Abstract
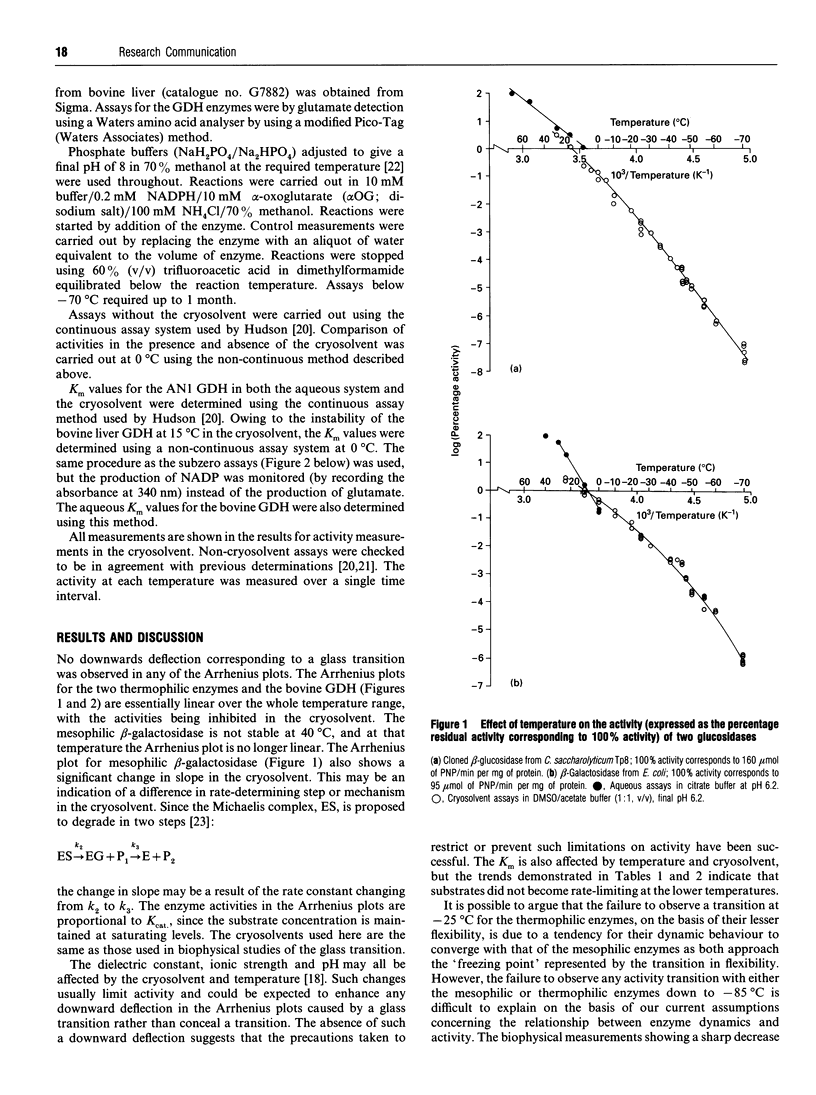
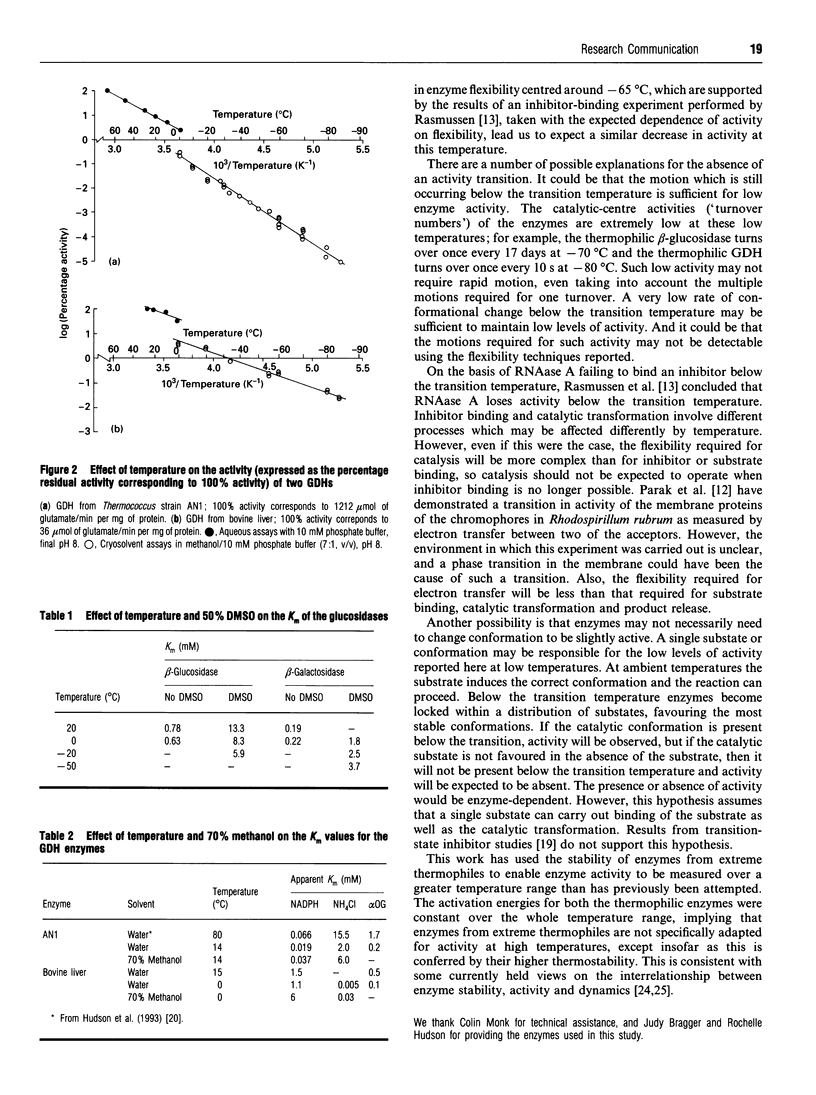
The stability of two enzymes from extreme thermophiles (glutamate dehydrogenase from Thermococcales strain AN1 and beta-glucosidase from Caldocellum saccharolyticum expressed in Escherichia coli) has been exploited to allow measurement of activity over a 175 degrees C temperature range, from +90 degrees C to -85 degrees C for the glutamate dehydrogenase and from +90 degrees C to -70 degrees C for the beta-glucosidase. The Arrhenius plots of these enzymes, and those for two mesophilic enzymes (glutamate dehydrogenase from bovine liver and beta-galactosidase from Escherichia coli), exhibit no downward deflection corresponding to the glass transition, found by biophysical measurements of several non-enzymic mesophilic proteins at about -65 degrees C and reflecting a sharp decrease in protein flexibility as the overall motion of groups of atoms ceases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bauminger E. R., Cohen S. G., Nowik I., Ofer S., Yariv J. Dynamics of heme iron in crystals of metmyoglobin and deoxymyoglobin. Proc Natl Acad Sci U S A. 1983 Feb;80(3):736–740. doi: 10.1073/pnas.80.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Douzou P. Enzymology at subzero temperatures. Adv Enzymol Relat Areas Mol Biol. 1977;45:157–272. doi: 10.1002/9780470122907.ch3. [DOI] [PubMed] [Google Scholar]
- Fink A. L., Angelides K. J. The beta-galactosidase-catalyzed hydrolysis of o-nitrophenol-beta-D-galactoside at subzero temperatures: evidence for a galactosyl-enzyme intermediate. Biochem Biophys Res Commun. 1975 May 19;64(2):701–708. doi: 10.1016/0006-291x(75)90377-0. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R. C., Ruttersmith L. D., Daniel R. M. Glutamate dehydrogenase from the extremely thermophilic archaebacterial isolate AN1. Biochim Biophys Acta. 1993 Oct 6;1202(2):244–250. doi: 10.1016/0167-4838(93)90011-f. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Závodszky P. Proteins under extreme physical conditions. FEBS Lett. 1990 Aug 1;268(2):344–349. doi: 10.1016/0014-5793(90)81283-t. [DOI] [PubMed] [Google Scholar]
- Loncharich R. J., Brooks B. R. Temperature dependence of dynamics of hydrated myoglobin. Comparison of force field calculations with neutron scattering data. J Mol Biol. 1990 Oct 5;215(3):439–455. doi: 10.1016/s0022-2836(05)80363-8. [DOI] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Kononenko A. A., Mössbauer R. L., Goldanskii V. I., Rubin A. B. Evidence for a correlation between the photoinduced electron transfer and dynamic properties of the chromatophore membranes from Rhodospirillum rubrum. FEBS Lett. 1980 Aug 11;117(1):368–372. doi: 10.1016/0014-5793(80)80982-3. [DOI] [PubMed] [Google Scholar]
- Parak F., Knapp E. W., Kucheida D. Protein dynamics. Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982 Oct 15;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]
- Plant A. R., Oliver J. E., Patchett M. L., Daniel R. M., Morgan H. W. Stability and substrate specificity of a beta-glucosidase from the thermophilic bacterium Tp8 cloned into Escherichia coli. Arch Biochem Biophys. 1988 Apr;262(1):181–188. doi: 10.1016/0003-9861(88)90180-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. F., Stock A. M., Ringe D., Petsko G. A. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature. 1992 Jun 4;357(6377):423–424. doi: 10.1038/357423a0. [DOI] [PubMed] [Google Scholar]
- Tilton R. F., Jr, Dewan J. C., Petsko G. A. Effects of temperature on protein structure and dynamics: X-ray crystallographic studies of the protein ribonuclease-A at nine different temperatures from 98 to 320 K. Biochemistry. 1992 Mar 10;31(9):2469–2481. doi: 10.1021/bi00124a006. [DOI] [PubMed] [Google Scholar]
- Varley P. G., Pain R. H. Relation between stability, dynamics and enzyme activity in 3-phosphoglycerate kinases from yeast and Thermus thermophilus. J Mol Biol. 1991 Jul 20;220(2):531–538. doi: 10.1016/0022-2836(91)90028-5. [DOI] [PubMed] [Google Scholar]
- Vihinen M. Relationship of protein flexibility to thermostability. Protein Eng. 1987 Dec;1(6):477–480. doi: 10.1093/protein/1.6.477. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Correlation between the amide proton exchange rates and the denaturation temperatures in globular proteins related to the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):31–37. doi: 10.1016/0022-2836(79)90550-3. [DOI] [PubMed] [Google Scholar]
- Wrba A., Schweiger A., Schultes V., Jaenicke R., Závodszky P. Extremely thermostable D-glyceraldehyde-3-phosphate dehydrogenase from the eubacterium Thermotoga maritima. Biochemistry. 1990 Aug 21;29(33):7584–7592. doi: 10.1021/bi00485a007. [DOI] [PubMed] [Google Scholar]


