Summary
Bioelectronics provide efficient information exchange between living systems and man-made devices, acting as a vital bridge in merging the domains of biology and technology. Using functional fibers as building blocks, bioelectronics could be hierarchically assembled with vast design possibilities across different scales, enhancing their application-specific biointegration, ergonomics, and sustainability. In this work, the authors review recent developments in bioelectronic fiber elements by reflecting on their fabrication approaches and key performance indicators, including the life cycle sustainability, environmental electromechanical performance, and functional adaptabilities. By delving into the challenges associated with physical deployment and exploring innovative design strategies for adaptability, we propose avenues for future development of bioelectronics via fiber building blocks, boosting the potential of “Fiber of Things” for market-ready bioelectronic products with minimized environmental impact.
Keywords: bioelectronic, fiber, life cycle sustainability, human-machine interaction, additive manufacturing
Graphical abstract
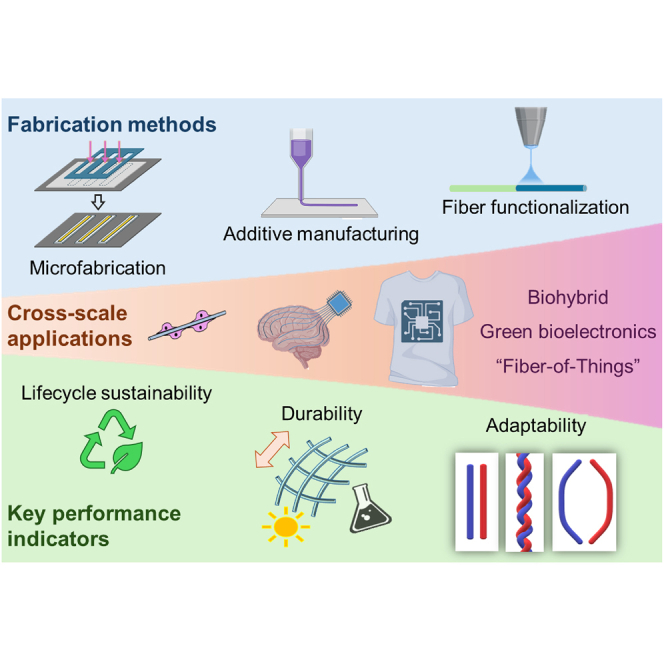
Bioelectronics, in the form of fibers, possess versatile design possibilities and favorable structural properties for biointegration across multiple scales. Here, Pan et al. review classic and emerging approaches to fabricate bioelectronics from fiber building blocks while evaluating the device life cycle sustainability and application-specific performance.
Introduction
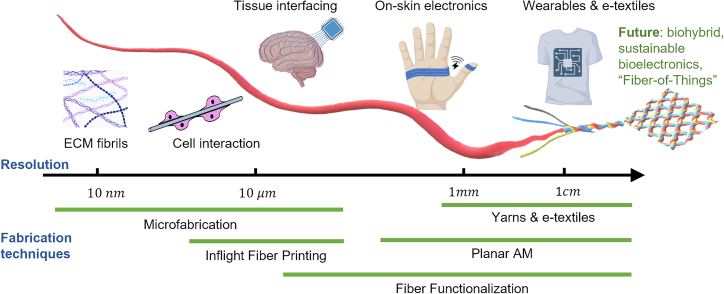
Bioelectronics is an interdisciplinary field that encompasses the development and application of electronic devices to interface biological entities.1,2,3,4,5,6,7 The employed device interface needs to be biocompatible for the duration of operation, ideally to minimize disruptions toward the natural states of the target biological systems while facilitating the desired bioelectronic functions. Fibers, one-dimensional (1D) structures with superior flexibility, widely exist as building blocks for various natural systems, such as extracellular matrices (ECMs) and spiderwebs.8,9 In addition, fibers could be woven into textiles that provide intimate and conformable contact with human bodies. Therefore, harnessing functional fibers as structural building blocks could unfold a range of bioelectronics across different size scales,10 from embedded sensing within biological cells and tissues both in vivo and in vitro11 to e-textiles and wearables7,11,12 (Figure 1).
Figure 1.
Size scale of bioelectronic fiber technologies
Shown is a scale-length illustration showing the typical fiber resolutions achieved by various fabrication techniques and exemplary applications with bioelectronic fibers of different sizes.
At the level of individual cells to connective tissues, fibers with micrometer-scale diameters could mimic the topology and global conformation of ECM fiber bundles to provide structural cues for in vitro culture.13,14 In addition, when interfaced with delicate tissues that exhibit electrophysiological activities, small-diameter bioelectronic fibers have the advantage of reducing adverse device-tissue interactions in bioelectronic neural implant devices.15,16 The large aspect ratio of bioelectronic fibers is beneficial for reducing their bending stiffness, thus invoking a less chronic immune response in the tissues due to reduced mechanical mismatch.15 At the macroscopic level, fibers could be woven and knitted into yarns and textiles with superior softness to enable ergonomic fitting (e.g., intimately following the complex contour of a body part) and wearing comfort.7,11,17
Bioelectronic fibers could also be multiplexed and consolidated into functional devices.18,19,20 Hierarchically assembling functional fibers into multi-fiber constructs, such as networks, meshes, yarns, or fabrics, could upscale their performance or enable additional functions on a structural level, such as triboelectric patch sensing, artificial muscles with directional control, and logic circuits.18,21,22 The process of creating multi-fiber constructs also allows convenient alterations of the designed porosity (e.g., hole sizes in a mesh), further enabling tunable air-moisture permeability, light transmission, matrix stiffness, and network interconnectivity.23,24 The structural and mechanical merits of individual fibers could also be retained in the multi-fiber constructs.7,17,25,26
Many bioelectronic devices contain single-use components due to health and safety considerations as well as challenges regarding sterilization and cross-contamination prevention required by their applications.27 As the deployment of these “transient” bioelectronic devices28 increases toward industrial scales, addressing the environmental impacts arising from the widespread use of consumable bioelectronics becomes a growing concern.29 To mitigate these impacts, it is crucial to establish key performance indicators (KPIs) for bioelectronic devices, considering factors such as sensing performance, eco-friendliness, materials efficiency, bio-integration, scalable customizability, and cost-effectiveness. Fiber-bioelectronics, whether employed as standalone bioelectronic elements or as fundamental building blocks for complex devices, hold particular promise. This review is motivated by the potential of fiber bioelectronics to explore concepts and methods that may lead to sustainable and adaptable bioelectronic fiber building blocks.
Fabricating bioelectronics from fiber building blocks
Bioelectronic fiber building blocks could be regarded as individual functional bioelectronic fibers, which could enable further manipulation, assembly, or scaling up for building bioelectronic devices and systems. In this regard, the device functionality should principally rely on the “fiber-shaped” individual components, which should not be rigid (otherwise they would be rods instead of fibers) or have lateral sizes markedly exceeding the sub-millimeter range (comparable to, e.g., fibers for smart garments, which are on the thicker end of the diameter range of state-of-the-art bioelectronic fibers7,11,17). Various techniques could be used to fabricate the bioelectronic fibers, ranging from microfabrication techniques to fiber drawing and “additive manufacturing.”3,7,12,17,25,26,27,28,30
The device functions arise from their structures and materials, which would determine the fabrication route choices. Functional materials, such as carbon, metal, and semiconducting materials, have been widely used to build bioelectronic fiber devices due to their diverse electronic functionalities and proven reliability.11,31 Microfabrication processes and fiber functionalization techniques are commonly used to process these materials into either fiber-format microfabricated devices32 or as coating layers on fiber substrates.33 Recently, conjugated polymers have emerged as alternative materials for producing bioelectronics with improved tissue integration and novel biointerfacing functionalities.1 Among them, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) is a notable bioelectronic polymer that exhibits a mixed ionic and electronic conductivity. Its unique features of accessible solution processing and its favorable electronic performance make it one of the most extensively studied and used materials for fabricating organic bioelectronic devices.3,34
At the device level, the function and performance could also be affected by the format and geometry of the bioelectronic materials. Small-diameter fibers usually possess larger surface-area-to-volume ratios, and this feature could be advantageous in certain applications for enhancing their sensitivity. For example, microfiber-based wearable gas sensors could outperform planar and solid bulky devices in terms of gas detection limitations and sensitivity.24,35 In addition, the low bending rigidity of small-diameter fibers is structurally favorable for direct tissue and cell integrations, and the topographical features of micrometer-level fibers could stimulate cell alignments and dynamics.24 However, it is important to note that the geometry of the bioelectronic materials could also alter their electronic performance. For example, in organic electrochemical transistors, the transistor gain could decrease as the channel aspect ratio increases.36 Therefore, a fiber-shaped transistor channel with a high aspect ratio would generally exhibit lower gain compared to traditional dot-shaped channels with all micrometer-scale dimensions.36 Therefore, when designing fiber bioelectronic devices, one should carefully balance the interplay between materials and structures so as to choose the suitable device designs and fabrication methods.
This section will catalog the features of typical fabrication methods capable of producing bioelectronic fiber building blocks while elaborating the interactions between fabrication methods and their produced fiber properties as well as the sustainability assessment.
Microfabrication
Although microfabrication is traditionally associated with conventional rigid electronics, in recent years, flexible polymeric electronics have also been fabricated using microfabrication techniques.37 In general, microfabrication provides precision manufacturing for devices with sub-micrometer feature sizes and intricate functionalities, so its products can contain pre-made functional structures, such as the highly complex circuits on small silicon wafer pieces in integrated circuits (ICs).37 In addition to plate- and mesh-like devices, microfabrication can also create quasi-1D devices in the shape of strips or fibers; one example is a type of insertion implant electrode that is flexible by design and embodies the “fiber” format with micrometer-level lateral sizes and high aspect ratios.15,32
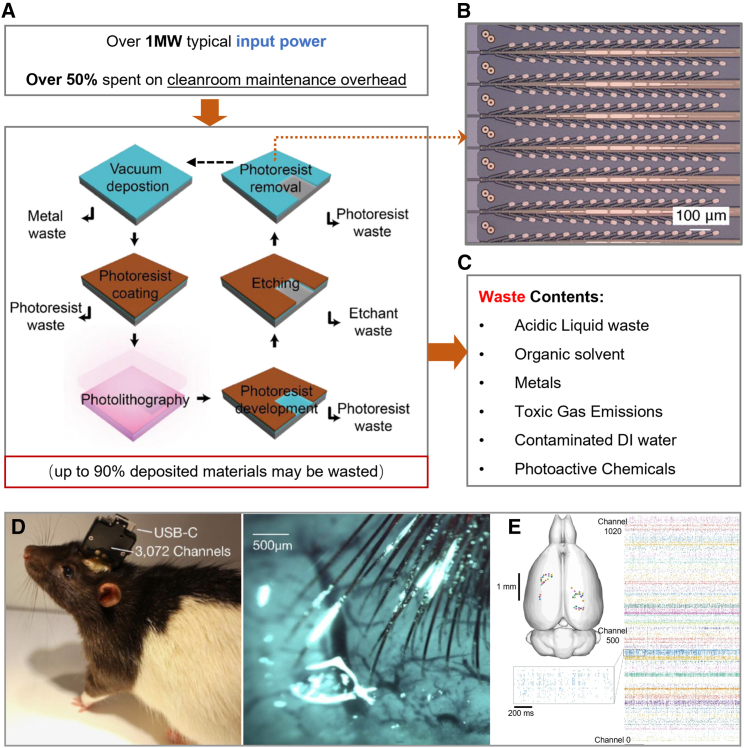
Microfabricated devices are usually not individually produced; instead, they are produced in batches of multiple devices; e.g., many ICs are populated on one wafer during chip production, and this helps to achieve cost-efficient fabrication.37 The microscopy image in Figure 2B provides an example of batch-processed 1D electrode devices in the shape of ribbons or narrow strips, which consist of gold interconnect traces, a parylene passivation layer, and electrode surface modifications of PEDOT:PSS or iridium oxide;32 similar devices can also be created using any other metallic, carbon, semiconductor, or polymeric materials compatible with cleanroom processes and offer adequate biocompatibility;37 for example, another device design similar to that shown in Figure 2B is reportedly created using SU-8 substrate (a commonly used epoxy-based negative photoresist) and platinum.38 The examples show a typical feature of microfabricated devices: they are usually planar, since microfabrication processes are usually based on additive and subtractive manipulations of layered thin films37 (Figure 2A). Additionally, due to the requirement of a cleanroom environment and a suite of dedicated equipment, microfabrication does not usually support in situ device fabrication in bioelectronics usage environments.
Figure 2.
Production and application of microfabricated bioelectronic fibers
(A) Input power allocation of a typical cleanroom (top),39 with an example of a typical lithography-assisted subtractive process, sources of generated wastes40 (middle flow chart) and the possible range of material wastage41 (bottom), reproduced with permission.40 Copyright 2020, Sui and Zorman.
(B) Photo of a batch of microfabricated fiber-shaped implant electrodes with multiple electrode pixels per fiber, reproduced with permission.32 Copyright 2019, Elon Musk, Neuralink.
(C) A non-exhaustive list of waste types that could originate from an active cleanroom.42,43
(D) A rat chronically implanted with the fiber electrodes in (B) as parts of a packaged implant device (left) and a perioperative photo showing the implanted flexible fiber electrodes inserted into the brain tissue (right). Reproduced with permission.32 Copyright 2019, Elon Musk, Neuralink.
(E) Example of implant insertion location marked on the rat brain (top) and raster plot from the electrical activities recorded from the channels on multiple implanted fiber electrodes (right). Raster plots are recorded electrical spike/events against time, which can reach millisecond-level time resolution (inset). Reproduced with permission.32 Copyright 2019, Elon Musk, Neuralink.
Microfabrication can achieve sub-nanometer process control (e.g., in coating) and provide micro- to nanometer-level horizontal feature size resolutions while offering high standards for product quality control.37 However, this process may fall short regarding prototyping flexibility and convenience because minor design changes could require extensive changes in the patterning mask designs and fabrication programs.37 As summarized in Figures 2A and 2C, conventional microfabrication, operated by expensive equipment in cleanroom environments, usually require high and continuous energy input as well as potentially wasteful material input; the by-products and wastes could be hazardous depending on the device design.37,41 To dilute the production costs and environmental impacts of microfabrication, the device number per batch usually needs to be maximized, and the products may also be designed for more long-term applications (e.g., reusable devices, neural implants; the example device is indeed a chronic implant with a flexible pixelated electrode that records temporally resolved neural activity, as seen in Figures 2D and 2E).15,16
Additive manufacturing
Additive manufacturing (AM) is a category of various fabrication techniques, and when used for creating electronic circuits, it boasts the feature of minimizing subtractive patterning.44,45 AM bioelectronic devices can largely be divided, by product format features, into three groups: planar or two-dimensional (2D), three-dimensional (3D), and quasi-1D.
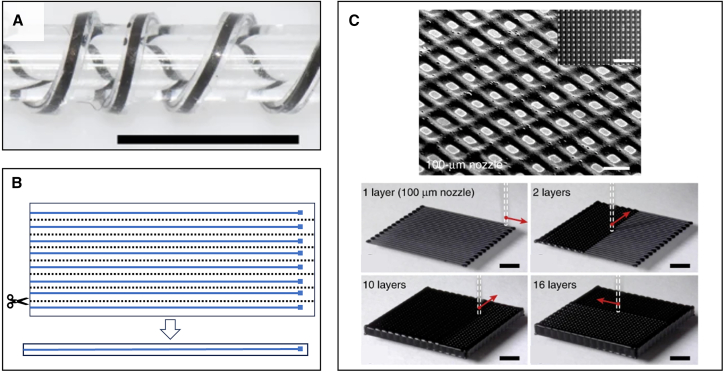
One popular example of planar AM is inkjet printing, where bioelectronic inks can be printed on an (often flexible) substrate to form a patch-type device, and the reported print resolution could be down to micrometer level, although the product fibers’ lateral sizes are often in the sub-millimeter range.40,44 Planar AM usually creates structurally flat devices consisting of thin layers, as shown by an example in Figure 3A.46 The substrate of these fiber-shaped devices usually needs to be processed separately; one simple example is “cutting out” individual fibers from a large substrate after printing, as shown in Figure 3B. The mechanical properties of these fiber devices usually depend on the substrate, like most other patch-type, inkjet-printed devices (unless the ink also functions as a mechanical property modifier).
Figure 3.
Examples of additively manufacturing bioelectronics
(A) A planar inkjet-printed flexible bioelectronic fiber on silicone backbone with sub-millimeter width, as a neuromuscular implant electrode (scale bar, 4 mm). Adapted with permission.46 Copyright 2020, Springer Nature.
(B) Example schematic of separately processing the substrate of an inkjet-printed fiber: separating a fiber-shaped device from a planar inkjet-printed sheet, i.e., “print and cut.” Blue color marks bioelectronic material ink; a dashed line marks designed cutting locations.
(C) A 3D resin printing product with complex merged internal structures. The top view shows a microscopy image of its indivisibly merged internal structures (scale bar, 200 μm; inset scale bar, 1 mm), and the bottom photos show the stacking structure formation using layer-by-layer 3D resin printing (scale bars, 2 mm). Adapted with permission.47 Copyright 2020, Yuk et al.
As for 3D AM, the most popular method is 3D resin printing, which can generate complex self-standing 3D geometries by procedural deposition of solidifying viscous liquid materials44,47 or by 3D polymerization lithography.48 These techniques usually produce internally merged and indivisible structures with sub-millimeter-level resolutions (Figure 3C).45 Therefore, although many studies reported fully 3D-printed bioelectronics, it is rare for the reported designs to embody bioelectronic functions powered by intricate circuitries that require non-merged internal structures.45,47,49 There are also techniques (fiber functionalization) that create individually handleable bioelectronic fibers that are designed for further assembly instead of in situ fabrication, which will be discussed separately in a later section.
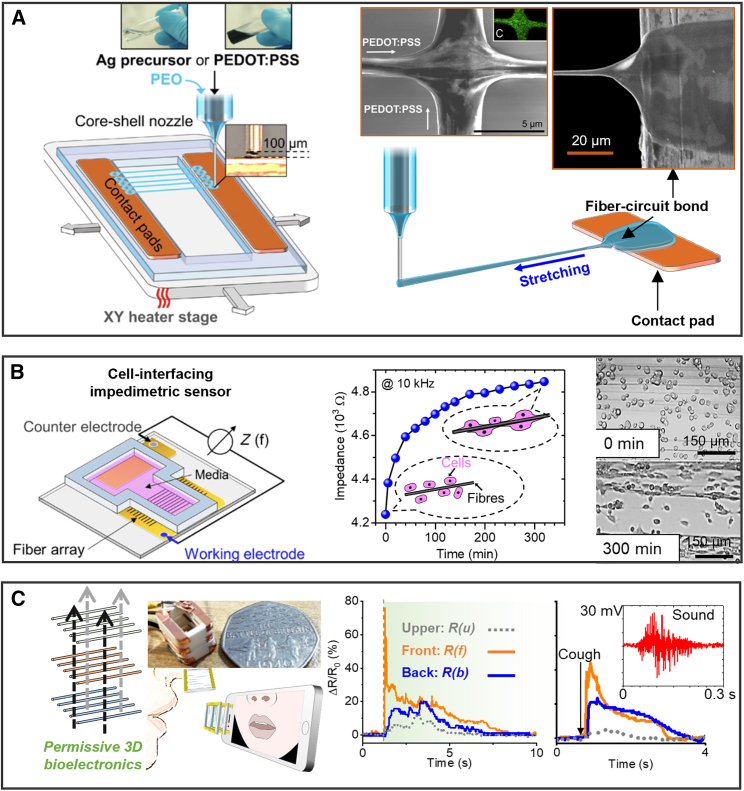
Finally, it is also possible to create and deploy individual quasi-1D fibers in situ through a one-step “printing and deployment” process,7,11,24,49,50 where in situ polymerization or gelation of conductive polymer (composites) can take place. For example, micropillars based on in situ polymerization of PEDOT:PSS can be formed directly on individual electrodes of multi-electrode arrays, which help couple the electrodes to cultured heart tissues to perform electrophysiological recording.50 Our research group also reports the inflight fiber printing technique (iFP) for creating structurally distinct and suspending high-aspect-ratio microfibers patterns (Figure 4A), which plainly invoke the concept of “using fibers as building blocks.” This technique does not require continuous planar surfaces or suspension baths, the fiber suspension is achieved as printed in situ by specially designed solution rheology.24,51 The fibers, composed with a PEDOT:PSS-based formulation, can be individually deposited as bioelectronic building blocks with tunable array patterns. This method enables direct fiber-to-circuit connection and structurally intricate functions, such as crossbar fiber junctions (Figure 4A). Figures 4B and4C demonstrate the PEDOT:PSS fibers printed by iFP as cell-interfacing bioelectronic sensors and a wearable breath analyzer.
Figure 4.
In situ bioelectronic fiber production by inflight fiber printing (iFP)
Shown is a one-step approach to print functional fibers with in situ circuit connections for building bioelectronic fiber devices.
(A) Schematic illustrations of iFP and the in situ fiber-circuit bond and fiber junction formation processes. Inset: electrically connected junction formed between two crossing PEDOT:PSS fibers.
(B) The printed PEDOT:PSS fiber array could be used as an impedimetric cell-interfacing sensor to monitor cell interactions with the fibers through impedance change.
(C) Hierarchical assembly with PEDOT:PSS fibers building blocks creating permissive 3D wearable bioelectronics, such as a multi-latitude breath analyzer to monitor exhalation strength (e.g., breathing, coughing) and sound. Adapted with permission.24 Copyright 2020, Wang et al.
The “additive” nature of AM offers potential advantages for efficient material usage. Furthermore, when a design overhaul is required, unlike microfabrication, which often relies on lithography masks, AM device designs are usually based on virtual models developed using computer-aided design tools, so the physical modification of production hardware (like lithography masks) is generally not needed. Overall, AM offers an energy- and material-efficient route for fabricating fiber building blocks at micro- to millimeter-scale resolutions while enabling flexible and cost-efficient customization for prototyping bioelectronic devices.
Fiber functionalization
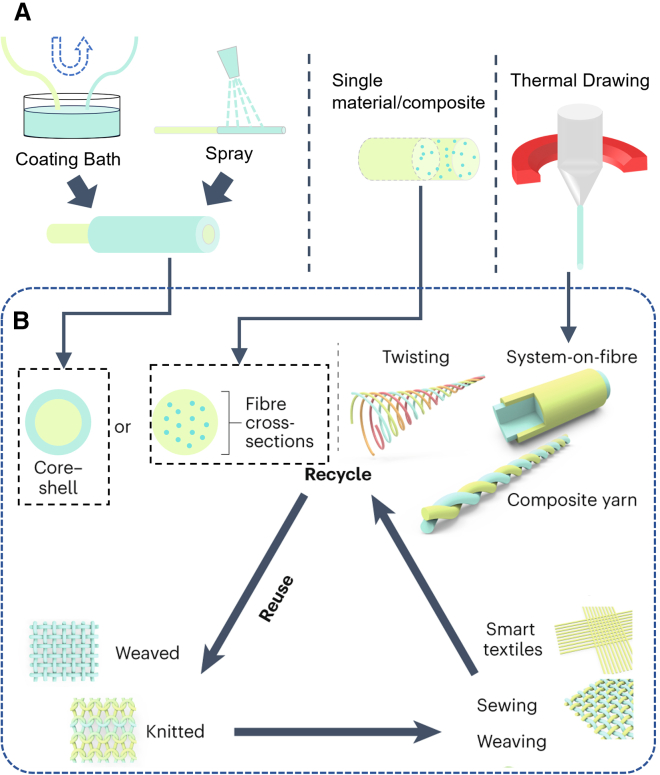
Bioelectronic fiber-based building blocks can be fabricated through functionalizing filaments, yarns, or threads, resembling processes in the textile industry. Their product is individually handleable fibers that can be sutured, sewn, or knitted (Figure 5). As shown in Figure 5A, extrusion-type wet spinning methods (assisted by coagulation baths) can create PEDOT:PSS composite fibers.11 Other conductive materials, such as carbon nanotube yarn, carbon fiber, silicon, and flexible conductive metals (e.g., gold), may also form individually handleable conductive fibers for bioelectronics.11,19 The other category instead creates fibers consisting of multiple material “layers,” including at least a core and one coated shell layer; the bioelectronic function is often embodied in the outer shell layer. For example, a PEDOT:PSS shell can be applied by bath or spray coating on insulating substrates;45,52 alternatively, electro-polymerization coating can also coat PEDOT:PSS with electrically tunable thickness, but this process requires an electrical conductor substrate or core.53 Other conjugated polymers (e.g., polypyrrole54 and PANI(polyaniline),19 could also be processed in a similar manner; when a conductor core is already present, subsequent layers based on, e.g., various conjugated polymer chemistries may be incorporated for additional functions, such as introducing non-electrical stimulation, facilitating catalysis, as well as appending further biochemical functionalization.1 In addition to solution processing, thermal drawing is a scalable quasi-1D fiber production approach that draws micro- to millimeter thickness fibers from solid macroscale preforms. The preforms could contain complex internal layers made from different materials, such as metals, elastomers, and even ceramics. Thermal drawing offers a convenient approach to composite different materials with designable structures from macroscale to microscale.12,55,56 This approach enables versatile internal microstructure design for the fibers and integration with textile systems. For example, piezoelectric multi-layer preform could be thermally drawn into sub-millimeter fibers for wearable acoustic sensors in smart garments.12
Figure 5.
Fiber functionalization and functional fiber construct assembly
(A) Functionalized fiber building blocks. Left: coating functionalization of a fiber-shaped substrate. Middle: individually handleable fiber consisting of a single bioelectronic material or composite. Right: thermally drawn composite fiber.
(B) Principles for assembling bioelectronic functional fiber constructs using individually handleable functionalized fiber building blocks; their usage can form sustainable circular economies when recycling and reuse are supported. Adapted with permission.17 Copyright 2023, Springer Nature.
These individually handleable fibers have already been used as building blocks for assembling bioelectronic fiber constructs with distinct structural functionalities (e.g., computing architecture, triboelectric generator, pressure sensor, multiplexed sensors).7,11,19,21,25,57 Examples of functional fiber network assemblies are shown in the schematic in Figure 5B. Individually handleable fibers and their constructs are suited for certain bio-interfacing applications, including loose-fitting smart garments and 3D tissue scaffolds for in vivo or in vitro uses;11,17 these categories can capitalize on their individual handleability, the fiber geometry, and their natural compatibility with assembling complex functional network patterns.
Life cycle sustainability
Electronic waste has become the fastest growing waste stream in the world.17 The increasing demand for bioelectronic devices could exacerbate this trend in the future if their environmental footprints are not well managed and controlled. In order to provide a holistic sustainability analysis of bioelectronic fiber devices, this section will discuss the device “life cycle sustainability,” which encompasses the device environmental impact from their production to deployment and eventual retirement.
Production sustainability
Production sustainability refers to the environmental impact of the device manufacturing processes, including the use of resources, energy consumption, and waste generation. In terms of the three bioelectronic fiber production methods discussed by this review, microfabrication, which is a preferred method for producing bioelectronic implants, is typically resource intensive due to the environmental demands of cleanroom operations. In addition, the material wastages37,41 and the potential hazardous waste generations42,43 incurred during the process steps (e.g., deposition, lift-off, selective etching) should not be overlooked. AM offers a more tailored approach to enable “on-demand mass customizations,” thereby potentially reducing the by-product wastes and wastages from production redundancies. However, in some planar device designs, sacrificial transfer layers (e.g., supportive substrates) used in intermediate production steps could eventually be disposed, which is another source of wasted material.58 The effect of these material inefficiencies could become significant when the production volume scales up. Finally, fiber functionalization for e-textiles also reflects a mixed sustainability profile. Despite the functionalized fibers being able to be assembled with a certain level of design freedom for functional construct designs,17 outstanding material wastage could still be generated; e.g., from the dip coating and fiber coagulation processes.11,17,45
Deployment sustainability
The deployment phase of bioelectronic fiber devices could also affect their sustainability, and this is mainly based on the characteristics of their materials, formats, and application scenarios. For example, the suspended PEDOT:PSS microfibers produced by iFP could be susceptible to damage by environmental disturbances because of the fragility of the fibers,24 thus affecting the longevity of the devices and generating waste upon failure. In general, bioelectronic fiber devices intended for short-term or single-use applications could exhibit considerable environmental footprints during the deployment phase. On the other hand, devices designed for long-term or repeated use could offer more sustainable deployment by minimizing the need for frequent replacement, such as long-term implants15,16,26 or reusable in vitro devices.3,30 As for e-textiles, they could be designed to withstand machine washing to extend their product life;12,17 thus, wastes generated from the deployment phase of e-textiles could also be small to negligible.
Retirement sustainability
Retirement sustainability focuses on the end-of-life options for bioelectronic fibers, including their potential for recycling or destructive abatements. The rise of natural organic materials and biomaterials enables the creation of degradable bioelectronic fiber devices.37,59 For the devices that need to be recycled, the material composition and device formats would determine the extent of their recyclability and the sustainability of the recycling process. It is to note that recycling does not necessarily lead to improved sustainability over destructive retirement; for example, recycling of metal contents in bioelectronics could be an energy- and cost-intensive process that may also generate hazardous byproducts.60 Contrarily, if bioelectronics are designed to be incinerable with minimal hazardous emissions and residues, then they may achieve more favorable sustainability as fuels for carbon-negative incineration power stations.61
Comparative life cycle sustainability analysis
Overall, the sustainability and the environmental impact of bioelectronic fiber devices should be analyzed by considering the entire lifespan of bioelectronic fiber devices, from production through to retirement. Furthermore, it is imperative to evaluate the life cycle sustainability of devices by considering their differences in terms of material composition, device architecture, manufacturing methods, and intended application contexts because these factors all may affect the overall sustainability profile of each device design.
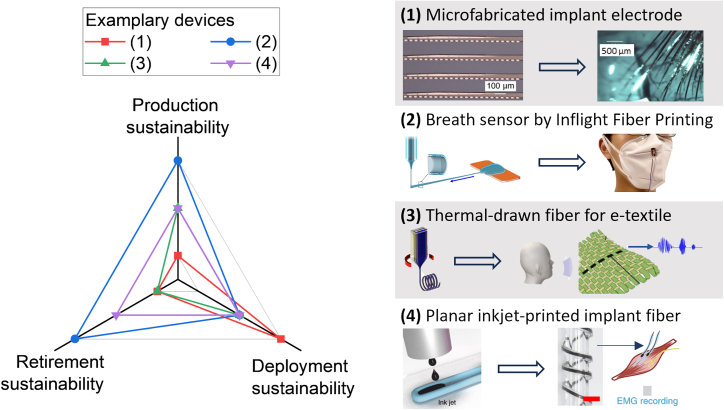
Figure 6 provides a comparison of the life cycle sustainability of several bioelectronic fiber device examples as an intuitive display of the life cycle evaluation framework. Their sustainability is rated at each of the life cycle stages using a 3-level rating system, graded as “inadequate” (level 1), “balanced” (level 2), or “favorable” (level 3). “Inadequate” is given when the device has more features that may impair the sustainability of a life cycle stage than features beneficial to sustainability, “favorable” is given to a device with evident favorable design features for sustainability and few detrimental features, and “balanced” is given to examples with balanced sustainability performance (including both pro- and anti-sustainability features) in a life cycle stage.
Figure 6.
Lifecycle Sustainability Comparison of Bioelectronic Fiber Examples
Shown is a radar chart comparing the life cycle sustainability of device production, deployment, and retirement phases, with schematics showing the bioelectronic fiber examples compared and their applications. (1) microfabricated pixelated electrode fibers for chronical neural implant. Reproduced with permission.32 Copyright 2019, Elon Musk, Neuralink. (2) PEDOT:PSS fiber array component created by iFP for wearable breath sensing, adapted with permission.24 Copyright 2020, Wang et al.. (3) Thermally drawn piezoelectric fiber for sewn-in acoustic sensing in e-textiles. Adapted with permission.12 Copyright 2022, Springer Nature. (4) Inkjet-printed planar fiber implant electrode for neuromuscular electrophysiology recording; scale bar, 1 mm. Adapted with permission.46 Copyright 2020, Springer Nature.
The four examples were chosen from typical bioelectronic fiber devices fabricated using different methods. The microfabricated fiber electrode implants32 face sustainability challenges due to cleanroom production processes and the use of unsustainable metals,62 leading to inadequate production and retirement sustainability despite favorable deployment sustainability realized by persistent long-term use. The wearable breath sensors produced by iFP24 have favorable production sustainability by efficiently creating fibers via AM; their deployment sustainability is balanced, with repairable damage offsetting fragility, and their retirement sustainability should also be favorable due to easy recycling or incineration. The thermally drawn acoustic sensor fibers12 have balanced production and deployment sustainability thanks to scalable manufacturing and lifespans aligning with smart garments (as a component), which balance the impact of energy-intensive production and less persistent usage; their retirement sustainability is inadequate due to inclusion of metal (and even heavy metal) materials. Finally, the planar electrophysiology implant fibers,46 produced via inkjet printing, have balanced sustainability in all life cycle stages; they are produced by on-demand inkjet printing but use unsustainable metals like platinum. Long-term intramuscular implant deployment may be hindered by internal delamination effects more common in printed electronics with fewer adhesion enhancer options than cleanroom processes;37,63,64 despite the metal content, these implants may still be recycled as lower-grade printing feedstock.17
The life cycle sustainability evaluations could also reveal inspirations for the future developments of bioelectronic fiber building blocks. The device durability during the deployment is often affected by environmental disturbances; therefore, the environmental electromechanical performance of fiber building blocks could have an indirect effect on its sustainability by affecting the failure rates. Similarly, designing the devices with improved adaptability (i.e., deployment in a wider range of environments) and the capability to support in situ repair and upgrade, wastage, and failures may also be further reduced. The next chapter will discuss the device stability considerations and adaptability designs for future developments.
Further development
In order to boost the potential of scaling up the production of market-ready bioelectronic fiber devices, it is crucial to consider several KPIs that are closely related to their application scenario and environmental impact. These include device life cycle sustainability, environmental electromechanical performance, and adaptability. Building upon the previously discussed aspects of production and sustainability, a harmonious balance can be achieved between adaptability and the framework of environmental electromechanical performance. Analyzing these KPIs not only sheds light on current challenges but also unveils potential avenues for the research and development of “next-generation” bioelectronic fiber building blocks.
Environmental electromechanical performance
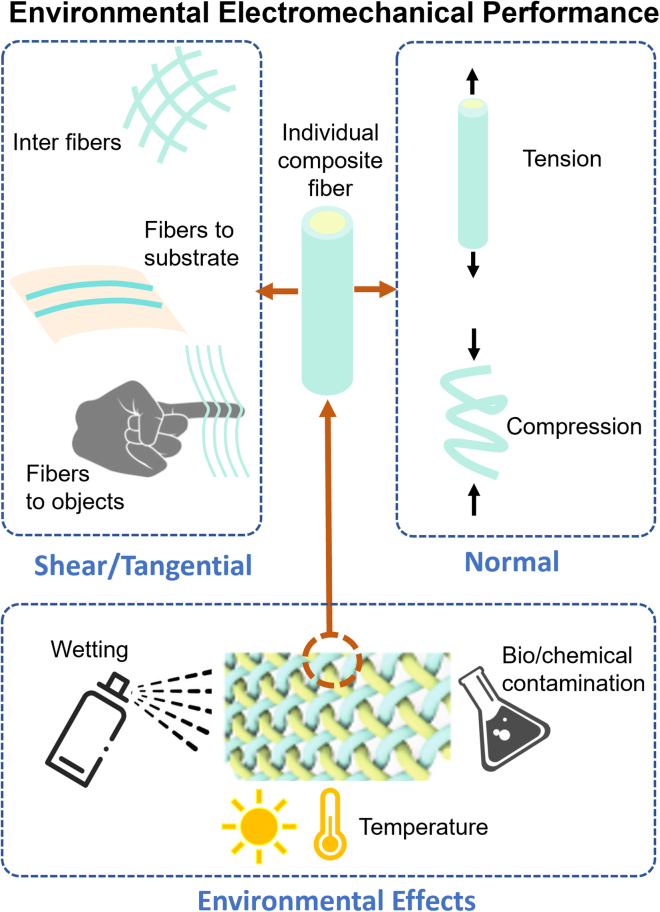
Bioelectronic fiber devices are to be directly interfaced with the periphery of human or other living structures, and inevitably, they are constantly exposed to a multitude of environmental disturbances, such as mechanical deformation (friction, extension, compression), humidity, and temperature fluctuations (Figure 7). Therefore, the environmental electromechanical performance, or durability, of the bioelectronic fiber devices is an essential KPI to ensure that the devices could serve their designed functions effectively over time. When functional fibers are used as the building blocks, the device durability could be obtained and tailored from material formulations, fiber synthesis approaches, and fiber architectural designs and assemblies. In this forward-looking section, we specifically focus on critical considerations pertinent to the applications of these fiber building blocks in large-area epidermis-interfaced devices.
Figure 7.
Electromechanical performance evaluations of bioelectronic fibers
Shown are schematic illustrations of evaluating the environmental electromechanical performance of bioelectronic fiber devices.
The device durability could be evaluated from various perspectives with different performance indicators, such as stretchability, wear resistance, and adhesion strength. For the device to be fit for purpose while minimizing the environmental footprints, one needs to carefully balance the weight of different durability indicators to be in alignment with the intended applications and usage context. By doing so, it could avoid over-engineering, which could lead to unnecessary resource use and costs, or under-engineering, which might result in failures or unsatisfactory performance. For example, an e-skin tactile sensor on the fingertip mainly needs to be designed against daily “touching” experience of the fingers and wetness,33,65 while a motion sensor on the joint mainly could require high stretchability to account for the large deformations and movements.66 The most important durability performance indicators, which could be regarded as the “weakest link” to affect the device lifespan, usually vary depending on the applications. This could make it difficult for people to compare and objectively evaluate the device’s performance. In addition, the device format and durability testing approaches from the literature usually differ a lot. Despite bioelectronic devices having been extensively reviewed, a standard to cross-compare the device environmental electromechanical performance is still lacking.
Therefore, Table 1 summarizes the environmental electromechanical performance of typical bioelectronic fiber devices by standardizing their testing conditions and the performance indicators. By doing so, it elucidates the difference of experimental methods used by the literature to provide a clear understanding of the device performance that reflects real-world application viability and sustainability.
Table 1.
Environmental electromechanical performance of state-of-the-art bioelectronic fiber devices
| Reference | Device format and materials | Environmental electromechanical performance (e.g., durability) |
Target applications | |||
|---|---|---|---|---|---|---|
| Unperturbed | Dry surface contact wear | Wet surface contact wear | Stretch/compress | |||
| Lee et al.,33 | 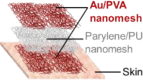 |
pressing (on a pressing rig) P = 19.6 kPa, 1,000 times ∼ stable Rubbing (on PU sheet/PU) FN = 5 N, 300 times ∼1, drops 9.7% |
stretching (nanomesh on an elastomer) ε = 14% 1 cycle ∼0.9 |
on-skin pressure sensor for fingertip in dry and non- sweating conditions | ||
| Miyamoto et al.,65 | 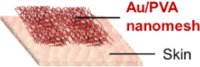 |
rubbing (finger/plastic) 150 kPa, 20 times no physical damage |
finger clenching (finger) 10,000 cycles, ∼2.7 stretching (on PU sheet) ε = 25%, 500 cycles drops 8% |
hand movement, temperature, and pressure sensor wearable biopotential sensor |
||
| Zhu et al.,67 | 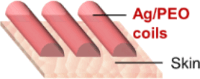 |
100% humidity (back of hand) 20 min for 4 cycles, ∼1 |
bending (on artificial skins) r = 52 mm, 1,000 cycles good adhesion |
3D printing wireless powered circuits on hand | ||
| Kim et al.,66 | 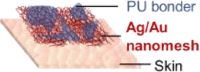 |
heat ∼40°C in water 3-h ambient wear (back of hand) motion recognition unaffected ( not reported) |
rubbing (porcine skin/rubber) FN = 0.2 N, 1,500 times. ∼1.2 |
rinsing (hand/running water) 20 s rinse, 4 cycles. ∼1.1 |
stretching ε = 30% 5,000 cycles ∼1 |
strain sensor for hand motion monitoring |
| Yan et al.,12 | 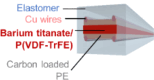 |
machine wash (fiber embroidery fabrics/running water) 10 cycles ∼1 |
bending (fiber on a film) min. r = 1.2 cm 1,000 cycles ∼1 twisting (standalone fiber) Max. angle 540°, 1,000 cycles ∼1 |
e-fabrics with sensitive audible microphone function | ||
The test condition is underlined for in situ durability tests.
C, capacitance; FN, normal force; G, conductivity; R, resistance; PDMS, Polydimethylsiloxane; PE, Polyethylene; PEO, Polyethylene glycol; PU, Polyurethane, PVA – Poly(vinyl alcohol), P(VDF-TrFE) – poly(vinylidene fluoride-co-trifluoroethylene), r – radius, Z – impedance, ε – normal strain. The fiber electrical property indicators C0, G0, R0, and Z0 refer to original properties while C, G, R, and Z refer to properties after all wearing test cycles.
Stretchability
Stretchability is often regarded as an important feature of bioelectronics, and numerous recent works have demonstrated highly stretchable bioelectronic fiber devices.59,65,68 Although enhancing the device stretchability could unlock applications to accommodate large and repeated deformations, excessive focus on high stretchability, regardless of the actual application scenarios, might lead to unnecessary over-engineering. For bioelectronic fiber devices that directly interface with the natural biological surfaces of human or other organisms, the device stretchability could be evaluated from two aspects: (1) the device stretchability should be sufficient so that it does not restrict the hosts’ normal biological functions and deformations (i.e., movements), and (2) repeated deformations would not affect the device sensing or other functional performance over the expected lifespans. With the above criteria, fibrous electronic skins for hand or human motion monitoring should possess high stretchability to accommodate the body movements (i.e., over ∼30% for hand gesture sensing66 and over ∼50% or even ∼100% for arm movement recognition69,70). For such devices, the stretchability also needs to be tested by high cycle numbers, usually over several thousand cycles, to ensure that the sensing performance is maintained for the designed usage period. On the other hand, for fibrous electronic skins that are to be applied on the fingertip or skin away from joints, high stretchability would not need to be prioritized because the intrinsic stretchability of human skin at these regions usually would not exceed 30%.71
The device stretchability of fiber building blocks could be obtained through customizing the fiber materials or fiber patterns. For example, intrinsically stretchable polymers and liquid metals could be used to produce electrospun fibrous electronic skins that exhibit maxima of 1,800% strain.70 If the fiber materials possess limited stretchability, then the device-level stretchability could still be enhanced by designing the fiber assembly, networks, and structures.69
Wear resistance
Device wear resistance refers to the device stability against surface contact wearing under normal usage conditions (i.e., dry and wet wearing). This performance indicator is most relevant with applications where the bioelectronic fibers are attached on skin that frequently experiences disturbances, such as “touching,” “rubbing,” and “wetting.” For example, nanomesh-based tactile sensors, usually adhered on the fingertip of the index finger, would constantly experience pressing and shear friction.33,65 Therefore, wear resistance is the “weakest link” among durability performance indicators of such devices. As seen in Table 1, the effects of pressing and rubbing have been purposely studied. Despite the stretchability of the nanomesh-based tactile sensors appearing to be moderate compared to other bioelectronic devices, it would not restrict their performance because the strains of fingertip skin are usually less than 10%.71
The effect of surface contact wear is closely related to the testing approaches and conditions. Therefore, in order to best reflect and mimic the wearing during the actual usage scenario, in situ testing has been favored by researchers.33,65,66 Another advantage of in situ testing is that the bioelectronic fiber devices are directly tested on the skin of human volunteers instead of artificial skins or skin replicas, which could have different surface and biological properties than living human skins (i.e., perspiration and hydrophilicity). Despite the fact that in situ testing may not be able to facilitate precision control over testing parameters such as pressing force and friction speed compared to ex situ testing, it provides a more holistic assessment of wearing behavior by incorporating environmental factors that could occur in real-world usage scenarios. For example, tests like in situ rubbing, finger clenching, or washing could combine mechanical disturbances and the potential effects of water exposure and sweating.
Adhesion strength
Adhesion is another important durability performance indicator that characterizes the material bonding strength between bioelectronic fibers with the biological surfaces.72,73 Appropriate adhesion is desirable to ensure that the bioelectronic fibers do not delaminate or peel off from the hosts during operations. While it may seem that stronger adhesion leads to better performance, over-engineering the adhesion strength may instead introduce unintended drawbacks. The most obvious drawback of excessive adhesion is that it may lead to skin irritation or damage, particularly when the attached skin electronics need to be removed due to device misplacement or for replacement.74 In addition, human skin naturally undergoes continuous shedding. Skin flakes are estimated to shed at a rate of 30–90 mg every hour.75 Taking the average surface areas of a person as 1.8 m2, about 0.03 mg of skin flakes are generated over 1 cm2 over a 6-h period. In other words, for a bioelectronic fiber “sheet” device with limited porosity, the durability of the device’s adhesion to living human skin is not entirely governed by the initial adhesion strength but also the cumulative skin shedding underneath the device that could still lead to delamination.
The standard characterization approach for adhesion strength is a peeling test, such as the 90° peeling test.74 Although the peeling test has been commonly used for research with film- or hydrogel-based skin electronics,74 it is rarely seen in the literature that such a test was performed on bioelectronic fibers. One possible reason could be that small-diameter fiber-based devices are usually fragile, with very low tensile strength to withstand the peeling forces; in other words, during peeling, the bioelectronic fibers would break along their axes prior to peeling off. However, many in situ wearing experiments would be sufficient to examine the adhesion strength of the bioelectronic fibers. For example, rubbing and washing experiments would be able to provide evidence regarding whether the bioelectronic fibers would delaminate from the skin due to insufficient adhesions in dry and wet conditions. A range of material couplings and strategies have been used to adhere bioelectronic fiber devices to the biological surfaces. Among them, surface wetting could be a facile and effective method to attach micro/nanofiber structures to human skin with appropriate adhesion strength. For example, water-soluble nanofiber meshes could be directly adhered to the skin by first moistening the skin area with water,33,65 and in situ fiber spinning allows for solvent-rich microfibers to wet onto the skin during deposition.76 Such a surface wetting attachment technique is advantageous for its simplicity and biocompatibility, as it avoids the need for potentially irritating or hazardous adhesive chemicals and ensures a conformal fit even down to microscale skin topologies. In addition, in situ wearing tests indicate that this surface wetting strategy offers adhesions that are robust enough to withstand everyday use while also facilitating easy removal of the devices.
Adaptability of fiber building blocks
Currently, many bioelectronic fiber devices are developed with a single fiber type for dedicated and specialized functions,7,25 lacking the potential to be adapted for different applications. Multi-fiber constructs have also been reported in the literature,18,25,77 but these constructs were also not designed to allow internal structural-functional reconfigurations for repair or upgrade. Based on life cycle sustainability analyses of bioelectronic fibers, the concept of “adaptability” could be introduced for further enhancing sustainability by reducing device failures or design overhauls due to variabilities in their application environments and requirements. This section aims to explore bioelectronic fiber design strategies for realizing functional adaptability. Adaptability includes (1) compatibility with a wider range of deployment environments and (2) enabling internal modifications of functional fiber structures. The former is named “deployment adaptability,” and the latter is “modular adaptability.”
Deployment adaptability
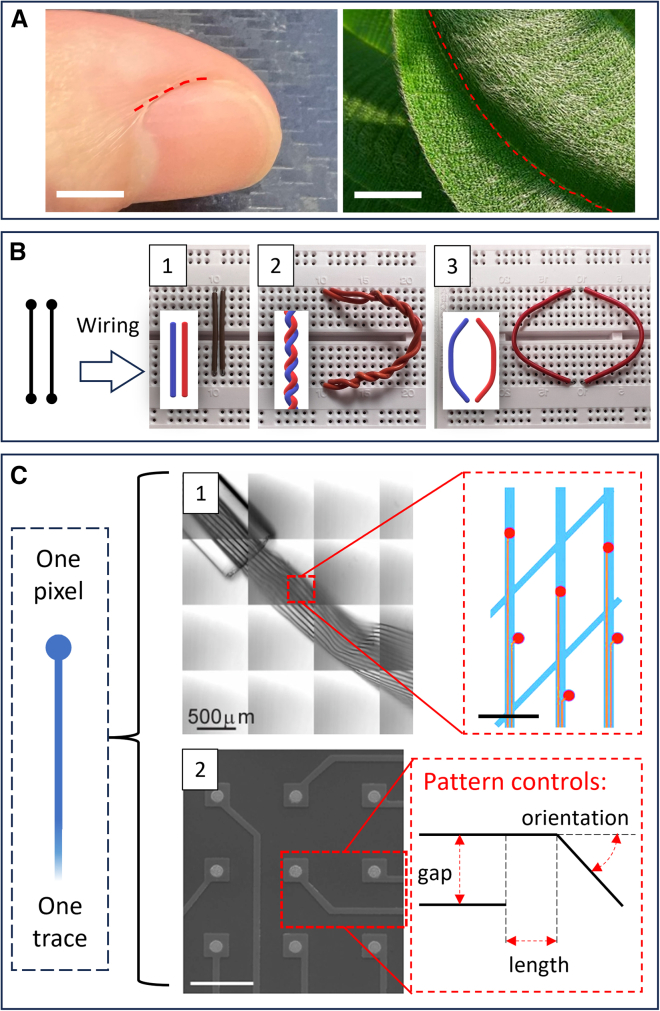
Deployment adaptability refers to device compatibility with a wider range of deployment environments. Its implementation can benefit from analyzing the example of bioelectronic paints. Paints can be used on continuous surfaces to which they can adhere for “writing or painting” bioelectronic circuitry.78 Other than the in situ fabrication feature, paints are also adaptive to soft and contoured surfaces, such as the skin crevices around finger joints.78,79,80 Conformity in bioelectronic paints therefore aids deployment adaptability, but it also has downsides, such as blocking skin pores and stimulating receptors due to their coverage over centimeter lengths and millimeter widths, which may lead to sensory irritation.81,82 Moreover, their adaptive adhesion can be challenged by jagged and rigid biological surface features, like plant trichomes, leaf edges, or human fingernail edges, where the painted structures may weaken or break, as shown in Figure 8A.
Figure 8.
Adaptability in bioelectronic fiber building blocks
(A) Example geometries on biological surfaces that may challenge reliable bioelectronic fiber deployment, demanding deployment adaptability. Left: sharp edge at the side of fingernails. Right: the edge between overlapping leaves as well as trichomes on leaf surfaces. Original photo adapted with permission.83 Copyright 2007, Clyde Robinson. Scale bars, 5 mm.
(B) Different entanglement configurations for a pair of wires with identical connection logic (neighboring parallel fibers, left). The physical jumper wires in the photos connect the top and bottom sockets of two adjacent columns; the wires are structurally mechanically comparable to individually handleable bioelectronic fibers. (1) Simple parallel fibers as the basic configuration. (2) The “twisted pair” configuration that reduces inductive noise between wires. (3) Using increased distance between wires for reducing capacitive noise.84 (Insets show generalized wire entanglement schematics.).
(C) Two structurally different bioelectronic pixel device examples with the same “one pixel, one trace” circuit topology that may support modular adaptability; each trace and pixel can be designed to be individually manipulable and replaceable using bioelectronic fiber building blocks. (1) Microscopy photo of a mesh-like bioelectronic device made with bundled bioelectronic fibers (left); pixel conductor traces (red lines) each connect to individual pixels (red circles) fabricated on the mesh filaments (blue). Scale bar, 50 μm (right). Reproduced with permission.85 Copyright 2017, Fu et al. (2) Example SEM photo of a planar bioelectrode pixel array with identical topology as in (1). Scale bar, 100 μm (left).86 The schematic shows key pattern control factors for arranging mutually separated pixel traces, which is also a deployment adaptability requirement for using fiber building blocks to construct pixel structures (right). Left: adapted with permission.86 Copyright 2020, Yoo et al.
These observations can be referenced when developing future bioelectronic fibers. In terms of surface conformity and imperceptibility, individually handleable fiber designs may often be non-ideal due to their size and robustness requirements (e.g., sub-millimeter-diameter fiber yarns21). As for deploying on complex structures, existing in situ AM techniques like iFP are challenged by line-of-sight breaking or simply large distances between points for fiber attachment. Therefore, for future bioelectronic fibers, structural factors like the abovementioned examples need to be considered for a range of possible deployment environments; the compatibility to variable deployment environments makes fiber building blocks more adaptive, which, in turn, may lead to improved reliability and reduced waste generation, thus improving sustainability.
Modular adaptability
Modular adaptability is the ability to add, remove, or replace functionalities as “modules” inside a bioelectronics system. In conventional electronics, modular adaptability can be realized through two different approaches: (1) “soft” customizability, represented by displays and FPGAs (field programmable gate arrays), and (2) “hard” customizability, represented by breadboards for electronic prototyping. Soft customization in displays and FPGAs involves built-in redundancy, with unused pixels or components remaining idle,87,88 leading to temporary wastages; the division (sharing) of a display or an FPGA chip between spatially distant projects is also not possible. Therefore, soft customizability is a less sustainable form of modular adaptability. Alternatively, hard customization allows for straightforward, reversible connections of standardized components, aspiring to a “plug-and-play” dynamic,89 such as the breadboard circuits.90 The transferable and reusable electronic components, along with their standardized interconnection designs, enable resource sharing across different circuits with minimal wastages, offering a solution for cost-effective and sustainable electronics assembly and adaptation.
The jumper wires in typical breadboard circuits could resemble the bioelectronic fibers, so their assembly into the circuitry could be inspirational for deploying bioelectronic fibers. Individual wires in the breadboard circuits could be easily inserted or repositioned, bioelectronic fibers could also be designed with modifiable connectivity. In addition, the jumper wires could be synergically coupled to add functionalities to themselves. For example, as shown in Figure 8B, the jumper wire arrangements have the same circuit connection logic (simply, two parallel wires that are neighboring but separate) but with different spatial relationships or entanglement states. The unusual configurations may enable additional functions, such as a “twisted pair” for reducing inductive noise, while the large separation can reduce capacitive coupling between wires.84 These reversible functional wire couplings depend on the high aspect ratios and mechanical flexibility of the jumper wires. Bioelectronic fibers also possess mechanical flexibility at different scales; thus, their assemblies could be engineered to leverage unique local fiber couplings. By thoughtfully designing local fiber geometries and arranging the spatial relationships among adjacent fibers, it is feasible to incorporate specialized functionalities.
For bioelectronic devices assembled with fiber building blocks, modular adaptability should be a favorable feature, as reversibly assembled bioelectronic fiber structures are more repairable and may enable in situ functional modifications and upgrades; this enables “hard customization,” which should constitute an upgrade to irreversible bioelectronic fiber structures. Individually handleable bioelectronic fibers are similar to both jumper wires and conventional textile fibers that may be designed to allow repair and modifications;17 these fibers should be primarily referenced for modular adaptability in future designs.
Considerations for bioelectronic pixels
When interfacing with biological systems that exhibit fine spatial signal dynamics, the pixel, or the smallest addressable interfacing element, is an important electronic functional structure or feature.3,7,15,30,78 In the context of bioelectronic devices, bioelectronic pixel devices are often utilized for multi-site sensing and stimulation of a 3D biological tissue or system.15,26 Bioelectronic fibers usually possess small diameters and versatile assembly designs. With individual fibers acting as independent conductor pathways at each pixel site, they could advantageously support the creation of both 2D and 3D pixelated sensing interfaces.19,46 Such pixel arrays, when combined with on-demand deployment and modular adaptability, could further allow for replacement or repair of single pixels, promising to improve overall system sustainability.
The basic requirement for creating pixels is the ability to create mutually separated interconnect traces for individual pixel signal channels. To meet this requirement, the bioelectronic fiber assembly process should allow for precise fiber patterning and independently connected fiber sensing pathways, as exemplified by the mesh bioelectronic devices in Figure 8C(1). In this pixelated sensing device, each channel is connected through a dedicated trace, physically and electronically separated from other channels, so that the signals transferred in the channels are distinguishable. Furthermore, when the sensing channels are made from fiber building blocks that are individually replaceable, each channel could be manipulated or repaired without needing to replace the overall device. Pixelated fiber bioelectronics, when possessing modular adaptability, may improve the device cost-effectiveness and deployment sustainability. The scanning electron microscopy (SEM) photo in Figure 8C(2) is another example of the pixelated bioelectronic device, showing that this principle applies to both planar86 (2D) and mesh-shaped85 (3D) device configurations. In both cases in Figure 8C, pixel channels are already connected through mutually separated fiber-shaped conductors. Although the current design does not explicitly facilitate individual manipulation of each conductor, it does provide a proof-of-concept perspective for the implementation of fiber building blocks in creating distinct pixel channels.
The deployment of fiber building blocks for creating pixels also requires specific compatibility with pattern control. Typical pattern control factors are displayed in the schematic in Figure 8C(2); when deploying traces (e.g., fibers), the length and orientation of each trace need to be controllable, and then the assembly mechanism should identify the locations of already deployed traces to accurately deploy subsequent traces in proximity. The production and deployment characteristics of the fiber building blocks should also be compatible with these controls. For example, if a type of functionalized fibers is too rigid (i.e., not practically bendable), or if an in situ AM fiber production method cannot freely create angles at arbitrary locations along the fiber, then it may be difficult to create the abovementioned pixel topology using these fiber building blocks.
Conclusions
Bioelectronic devices, built with functional fibers, have attracted increasing interests due to their favorable structural and mechanical properties for bio-interfacing applications. Advanced materials and versatile design formats have been used to build and assemble fiber bioelectronics covering multiple size scales for intimate bio-integration and expanded functionalities. Various techniques have been explored to fabricate the bioelectronic fiber building blocks and then to assemble them into functional devices. These techniques display different levels of complexity, precision, and efficiency. On-demand production can reduce wastage to improve sustainability. For fiber building blocks, the design customization of functional fiber structures may benefit from streamlined production, in situ fabrication, or an intuitively designed assembly process. With the projected growth of bioelectronic devices in the future, it is crucial to consider their entire life cycle sustainability with regard to their real-world application scenarios. Moreover, to ensure that these devices are practical, scalable, and market ready, their environmental electromechanical robustness and adaptability should also be respectfully developed.
Treating bioelectronic fibers as both individual devices and as building blocks for structures with intricate functionalities is a perspective guided by the principle of compartmentalization. It may offer a new approach for developing next-generation bioelectronics that are more versatile and sustainable. New opportunities may also arise in expanding the functional materials library and integrating artificial intelligence with advanced fabrication techniques to realize scalable and customizable production with minimized environmental footprints. As the portfolio of bioelectronic fiber building blocks expands, the potential for constructing a fiber-based ecosystem of bioelectronics and other peripheral electronics arises. This evolving ecosystem of fiber modules could be adaptively integrated into biological systems and the surrounding environment, offering a sustainable future lifestyle powered by “Fiber of Things.”
Acknowledgments
The authors acknowledge financial support from the European Research Council (ERC-StG, 758865). J.F.M. is supported by a W.D. Armstrong studentship.
Author contributions
Y.P., W.W., and Y.Y.S.H. conceptualized the ideas, reviewed the literature, and designed the structure of this review article. Y.P. drafted the main structure of this article, and W.W. wrote the electromechanical performance section. Y.P. and W.W. were mainly responsible for writing, editing, and visualization. Y.S. assisted with visualization. All authors reviewed and commented on the article.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Wenyu Wang, Email: wenyuwang@hkust-gz.edu.cn.
Yan Yan Shery Huang, Email: yysh2@cam.ac.uk.
References
- 1.Ohayon D., Inal S. Organic Bioelectronics: From Functional Materials to Next-Generation Devices and Power Sources. Adv. Mater. 2020;32 doi: 10.1002/adma.202001439. [DOI] [PubMed] [Google Scholar]
- 2.Pitsalidis C., Pappa A.-M., Boys A.J., Fu Y., Moysidou C.-M., Van Niekerk D., Saez J., Savva A., Iandolo D., Owens R.M. Organic Bioelectronics for In Vitro Systems. Chem. Rev. 2022;122:4700–4790. doi: 10.1021/acs.chemrev.1c00539. [DOI] [PubMed] [Google Scholar]
- 3.Someya T., Bao Z., Malliaras G.G. The rise of plastic bioelectronics. Nature. 2016;540:379–385. doi: 10.1038/nature21004. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Zhang Y., Liang Z., Cao Y., Han Z., Feng X. Flexible inorganic bioelectronics. npj Flex Electron. 2020;4:2. doi: 10.1038/s41528-020-0065-1. [DOI] [Google Scholar]
- 5.Dufil G., Bernacka-Wojcik I., Armada-Moreira A., Stavrinidou E. Plant Bioelectronics and Biohybrids: The Growing Contribution of Organic Electronic and Carbon-Based Materials. Chem. Rev. 2022;122:4847–4883. doi: 10.1021/acs.chemrev.1c00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majumder S., Sagor M.M.H., Arafat M.T. Functional electrospun polymeric materials for bioelectronic devices: a review. Mater. Adv. 2022;3:6753–6772. doi: 10.1039/D1MA01114F. [DOI] [Google Scholar]
- 7.Zhang Y., Zhou J., Zhang Y., Zhang D., Yong K.T., Xiong J. Elastic Fibers/Fabrics for Wearables and Bioelectronics. Adv. Sci. 2022;9 doi: 10.1002/advs.202203808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluge J.A., Rabotyagova O., Leisk G.G., Kaplan D.L. Spider silks and their applications. Trends Biotechnol. 2008;26:244–251. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbloom J., Abrams W.R., Mecham R. Extracellular matrix 4: The elastic fiber. Faseb. J. 1993;7:1208–1218. doi: 10.1096/fasebj.7.13.8405806. [DOI] [PubMed] [Google Scholar]
- 10.Wang W., Ka S.G.S., Pan Y., Sheng Y., Huang Y.Y.S. Biointerface Fiber Technology from Electrospinning to Inflight Printing. ACS Appl. Mater. Interfaces. 2023 doi: 10.1021/acsami.3c10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei L., Wang S., Shan M., Li Y., Wang Y., Wang F., Wang L., Mao J. Conductive fibers for biomedical applications. Bioact. Mater. 2023;22:343–364. doi: 10.1016/j.bioactmat.2022.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan W., Noel G., Loke G., Meiklejohn E., Khudiyev T., Marion J., Rui G., Lin J., Cherston J., Sahasrabudhe A., et al. Single fibre enables acoustic fabrics via nanometre-scale vibrations. Nature. 2022;603:616–623. doi: 10.1038/s41586-022-04476-9. [DOI] [PubMed] [Google Scholar]
- 13.Gill E.L., Li X., Birch M.A., Huang Y.Y.S. Multi-length scale bioprinting towards simulating microenvironmental cues. Biodes. Manuf. 2018;1:77–88. doi: 10.1007/s42242-018-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021;2 doi: 10.1016/j.xcrp.2021.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong G., Lieber C.M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019;20:330–345. doi: 10.1038/s41583-019-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salatino J.W., Ludwig K.A., Kozai T.D.Y., Purcell E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017;1:862–877. doi: 10.1038/s41551-017-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H.H., Pan Y., Xu L., Feng X., Wang W., Potluri P., Hu L., Hasan T., Huang Y.Y.S. Sustainable electronic textiles towards scalable commercialization. Nat. Mater. 2023;22:1294–1303. doi: 10.1038/s41563-023-01615-z. [DOI] [PubMed] [Google Scholar]
- 18.Xiong J., Chen J., Lee P.S. Functional Fibers and Fabrics for Soft Robotics, Wearables, and Human–Robot Interface. Adv. Mater. 2021;33 doi: 10.1002/adma.202002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Xie S., Wang Z., Liu F., Yang Y., Tang C., Wu X., Liu P., Li Y., Saiyin H., et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020;4:159–171. doi: 10.1038/s41551-019-0462-8. [DOI] [PubMed] [Google Scholar]
- 20.Canales A., Jia X., Froriep U.P., Koppes R.A., Tringides C.M., Selvidge J., Lu C., Hou C., Wei L., Fink Y., Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015;33:277–284. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., He J., Wang H., Qi K., Nan N., You X., Shao W., Wang L., Ding B., Cui S. Highly sensitive, self-powered and wearable electronic skin based on pressure-sensitive nanofiber woven fabric sensor. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamedi M., Forchheimer R., Inganäs O. Towards woven logic from organic electronic fibres. Nat. Mater. 2007;6:357–362. doi: 10.1038/nmat1884. [DOI] [PubMed] [Google Scholar]
- 23.Park S.-H., Lee H.B., Yeon S.M., Park J., Lee N.K. Flexible and Stretchable Piezoelectric Sensor with Thickness-Tunable Configuration of Electrospun Nanofiber Mat and Elastomeric Substrates. ACS Appl. Mater. Interfaces. 2016;8:24773–24781. doi: 10.1021/acsami.6b07833. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Ouaras K., Rutz A.L., Li X., Gerigk M., Naegele T.E., Malliaras G.G., Huang Y.Y.S. Inflight fiber printing toward array and 3D optoelectronic and sensing architectures. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng K., Shi X., Tang C., Liu T., Peng H. Design, fabrication and assembly considerations for electronic systems made of fibre devices. Nat. Rev. Mater. 2023;8:552–561. doi: 10.1038/s41578-023-00573-x. [DOI] [Google Scholar]
- 26.Dai X., Hong G., Gao T., Lieber C.M. Mesh Nanoelectronics: Seamless Integration of Electronics with Tissues. Acc. Chem. Res. 2018;51:309–318. doi: 10.1021/acs.accounts.7b00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J., Campbell A.S., de Ávila B.E.F., Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu K.K., Wang Z., Dai J., Carter M., Hu L. Transient Electronics: Materials and Devices. Chem. Mater. 2016;28:3527–3539. doi: 10.1021/acs.chemmater.5b04931. [DOI] [Google Scholar]
- 29.Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019;146 doi: 10.1016/j.bios.2019.111758. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Li X., Chen J., Yuan C. Micro/Nano Electrode Array Sensors: Advances in Fabrication and Emerging Applications in Bioanalysis. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.573865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang A., Lieber C.M. Nano-Bioelectronics. Chem. Rev. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musk E., Neuralink An Integrated Brain-Machine Interface Platform With Thousands of Channels. J. Med. Internet Res. 2019;21 doi: 10.2196/16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S., Franklin S., Hassani F.A., Yokota T., Nayeem M.O.G., Wang Y., Leib R., Cheng G., Franklin D.W., Someya T. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science. 2020;370:966–970. doi: 10.1126/science.abc9735. [DOI] [PubMed] [Google Scholar]
- 34.Rivnay J., Inal S., Collins B.A., Sessolo M., Stavrinidou E., Strakosas X., Tassone C., Delongchamp D.M., Malliaras G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016;7 doi: 10.1038/ncomms11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morais P.V., Suman P.H., Silva R.A., Orlandi M.O. High gas sensor performance of WO3 nanofibers prepared by electrospinning. J. Alloys Compd. 2021;864 doi: 10.1016/j.jallcom.2021.158745. [DOI] [Google Scholar]
- 36.Rivnay J., Inal S., Salleo A., Owens R.M., Berggren M., Malliaras G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018;3 doi: 10.1038/natrevmats.2017.86. [DOI] [Google Scholar]
- 37.Franssila S. 2nd ed. John Wiley & Sons; 2010. Introduction to Microfabrication. [Google Scholar]
- 38.Fan B., Rodriguez A.V., Vercosa D.G., Kemere C., Robinson J.T. Sputtered porous Pt for wafer-scale manufacture of low-impedance flexible microelectrodes. J. Neural. Eng. 2020;17 doi: 10.1088/1741-2552/ab965c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branham M.S., Gutowski T.G. Deconstructing Energy Use in Microelectronics Manufacturing: An Experimental Case Study of a MEMS Fabrication Facility. Environ. Sci. Technol. 2010;44:4295–4301. doi: 10.1021/es902388b. [DOI] [PubMed] [Google Scholar]
- 40.Sui Y., Zorman C.A. Review—Inkjet Printing of Metal Structures for Electrochemical Sensor Applications. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111/ab721f. [DOI] [Google Scholar]
- 41.Zendehdel M., Yaghoobi Nia N., Paci B., Generosi A., Di Carlo A. Zero-Waste Scalable Blade–Spin Coating as Universal Approach for Layer-by-Layer Deposition of 3D/2D Perovskite Films in High-Efficiency Perovskite Solar Modules. Sol. RRL. 2022;6 doi: 10.1002/solr.202100637. [DOI] [Google Scholar]
- 42.Williams E.D., Ayres R.U., Heller M. The 1.7 Kilogram Microchip: Energy and Material Use in the Production of Semiconductor Devices. Environ. Sci. Technol. 2002;36:5504–5510. doi: 10.1021/es025643o. [DOI] [PubMed] [Google Scholar]
- 43.Choi S., Yoon C., Kim S., Kim W., Ha K., Jeong J., Kim J., Shin J., Park D. Comprehensive Evaluation of Hazardous Chemical Exposure Control System at a Semiconductor Manufacturing Company in South Korea. IJERPH. 2018;15:1162. doi: 10.3390/ijerph15061162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Criado-Gonzalez M., Dominguez-Alfaro A., Lopez-Larrea N., Alegret N., Mecerreyes D. Additive Manufacturing of Conducting Polymers: Recent Advances, Challenges, and Opportunities. ACS Appl. Polym. Mater. 2021;3:2865–2883. doi: 10.1021/acsapm.1c00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan K.R., Down M.P., Hurst N.J., Keefe E.M., Banks C.E. Additive manufacturing (3D printing) of electrically conductive polymers and polymer nanocomposites and their applications. eScience. 2022;2:365–381. doi: 10.1016/j.esci.2022.07.003. [DOI] [Google Scholar]
- 46.Afanasenkau D., Kalinina D., Lyakhovetskii V., Tondera C., Gorsky O., Moosavi S., Pavlova N., Merkulyeva N., Kalueff A.V., Minev I.R., Musienko P. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 2020;4:1010–1022. doi: 10.1038/s41551-020-00615-7. [DOI] [PubMed] [Google Scholar]
- 47.Yuk H., Lu B., Lin S., Qu K., Xu J., Luo J., Zhao X. 3D printing of conducting polymers. Nat. Commun. 2020;11:1604. doi: 10.1038/s41467-020-15316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maibohm C., Silvestre O.F., Borme J., Sinou M., Heggarty K., Nieder J.B. Multi-beam two-photon polymerization for fast large area 3D periodic structure fabrication for bioapplications. Sci. Rep. 2020;10:8740. doi: 10.1038/s41598-020-64955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinrich M.A., Liu W., Jimenez A., Yang J., Akpek A., Liu X., Pi Q., Mu X., Hu N., Schiffelers R.M., et al. 3D Bioprinting: from Benches to Translational Applications. Small. 2019;15 doi: 10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q., Zhang P., O’Leary G., Zhao Y., Xu Y., Rafatian N., Okhovatian S., Landau S., Valiante T.A., Travas-Sejdic J., Radisic M. Flexible 3D printed microwires and 3D microelectrodes for heart-on-a-chip engineering. Biofabrication. 2023;15 doi: 10.1088/1758-5090/acd8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill E.L., Wang W., Liu R., Huang Y.Y.S. Additive batch electrospinning patterning of tethered gelatin hydrogel fibres with swelling-induced fibre curling. Addit. Manuf. 2020;36 doi: 10.1016/j.addma.2020.101456. [DOI] [Google Scholar]
- 52.Gong F., Meng C., He J., Dong X. Fabrication of highly conductive and multifunctional polyester fabrics by spray-coating with PEDOT:PSS solutions. Prog. Org. Coating. 2018;121:89–96. doi: 10.1016/j.porgcoat.2018.04.006. [DOI] [Google Scholar]
- 53.Koutsouras D.A., Gkoupidenis P., Stolz C., Subramanian V., Malliaras G.G., Martin D.C. Impedance Spectroscopy of Spin-Cast and Electrochemically Deposited PEDOT:PSS Films on Microfabricated Electrodes with Various Areas. Chemelectrochem. 2017;4:2321–2327. doi: 10.1002/celc.201700297. [DOI] [Google Scholar]
- 54.Kalambate P.K., Dar R.A., Karna S.P., Srivastava A.K. High performance supercapacitor based on graphene-silver nanoparticles-polypyrrole nanocomposite coated on glassy carbon electrode. J. Power Sources. 2015;276:262–270. doi: 10.1016/j.jpowsour.2014.11.130. [DOI] [Google Scholar]
- 55.Sahasrabudhe A., Rupprecht L.E., Orguc S., Khudiyev T., Tanaka T., Sands J., Zhu W., Tabet A., Manthey M., Allen H., et al. Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits. Nat. Biotechnol. 2023 doi: 10.1038/s41587-023-01833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loke G., Yan W., Khudiyev T., Noel G., Fink Y. Recent Progress and Perspectives of Thermally Drawn Multimaterial Fiber Electronics. Adv. Mater. 2020;32 doi: 10.1002/adma.201904911. [DOI] [PubMed] [Google Scholar]
- 57.Wang T., Meng J., Zhou X., Liu Y., He Z., Han Q., Li Q., Yu J., Li Z., Liu Y., et al. Reconfigurable neuromorphic memristor network for ultralow-power smart textile electronics. Nat. Commun. 2022;13:7432. doi: 10.1038/s41467-022-35160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pham T., Nguyen T., Vadivelu R.K., Dinh T., Qamar A., Yadav S., Yamauchi Y., Rogers J.A., Nguyen N., Phan H. A Versatile Sacrificial Layer for Transfer Printing of Wide Bandgap Materials for Implantable and Stretchable Bioelectronics. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202004655. [DOI] [Google Scholar]
- 59.Wang C., Yokota T., Someya T. Natural Biopolymer-Based Biocompatible Conductors for Stretchable Bioelectronics. Chem. Rev. 2021;121:2109–2146. doi: 10.1021/acs.chemrev.0c00897. [DOI] [PubMed] [Google Scholar]
- 60.Nithya R., Sivasankari C., Thirunavukkarasu A. Electronic waste generation, regulation and metal recovery: a review. Environ. Chem. Lett. 2021;19:1347–1368. doi: 10.1007/s10311-020-01111-9. [DOI] [Google Scholar]
- 61.Tabata T. Waste-to-energy incineration plants as greenhouse gas reducers: A case study of seven Japanese metropolises. Waste Manag. Res. 2013;31:1110–1117. doi: 10.1177/0734242X13502385. [DOI] [PubMed] [Google Scholar]
- 62.Althaf S., Babbitt C.W. Disruption risks to material supply chains in the electronics sector. Resour. Conserv. Recycl. 2021;167 doi: 10.1016/j.resconrec.2020.105248. [DOI] [Google Scholar]
- 63.Brenneman J., Tansel D.Z., Fedder G.K., Panat R. Interfacial delamination and delamination mechanism maps for 3D printed flexible electrical interconnects. Extreme Mechanics Letters. 2021;43 doi: 10.1016/j.eml.2021.101199. [DOI] [Google Scholar]
- 64.Kuliasha C.A., Judy J.W. The Materials Science Foundation Supporting the Microfabrication of Reliable Polyimide–Metal Neuroelectronic Interfaces. Adv. Mater. Technol. 2021;6 doi: 10.1002/admt.202100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyamoto A., Lee S., Cooray N.F., Lee S., Mori M., Matsuhisa N., Jin H., Yoda L., Yokota T., Itoh A., et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017;12:907–913. doi: 10.1038/nnano.2017.125. [DOI] [PubMed] [Google Scholar]
- 66.Kim K.K., Kim M., Pyun K., Kim J., Min J., Koh S., Root S.E., Kim J., Nguyen B.-N.T., Nishio Y., et al. A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition. Nat. Electron. 2022 doi: 10.1038/s41928-022-00888-7. [DOI] [Google Scholar]
- 67.Zhu Z., Guo S.Z., Hirdler T., Eide C., Fan X., Tolar J., McAlpine M.C. 3D Printed Functional and Biological Materials on Moving Freeform Surfaces. Adv. Mater. 2018;30 doi: 10.1002/adma.201707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guan S., Wang J., Yang Y., Zhu X., Zhou J., Ye D., Chen R., Dai H., Liao Q. Highly Stretchable and Flexible Electrospinning-Based Biofuel Cell for Implantable Electronic. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202303134. [DOI] [Google Scholar]
- 69.Gao Z., Xiao X., Carlo A.D., Yin J., Wang Y., Huang L., Tang J., Chen J. Advances in Wearable Strain Sensors Based on Electrospun Fibers. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202214265. [DOI] [Google Scholar]
- 70.Ma Z., Huang Q., Xu Q., Zhuang Q., Zhao X., Yang Y., Qiu H., Yang Z., Wang C., Chai Y., Zheng Z. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 2021;20:859–868. doi: 10.1038/s41563-020-00902-3. [DOI] [PubMed] [Google Scholar]
- 71.Manschot J.F., Brakkee A.J. The measurement and modelling of the mechanical properties of human skin in vivo—I. The measurement. J. Biomech. 1986;19:511–515. doi: 10.1016/0021-9290(86)90124-7. [DOI] [PubMed] [Google Scholar]
- 72.Chen F., Zhuang Q., Ding Y., Zhang C., Song X., Chen Z., Zhang Y., Mei Q., Zhao X., Huang Q., Zheng Z. Wet-Adaptive Electronic Skin. Adv. Mater. 2023;35 doi: 10.1002/adma.202305630. [DOI] [PubMed] [Google Scholar]
- 73.Hao Y., Yan Q., Liu H., He X., Zhang P., Qin X., Wang R., Sun J., Wang L., Cheng Y. A Stretchable, Breathable, And Self-Adhesive Electronic Skin with Multimodal Sensing Capabilities for Human-Centered Healthcare. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202303881. [DOI] [Google Scholar]
- 74.He X., Wang W., Yang S., Zhang F., Gu Z., Dai B., Xu T., Huang Y.Y.S., Zhang X. Adhesive tapes: From daily necessities to flexible smart electronics. Appl. Phys. Rev. 2023;10 doi: 10.1063/5.0107318. [DOI] [Google Scholar]
- 75.Weschler C.J., Langer S., Fischer A., Bekö G., Toftum J., Clausen G. Squalene and Cholesterol in Dust from Danish Homes and Daycare Centers. Environ. Sci. Technol. 2011;45:3872–3879. doi: 10.1021/es103894r. [DOI] [PubMed] [Google Scholar]
- 76.Wang W., Pan Y., Shui Y., Hasan T., Lei I.M., Ka S.G.S., Velasco-Bosom S., Cao Y., McLaren S.B.P., Cao Y., et al. 2023. Sustainable and Imperceptible Augmentation of Living Structures with Organic Bioelectronic Fibres. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanley J., Hunt J.A., Kunovski P., Wei Y. A review of connectors and joining technologies for electronic textiles. Engineering Reports. 2022;4 doi: 10.1002/eng2.12491. [DOI] [Google Scholar]
- 78.Ershad F., Patel S., Yu C. Wearable bioelectronics fabricated in situ on skins. npj Flex. Electron. 2023;7 doi: 10.1038/s41528-023-00265-0. 32–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benight S.J., Wang C., Tok J.B., Bao Z. Stretchable and self-healing polymers and devices for electronic skin. Prog. Polym. Sci. 2013;38:1961–1977. doi: 10.1016/j.progpolymsci.2013.08.001. [DOI] [Google Scholar]
- 80.Wang Y., Haick H., Guo S., Wang C., Lee S., Yokota T., Someya T. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 2022;51:3759–3793. doi: 10.1039/D2CS00207H. [DOI] [PubMed] [Google Scholar]
- 81.Millington P.F., Wilkinson R. Cambridge University Press; 1983. Skin. [Google Scholar]
- 82.Gorb S. Springer; 2009. Functional Surfaces in Biology: Little Structures with Big Effects. [Google Scholar]
- 83.giant hairy leaf | Clyde Robinson | Flickr https://www.flickr.com/photos/85549619@N00/1321743619.
- 84.Paul C.R. Wiley; 2012. Transmission Lines in Digital Systems for EMC Practitioners. [Google Scholar]
- 85.Fu T.-M., Hong G., Viveros R.D., Zhou T., Lieber C.M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl. Acad. Sci. USA. 2017;114:E10046–E10055. doi: 10.1073/pnas.1717695114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long-term Intracellular Recording of Optogenetically-induced Electrical Activities using Vertical Nanowire Multi Electrode Array | Sci. Rep. https://www.nature.com/articles/s41598-020-61325-3. [DOI] [PMC free article] [PubMed]
- 87.Maxfield C. 2008. FPGAs: Instant Access (Newnes) [Google Scholar]
- 88.Graf R.F. 7th ed. 1999. Modern Dictionary of Electronics. rev.updated. (Newnes) [Google Scholar]
- 89.Jiang Y., Ji S., Sun J., Huang J., Li Y., Zou G., Salim T., Wang C., Li W., Jin H., et al. A universal interface for plug-and-play assembly of stretchable devices. Nature. 2023;614:456–462. doi: 10.1038/s41586-022-05579-z. [DOI] [PubMed] [Google Scholar]
- 90.Nussey J. Second edition. 2018. Arduino for Dummies. (For Dummies) [Google Scholar]