Abstract
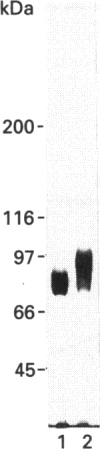
Previous studies from this laboratory have identified rat epididymal luminal fluid acid beta-D-galactosidase activity which also optimally hydrolyses a glycoprotein substrate at neutral pH [Skudlarek, Tulsiani and Orgebin-Crist (1992) Biochem. J. 286, 907-914]. We have now separated the luminal fluid beta-D-galactosidase into two molecular forms by ion-exchange chromatography on a column of DE-52. The separated enzyme activities were purified to an apparent homogeneity by molecular-sieve chromatography followed by affinity chromatography on a column of immobilized p-nitrophenyl beta-D-thiogalactopyranoside. The purified forms, when resolved by SDS/PAGE under reducing conditions, showed apparent molecular masses of 84 and 97 kDa. Kinetic studies, including a pH-dependent substrate preference and pH-dependent association/dissociation, disclosed no differences between these two forms. The two forms had identical N-terminal amino acid sequences. However, the 97 kDa form contained much more total carbohydrate and sialic acid than the 84 kDa form. The carbohydrate moieties in the two forms were assessed by comparing their size on SDS/PAGE before and after treatment with endo-enzymes. The removal of N-linked glycans by treatment with N-glycanase or endoglycosidase F generated de-N-glycosylated polypeptides of an apparent molecular mass of 70 kDa, and indicated that the two forms contained varying amounts of asparagine (N)-linked high mannose/hybrid-type and biantennary complex-type oligosaccharides. This result and the fact that the two molecular forms had identical N-terminal amino acid sequences indicated that the two forms probably have identical or very similar polypeptides. The potential role of the enzyme in modification of sperm plasma membrane (PM) glycoproteins was examined by resolving caput sperm PM proteins (before and after treatment in vitro of the membranes with the purified beta-D-galactosidase) on SDS/PAGE, followed by staining with peanut agglutinin (PNA), a lectin which preferentially binds to Gal beta 1,3GalNAc-linkages found in O-linked glycoproteins. The evidence presented in this report has indicated that a PNA-positive glycoprotein of an apparent molecular mass of 135-150 kDa present on the caput (but not cauda) sperm PM is degalactosylated by the digestion in vitro of the membranes with purified luminal fluid beta-D-galactosidase. This result suggests a possible role for the epididymal luminal fluid beta-D-galactosidases.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Orgebin-Crist M. C., Tulsiani D. R. Qualitative characterization of oligosaccharide chains present on the rat zona pellucida glycoconjugates. Biol Reprod. 1992 May;46(5):912–919. doi: 10.1095/biolreprod46.5.912. [DOI] [PubMed] [Google Scholar]
- CONCHIE J., FINDLAY J., LEVVY G. A. Mammalian glycosidases; distribution in the body. Biochem J. 1959 Feb;71(2):318–325. doi: 10.1042/bj0710318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., Hull S. R. O-glycosylation pathway for mucin-type glycoproteins. Bioessays. 1989 Apr;10(4):117–121. doi: 10.1002/bies.950100406. [DOI] [PubMed] [Google Scholar]
- Cheetham P. S., Dance N. E. The separation and characterization of the methylumbelliferyl beta-galactosidases of human liver. Biochem J. 1976 Jul 1;157(1):189–195. doi: 10.1042/bj1570189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. Glycolipid and glycoprotein degradation. Adv Enzymol Relat Areas Mol Biol. 1987;60:89–216. doi: 10.1002/9780470123065.ch3. [DOI] [PubMed] [Google Scholar]
- Frost R. G., Holmes E. W., Norden A. G., O'Brien J. S. Characterization of purified human liver acid beta-D-galactosidases A2 and A3. Biochem J. 1978 Oct 1;175(1):181–188. doi: 10.1042/bj1750181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Spooncer E., Oates J. E., Dell A., Klock J. C. Structure of sialylated fucosyl lactosaminoglycan isolated from human granulocytes. J Biol Chem. 1984 Sep 10;259(17):10925–10935. [PubMed] [Google Scholar]
- Hammerstedt R. H., Parks J. E. Changes in sperm surfaces associated with epididymal transit. J Reprod Fertil Suppl. 1987;34:133–149. [PubMed] [Google Scholar]
- Heyworth C. M., Neumann E. F., Wynn C. H. The stability and aggregation properties of human liver acid beta-D-galactosidase. Biochem J. 1981 Mar 1;193(3):773–779. doi: 10.1042/bj1930773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen A. T., Verheijen F. W., Galjaard H. The relation between human lysosomal beta-galactosidase and its protective protein. J Biol Chem. 1983 Oct 25;258(20):12143–12146. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nanba E., Suzuki K. Molecular cloning of mouse acid beta-galactosidase cDNA: sequence, expression of catalytic activity and comparison with the human enzyme. Biochem Biophys Res Commun. 1990 Nov 30;173(1):141–148. doi: 10.1016/s0006-291x(05)81033-2. [DOI] [PubMed] [Google Scholar]
- Rankin T. L., Holland M. K., Orgebin-Crist M. C. Lectin binding characteristics of mouse epididymal fluid and sperm extracts. Gamete Res. 1989 Dec;24(4):439–451. doi: 10.1002/mrd.1120240410. [DOI] [PubMed] [Google Scholar]
- Skudlarek M. D., Orgebin-Crist M. C. Effect of swainsonine on rat epididymal glycosidases. J Reprod Fertil. 1988 Nov;84(2):611–617. doi: 10.1530/jrf.0.0840611. [DOI] [PubMed] [Google Scholar]
- Skudlarek M. D., Orgebin-Crist M. C. Glycosidases in cultured rat epididymal cells: enzyme activity, synthesis and secretion. Biol Reprod. 1986 Aug;35(1):167–178. doi: 10.1095/biolreprod35.1.167. [DOI] [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Biosynthesis of two lysosomal enzymes in macrophages. Evidence for a precursor of beta-galactosidase. J Biol Chem. 1979 Oct 25;254(20):9939–9942. [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Turnover of two lysosomal enzymes in macrophages. J Biol Chem. 1981 Oct 10;256(19):10137–10144. [PubMed] [Google Scholar]
- Skudlarek M. D., Tulsiani D. R., Nagdas S. K., Orgebin-Crist M. C. Beta-D-galactosidase of rat spermatozoa: subcellular distribution, substrate specificity, and molecular changes during epididymal maturation. Biol Reprod. 1993 Aug;49(2):204–213. doi: 10.1095/biolreprod49.2.204. [DOI] [PubMed] [Google Scholar]
- Skudlarek M. D., Tulsiani D. R., Orgebin-Crist M. C. Rat epididymal luminal fluid acid beta-D-galactosidase optimally hydrolyses glycoprotein substrate at neutral pH. Biochem J. 1992 Sep 15;286(Pt 3):907–914. doi: 10.1042/bj2860907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Olson G. E. Glycoprotein changes in the rat sperm plasma membrane during maturation in the epididymis. Mol Reprod Dev. 1991 Aug;29(4):357–364. doi: 10.1002/mrd.1080290407. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Buschiazzo H. O., Tolbert B., Touster O. Changes in plasma hydrolase activities in hereditary and streptozotocin-induced diabetes. Arch Biochem Biophys. 1977 May;181(1):216–227. doi: 10.1016/0003-9861(77)90500-8. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Keller R. K., Touster O. The preparation and chemical composition of the multiple forms of beta-glucuronidase from the female rat preputial gland. J Biol Chem. 1975 Jun 25;250(12):4770–4776. [PubMed] [Google Scholar]
- Tulsiani D. R., Skudlarek M. D., Holland M. K., Orgebin-Crist M. C. Glycosylation of rat sperm plasma membrane during epididymal maturation. Biol Reprod. 1993 Feb;48(2):417–428. doi: 10.1095/biolreprod48.2.417. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Skudlarek M. D., Nagdas S. K., Orgebin-Crist M. C. Purification and characterization of rat epididymal-fluid alpha-D-mannosidase: similarities to sperm plasma-membrane alpha-D-mannosidase. Biochem J. 1993 Mar 1;290(Pt 2):427–436. doi: 10.1042/bj2900427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Skudlarek M. D., Orgebin-Crist M. C. Human sperm plasma membranes possess alpha-D-mannosidase activity but no galactosyltransferase activity. Biol Reprod. 1990 May-Jun;42(5-6):843–858. doi: 10.1095/biolreprod42.5.843. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]