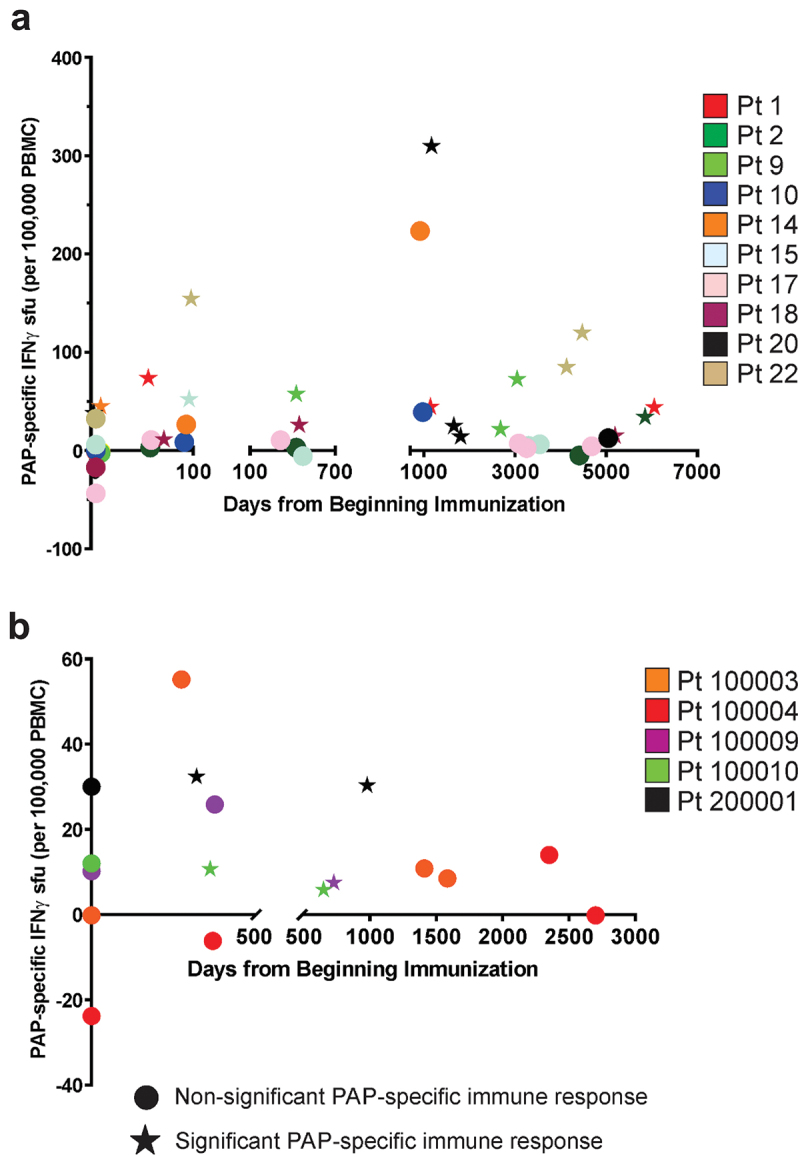
Long-term T cell immune response to PAP target antigen detectable after immunization. PBMCs from patients with nmCSPC (n = 10, panel A) or nmCRPC (n = 5, panel B) obtained within days pre-treatment, at the end of the vaccination period, and at late time points years later were evaluated by ELISPOT for IFNγ release in response to PAP antigen stimulation. pap-specific spot-forming units (sfu) shown are the mean among 6 replicates, and subtracted for contribution from media alone. Circles indicate non-significant difference from media alone control, and star shapes represent those significantly different from media alone. Colors correspond to individual subjects.
Discussion
We report the long-term follow-up of patients with prostate cancer treated with a DNA vaccine encoding PAP (pTVG-HP) for non-metastatic recurrent prostate cancer. At the time the first trial was initiated in 2005, there had been relatively little experience administering recombinant DNA vaccines to human patients. As such, FDA requested 15 y of long-term follow-up for potential late complications. While the total sample size was relatively small, no long-term safety concerns emerged. The median overall survival was 12.3 y, and the prostate cancer-specific survival was over 15 y, consistent with this early stage of disease. Of note, while the median time to metastatic disease was 8.2 y, this time was 6.8 y in the subset of 15 patients with a pre-treatment PSA doubling time of less than 12 months. This is longer than expected based on published reports of the natural history of prostate cancer in this population;5,21 however, it should be noted that most patients began androgen deprivation prior to the development of metastases, which certainly prolonged this metastasis-free interval. Similarly, patients with nmCRPC had a median overall survival of 4.5 y and 1.4 y median time to metastases. This was as long or longer than expected, given the natural history of this stage of disease from what has been previously published, in particular given that this population had rapidly progressive disease with a short pre-treatment PSA doubling time.3,4 Again, no long-term safety signals emerged in this population either. A major limitation of our study, however, was that these were both very small trials conducted at a single center. Larger trials will be necessary to assess safety and long-term efficacy in more diverse patient populations.
We subsequently conducted a randomized phase 2 trial using pTVG-HP with GM-CSF adjuvant versus GM-CSF adjuvant alone in patients with nmCSPC (NCT01341652), designed to determine whether vaccination increased the time to metastatic disease progression.15 In that trial, there was no overall difference in time to metastases detected. This is consistent with what has been observed with other vaccine trials for prostate cancer, notably trials using PROSTVAC or Sipuleucel-T.22,23 Studies with other vaccines have suggested that they might favorably impact the treatment effect of subsequent therapies. For example, in an early phase trial of patients with non-small cell lung cancer, 62% of patients had greater than expected objective response to salvage chemotherapy initiated after vaccine treatments.24 Similarly, patients who received sipuleucel-T prior to docetaxel demonstrated a longer survival compared to patients receiving placebo prior to docetaxel (p = .023).25 Furthermore, in another trial of 34 patients with mCRPC who were treated with the whole tumor-cell vaccine GVAX, 13 patients went on to receive a taxane-based chemotherapy after receiving vaccine. These individuals had a mean overall survival of 35.2 months vs. 17.2 months for those who did not receive chemotherapy.26 In the current small trials, we did observe that patients with nmCSPC were on androgen deprivation for a median of 6.5 y, and for those that began another therapy subsequently, the median time to death was 4.8 y. This was longer, albeit not significantly, than what was observed in patients with nmCRPC in whom the median time from subsequent therapy to death was 2.5 y. This observation suggests that immunization early in the course of disease, prior to the use of androgen deprivation, may be preferred, as has been suggested by studies in murine models27 and other human trials.28,29 The potential impact on the benefit from subsequent therapies could explain why survival may be a better endpoint for vaccine therapies. We will consequently plan to determine whether patients who received pTVG-HP for nmCSPC in the randomized phase 2 trial15 experienced a longer survival than patients who received adjuvant alone.
There has been very little information about the durability of T cell immunity to cancer vaccine antigens. While the current study was small and samples were only collected where feasible, we found that vaccine antigen-specific T cells were detectable in the majority of individuals and could be identified many years later. These responses were low in frequency, in the range of 10–300 per 100,000 T cells, frequencies lower than those typically detected to foreign viral antigens, but at similar frequencies as were detected shortly after vaccination. In general, most cancer vaccines studies have failed to show that immune response detected shortly after vaccination predicts longer time to progression or survival. However, it is conceivable that persistent immunity may be a better measure of effective vaccination, and more associated with the clinical outcome than short-term immunity, suggesting it should be implemented in the evaluation of anti-tumor vaccines. The number of samples for immune analysis in the current studies was too small to make these associations with clinical outcome. Notwithstanding, immune response to the vaccination target antigen does demonstrate biological activity of the vaccine, and the findings here suggest that the method of vaccination, using plasmid DNA, was able to elicit long-term T cell immunity in humans. Other methods of immunization, such as viral or mRNA vaccines, have been generally favored over DNA vaccines given the modest magnitude of immune response observed following DNA immunization. However, it is conceivable that the durability of response could be different for different types of vaccine approaches, and this could have treatment advantages. Given the ease of preparation and low cost of DNA vaccines relative to protein, viral, or mRNA vaccines, it will be of interest in future studies to determine whether DNA provides an advantage in terms of durability of immune response, and long-term clinical benefit, in humans. This may be most discernable following vaccination for COVID-19, since viral, mRNA, and DNA vaccines have all been approved targeting the same COVID-19 antigen.
In general, anti-cancer vaccines have shown only modest clinical activity when used as monotherapies. Despite the approval of sipuleucel-T based on improved survival observed in a phase 3 trial,23 other phase 3 trials in patients with advanced prostate cancer using poxviral vaccines, a multi-peptide vaccine, a GM-CSF-expressing allogeneic cellular vaccine, or a dendritic cell vaccine, failed to demonstrate improved survival when these treatments were used as monotherapies.30–33 We have demonstrated that CD8 T cells activated by vaccination express multiple immune checkpoint receptors, including PD-1.34 Blockade of these immune checkpoint receptors at the time of vaccination using DNA vaccines led to improved anti-tumor responses in murine models.34–37 We have also demonstrated that PD-1 blockade, when used with pTVG-HP administration, can lead to PSA declines and objective decreases in prostate tumor volume in patients with recurrent and advanced prostate cancer.16,20,38 Consequently, it is likely that the best use of anti-tumor vaccines such as pTVG-HP will be in combination with other immune modulating agents, including PD-1 blockade. The ability of vaccines to activate and expand antigen-specific subsets of T cells makes them particularly well poised to be used as parts of combination approaches where it may be desirable to more specifically activate tumor-specific T cells. The long-term outcome of these combination approaches and specifically whether pTVG-HP combined with PD-1 blockade can lead to greater overall survival are the objectives of ongoing and planned clinical trials.
Supplementary Material
Acknowledgments
We thank the participating patients and clinical trial research staff.
Biography
Douglas G. McNeel MD PhD, is a tenured Professor of Medicine at the University of Wisconsin, Madison. He is a genitourinary medical oncologist with a laboratory and clinical research program that has focused on prostate cancer immunology since 2001. The long-term goal of these efforts is to develop effective anti-tumor immunotherapies as treatments for prostate cancer. Shorter term goals of this research have been to identify immunologically recognized proteins of the prostate, characterize these as tumor target antigens, evaluate anti-tumor DNA-based vaccines in pre-clinical models to understand mechanisms of action and resistance, and translate these findings to early phase human clinical trials.
Funding Statement
Grant support was provided by NIH [P50 CA269011, P30 CA014520, and R21 CA132267] and by the US Army Medical Research and Materiel Command Prostate Cancer Research Program [W81XWH-05-1-0404].
Disclosure statement
DGM has ownership interest, has received research support, and serves as consultant to Madison Vaccines, Inc which has licensed material described in this manuscript. None of the other authors have relevant potential conflicts of interest.
Authors’ contributions
TPT and LEJ conducted laboratory studies and edited the manuscript. GL edited the manuscript. JCE was the biostatistician for the trials, analyzed all results, and edited the manuscript. DGM oversaw the original experimental designs and immunological analyses.
Data availability statement
The data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Study protocols that permitted treatment, and collection and use of human blood samples, were reviewed and approved by the University of Wisconsin Human Subjects’ Review Board (IRB). All patients gave written informed consent for use of blood products for research. The trials were registered with ClinicalTrials.gov as NCT00582140 and NCT00849121.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2395680
References
- 1.Oefelein MG, Smith ND, Grayhack JT, Schaeffer AJ, McVary K.. Long-term results of radical retropubic prostatectomy in men with high grade carcinoma of the prostate. J Urol. 1997;158(4):1460–9. [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281(17):1591–1597. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, Wynne C, Murray R, Zinner NR, Schulman C, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. [2005 May 1];23(13):2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 4.Hird AE, Dvorani E, Saskin R, Emmenegger U, Herschorn S, Kodama R, Kulkarni GS, Nam RK. Prevalence and natural history of non-metastatic castrate resistant prostate cancer: a population-based analysis. Clin Genitourin Cancer. 2023. Apr;21(2):27–34. [DOI] [PubMed] [Google Scholar]
- 5.Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, Walsh PC, Eisenberger MA. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2011. Jan;109(1):32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis- free survival in men with psa-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. [2012 Mar 15];118(6):1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastogi I, Muralidhar A, McNeel DG. Vaccines as treatments for prostate cancer. Nat Rev Urology. 2023. Mar;6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovin SF, Ragupathi G, Fernandez C, Jefferson MP, Diani M, Wilton AS, Powell S, Spassova M, Reis C, Clausen H, Danishefsky S. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 or QS-21. Vaccine. [2005 May 2];23(24):3114–3122. [DOI] [PubMed] [Google Scholar]
- 10.Wood LV, Fojo A, Roberson BD, Hughes MS, Dahut W, Gulley JL, Madan RA, Arlen PM, Sabatino M, Stroncek DF, et al. TARP vaccination is associated with slowing in PSA velocity and decreasing tumor growth rates in patients with stage D0 prostate cancer. Oncoimmunology. 2016. Aug;5(8):e1197459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madan RA, Bilusic M, Stein MN, Donahue RN, Arlen PM, Karzai F, Plimack E, Wong Y-N, Geynisman DM, Zibelman M, et al. Flutamide with or without PROSTVAC in non-metastatic castration resistant (M0) prostate cancer. Oncologist. [2023 Jul 5];28(7):642–e561. doi: 10.1093/oncolo/oyad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. [2009 Sep 1];27(25):4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahm CD, Colluru VT, McNeel DG. DNA vaccines for prostate cancer. Pharmacol Ther. 2017. June;174:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LE, Frye TP, Arnot AR, Marquette C, Couture LA, Gendron-Fitzpatrick A, McNeel DG. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP). Vaccine. [2006 Aug 19];24:293–303. [DOI] [PubMed] [Google Scholar]
- 15.McNeel DG, Eickhoff JC, Johnson LE, Roth AR, Perk TG, Fong L, Antonarakis ES, Wargowski E, Jeraj R, Liu G, et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-hp [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol. [2019 Dec 20];37(36):3507–3517. doi: 10.1200/JCO.19.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeel DG, Emamekhoo H, Eickhoff JC, Kyriakopoulos CE, Wargowski E, Tonelli TP, Johnson LE, Liu G. Phase 2 trial of a DNA vaccine (pTVG-hp) and nivolumab in patients with castration-sensitive non-metastatic (M0) prostate cancer. J Immunother Cancer. [2023 Dec 14];11(12):e008067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autio KA, Higano CS, Nordquist LT, Appleman LJ, Zhang T, Zhu X, Babiker HM, Vogelzang NJ, Prasad S, Schweizer MT, et al. First-in-human, phase I study of PF-06753512, a vaccine-based immunotherapy regimen (PrCa VBIR), in biochemical relapse (BCR) and metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39(15_suppl):2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010. Jul;33(6):639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley E, Pohlkamp I, Staab MJ, Liu G, Wilding G, Olson BM, et al. Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res. [2014 Jul 15];20(14):3692–3704. doi: 10.1158/1078-0432.CCR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeel DG, Eickhoff JC, Wargowski E, Johnson LE, Kyriakopoulos CE, Emamekhoo H, Lang JM, Brennan MJ, Liu G. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Immunother Cancer. 2022. Mar;10(3):e004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. [2005 Dec 15];11(24 Pt 1):8669–8673. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 22.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based psa-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. [2010 Mar 1]; 28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. [2010 Jul 29];363(5):411–422. [DOI] [PubMed] [Google Scholar]
- 24.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee J-H, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. [2006 Feb 1];12(3 Pt 1):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 25.Petrylak D. Defiing the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel-T (PROVENGE) followed by docetaxel derive the greatest survival benefit. 14th Annual Meeting of the Chemotherapy Foundation Symposium; 2006. New York (NY). [Google Scholar]
- 26.Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, et al. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. [2007 Jul 1];13(13):3883–3891. [DOI] [PubMed] [Google Scholar]
- 27.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. [2009 May 1];69(6):571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonarakis ES, Kibel AS, Yu EY, Karsh LI, Elfiky A, Shore ND, Vogelzang NJ, Corman JM, Millard FE, Maher JC, et al. Sequencing of Sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin Cancer Res. [2017 May 15];23(10):2451–2459. doi: 10.1158/1078-0432.CCR-16-1780. [DOI] [PubMed] [Google Scholar]
- 29.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005. Aug;174(2):539–546. [DOI] [PubMed] [Google Scholar]
- 30.Ward JE, McNeel DG. GVAX: an allogeneic, whole-cell, GM-CSF-secreting cellular immunotherapy for the treatment of prostate cancer. Expert Opin Biol Ther. 2007. Dec;7(12):1893–1902. doi: 10.1517/14712598.7.12.1893. [DOI] [PubMed] [Google Scholar]
- 31.Vogelzang NJ, Beer TM, Gerritsen W, Oudard S, Wiechno P, Kukielka-Budny B, Samal V, Hajek J, Feyerabend S, Khoo V, et al. Efficacy and safety of autologous dendritic cell-based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: the VIABLE phase 3 randomized clinical trial. JAMA Oncol. [2022 Apr 1];8(4):546–552. doi: 10.1001/jamaoncol.2021.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noguchi M, Fujimoto K, Arai G, Uemura, H, Hashine, K, Matsumoto, H, Fukasawa, S, Kohjimoto, Y, Nakatsu, H, Takenaka, A, et al. A randomized phase III trial of personalized peptide vaccination for castrationresistant prostate cancer progressing after docetaxel. Oncol Rep. 2021. Jan;45(1):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. [2019 May 1];37(13):1051–1061. doi: 10.1200/JCO.18.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T cells. Cancer Immunol Res. 2017. Aug;5(8):630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rekoske BT, Olson BM, McNeel DG. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 2016. June;5(6):e1165377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahm CD, Moseman JE, Delmastro LE, Mcneel D. PD-1 and LAG-3 blockade improves anti-tumor vaccine efficacy. Oncoimmunology. 2021;10:e1912892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colluru VT, Zahm CD, McNeel DG. Mini-intronic plasmid vaccination elicits tolerant LAG3+ CD8+ T cells and inferior antitumor responses. Oncoimmunology. 2016;5(10):e1223002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2018. May 22;P9(39):25586–25596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and/or analyzed during this study are available from the corresponding author on reasonable request.


