Highlights
-
•
Introducing honey bees into protected areas can have detrimental effects on wild pollinators.
-
•
This competition risk is mainly explored between honey bees and wild bees.
-
•
Non-bee pollinator species could also suffer this detrimental competitive effect from honey bees.
-
•
Neglecting non-bee pollinators may underestimate the competition risk among pollinators.
-
•
The integration of non-bee pollinators into scientific studies and conservation plans is urgently required.
Keywords: Wild bees, Beekeeping, Apis mellifera, Interspecific competition, Insect pollinators, Biodiversity conservation
Abstract
Due to the increasing pressures on bees, many beekeepers currently wish to move their managed livestock of Apis mellifera into little disturbed ecosystems such as protected natural areas. This may, however, exert detrimental competitive effects upon local wild pollinators. While it appears critical for land managers to get an adequate knowledge of this issue for effective wildlife conservation schemes, the frequency of this competition is not clear to date. Based on a systematic literature review of 96 studies, we assessed the frequency of exploitative competition between honey bees and wild pollinators. We found that 78% of the studies highlighted exploitative competition from honey bees to wild pollinators. Importantly, these studies have mostly explored competition with wild bees, while only 18% of them considered other pollinator taxa such as ants, beetles, bugs, butterflies, flies, moths, and wasps. The integration of non-bee pollinators into scientific studies and conservation plans is urgently required as they are critical for the pollination of many wild plants and crops. Interestingly, we found that a majority (88%) of these studies considering also non-bee pollinators report evidence of competition. Thus, neglecting non-bee pollinators could imply an underestimation of competition risks from honey bees. More inclusive work is needed to estimate the risks of competition in its entirety, but also to apprehend the context-dependency of competition so as to properly inform wildlife conservation schemes.
Graphical abstract
1. Introduction
Recent studies have highlighted a global decline in wild bee populations (Biesmeijer et al., 2006; Koh et al., 2016; Zattarra and Aizen, 2021) and increased mortality rates in the livestock of managed honey bees Apis mellifera (Requier et al., 2018; Gray et al., 2023; Bruckner et al., 2023; Requier et al., 2024). These colony losses force beekeepers to increase their livestock to compensate for the mortalities, resulting in a global increase in the number of managed honey bee colonies (Phiri et al., 2022). Threats to bee populations have alarmed scientists and public authorities given their ecological and economic importance (Potts et al., 2016; Pérez-Méndez et al., 2020). The safeguard of wild and managed bees is essential for the maintenance of pollination services, food security and associated human well-being (Potts et al., 2016). Yet, this major ecosystem service is threatened by land use change, intensification and associated disturbances that strongly impact both wild and managed pollinators.
Land use changes and intensification have greatly modified many habitats including agricultural landscapes, confronting bees (and other pollinator insects) to disturbances in food resource availability (Requier et al., 2015, 2017, 2020; Timberlake et al., 2019) and exposure to pesticides (Henry et al., 2012, 2015; Prado et al., 2019; Barascou et al., 2022). Consequently, many agricultural dominated landscapes are today inhospitable for pollinators and beekeepers (Otto et al., 2016; Dixon et al., 2021). In contrast, due to their rich and diversified (floral) food resources and their lower exposure to pesticides, protected natural areas are of great interest to beekeepers seeking to minimize colony loss (Geldmann and González-Varo, 2018; Henry and Rodet, 2018; Valido et al., 2019). Thus, more and more beekeepers place or move their managed honey bee colonies to protected natural areas (Geldmann and González-Varo, 2018). However, these preserved areas are also considered as sanctuaries for the conservation of wild pollinators (Öckinger et al., 2007; Morandin and Kremen, 2013; Ropars et al., 2020a).
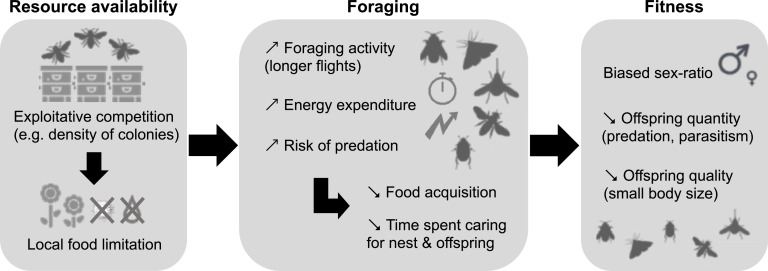
Recent studies have shown that the introduction of honey bee colonies into protected areas could deplete floral resources with detrimental effects on populations of wild pollinators (e.g., Magrach et al., 2017; Henry and Rodet, 2018; Valido et al., 2019; Ropars et al., 2020b, 2022; Page and Williams, 2023a, 2023b; MacInnis et al., 2023; Prendergast and Ollerton, 2022; Weaver et al., 2022; Casanelles‐Abella et al., 2023; MacKell et al., 2023; Mouillard-Lample et al., 2023; Sponsler et al., 2024). In particular, studies suggest potential causal links between this exploitative competition and the fitness of wild pollinators (e.g., Cane and Tepedino, 2017; Fig. 1). Exploitative competition from honey bees can lead to local food limitation in nectar and pollen (Carneiro and Martins, 2012; Sponsler et al., 2024) with consequences for wild pollinator foraging, such as an increase foraging activity and energy expenditure by exploring more distant resources and longer flights (Thomson, 2004; Zurbuchen et al., 2010), an increase in predation risk (Sponsler et al., 2023), which can lead to a decrease in food acquisition (Carneiro and Martins, 2012; Cane and Tepedino, 2017), and a decrease in time spent caring for nest construction and offspring (Sponsler et al., 2023; Goodell, 2003; Fig. 1). In turn, these foraging disruptions can lead to detrimental effects on the fitness of wild pollinators (Cane and Tepedino, 2017), such as male-biased sex ratios (Peterson and Roitberg, 2006; Bosch, 2008), a decrease in the quantity of offspring due to predation and parasitism (Goodell, 2003), and a decrease in the quality of offspring with, for example, smaller body size (Tepedino and Torchio, 1982; Bosch, 2008; Fig. 1). There are also two other competition processes at play: apparent competition and interference competition (Zakardjian et al., 2022; Geslin et al., 2023). In the apparent competition, managed and wild pollinators may indirectly compete with each other due to the effect of shared pathogens that may spread among individuals through successive visits of the same flowers (Alger et al., 2019a, 2019b; Dalmon et al., 2021; Cilia et al., 2022). Finally, in interference competition, individuals may compete among each other through direct physical fight, while accessing the same flower, or through cleptolecty whereby the largest or most aggressive individuals or the robber are generally favored (Cairns et al., 2005; Londei and Marzi, 2024).
Fig. 1.
Potential causal links between exploitative competition and the fitness of wild pollinators. Icons used from www.freepik.com.
Of major importance, the competition issue is becoming more and more prominent in conservation science. While reviews exist on the potential risks and disturbances of introducing honey bee colonies on the ecology of wild pollinators (e.g., Geslin et al., 2017, 2023; Mallinger et al., 2017; Agüero et al., 2018; Wojcik et al., 2018; Iwasaki and Hogendoorn, 2022; Sponsler et al., 2023), most have focused on the potential impact of honey bees on other Anthophila (i.e., wild bees). Little is known about the proportion of studies reporting evidence of competition between honey bees and wild pollinators when considering a larger diversity of pollinator taxa, such as the inclusion of ants, beetles, bugs, butterflies, flies, moths, and wasps, whose contribution to plant pollination and crop yields is demonstrated in an increasing number of studies (e.g., Rader et al. 2016, Lucas et al., 2018a; Rader et al., 2020; Doyle et al., 2020, Page et al., 2021, 2023a, Travis and Kohn, 2023, Requier et al., 2023, Muinde and Katumo, 2024).
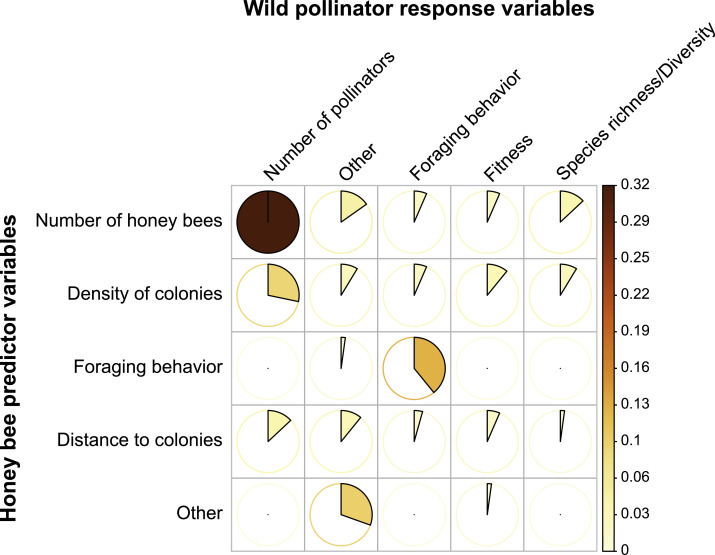
For that purpose, we performed an extensive literature search using the Web of Science section of the bibliographic database ISI Web of Knowledge (Web of Knowledge, 2022). We used the TS function to define the search strings (i.e. the criteria keywords) in order to separate articles that had studied the topic of interest from those which only mentioned it. We included all literature from 1945 until May 2020. The complete search string was "(TS=Apis mellifera OR TS=honeybee* OR TS=honey bee*) AND (TS=wild OR TS=native) AND (TS=competit* OR (TS=pathogen* AND (TS=transmission* OR TS=spill-over* OR TS=spillover*)))”. This search revealed 325 studies. Abstracts and full texts were reviewed for relevance, in order to select empirical studies using original data and to exclude review papers and simulation-based studies. Lastly, given that it is acknowledged that combining search methods improves the robustness and completeness of a systematic literature review (Booth et al., 2021), we included 29 additional studies not found in the automatic process described above, based on the authors’ expertise (e.g. found in the references of selected publications). A total of 96 studies estimating the risk of exploitative competition between honey bees and wild pollinators was therefore selected for the review (see the complete list in Supporting Information, Appendix A). Response variables of wild pollinators commonly measured for quantifying competition were classified in five variables including number of pollinators, richness/diversity of pollinators, foraging behavior (e.g. time of flower visit and part of the flower that was visited), fitness (e.g. offspring quantity and quality) and other (i.e. different of the four other variables) (Fig. 2). The predictor variables often used to quantify the degree of honey bee competition pressure were classified in five variables including number of honey bees, foraging behavior (e.g. time of flower visit and part of the flower that was visited), density of colonies, distance to colonies and other (i.e. different of the four other variables) (Fig. 2).
Fig. 2.
Diversity of variables usually used to study the risk of competition between honey bee predictor variables (i.e. the predictor variables often used to quantify the degree of honey bee competition pressure) and wild pollinator response variables. Color gradient and pie charts represent the frequency of occurrence of variables based on the articles considered in this review (n = 96 articles).
2. Frequency of the competition among pollinators
Among the 96 articles considered, only one study found a positive effect of honey bees on wild pollinators (Nielsen et al., 2012). This study analyzed whether the presence of honey bees affected the visitation rate of wild pollinators on plant species in European countries. The authors found a positive relationship between visitation frequencies of honey bees and bumble bees, suggesting that the presence of honey bees enhanced the bumble bee visitation rate in the study sites considered. However, they noted that this does not indicate a general absence of competition, as other relationships (positive, negative, and neutral) were found with hoverflies and solitary bees. The study highlighted the need to consider multiple factors such as flower abundance, diversity of pollinators, and climate when analyzing competition risk, indicating that it is a complex and potentially context-dependent phenomenon.
On the other hand, we found that 78% (n = 75 articles) of the articles identified an exploitative competition from honey bees to wild pollinators. This estimate confirms previous finding (66% in Iwasaki and Hogendoorn, 2022). These studies highlighted that honey bees could have a negative effect on pollination services driven by wild pollinators by reducing the diversity of wild pollinators and their foraging activity (e.g. Valido et al., 2019; Leguizamón et al., 2021). The other 22% (n = 21 articles) of the articles found no effect of honey bees on wild pollinators (e.g. Roubik et al., 1986; Steffan-Dewenter and Tscharntke, 2000; Roubik and Wolda, 2001). This result suggests that competition between honey bees and wild pollinators is frequently observed but is not systematic (see also Wojcik et al., 2018), and potentially context dependent. It is also possible that a part of those studies was carried out under conditions of low beekeeping density, below certain ecological threshold liable to trigger competition per se (e.g., Henry and Rodet, 2018; McCune et al., 2020).
It is important to note that there is currently no standardized method to study competition risk among pollinators. For instance, we found a wide diversity of honey bee variables and wild pollinators response variable used across the 96 studies considered in the review (Fig. 2). The main metric used is the number of honey bees observed visiting flowers (Hudewenz and Klein, 2015; Torné-Noguera et al., 2016), that may be further weighted by the distance to nearest honey bee colonies (Ropars et al. 2022). Other candidate metrics are the distance to honey bee colonies, the density of honey bee colonies in the surrounding landscape, and the foraging behavior (Steffan-Dewenter and Tscharntke, 2000; Goras et al., 2016; Ropars et al. 2019; Fig. 2). Response variable used could either refer to the number, the foraging behavior, the species richness and diversity of wild pollinators or to bipartite network metrics (Torné-Noguera et al., 2016; Ropars et al., 2019, 2022; Fig. 2). The use of such a wide diversity of methods makes it difficult to robustly assess the frequency of competition risk among pollinators and may bias estimates of competition. Thus, methodological standardizations are urgently required (see also Henry and Rodet, 2018).
3. Beyond the risk of competition between Apis and non-Apis bees
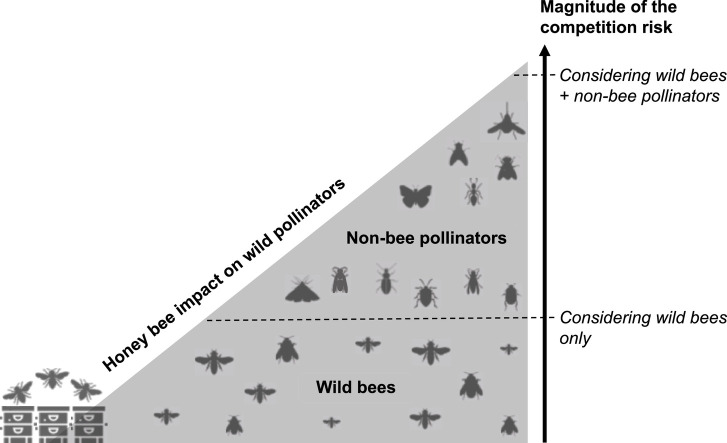
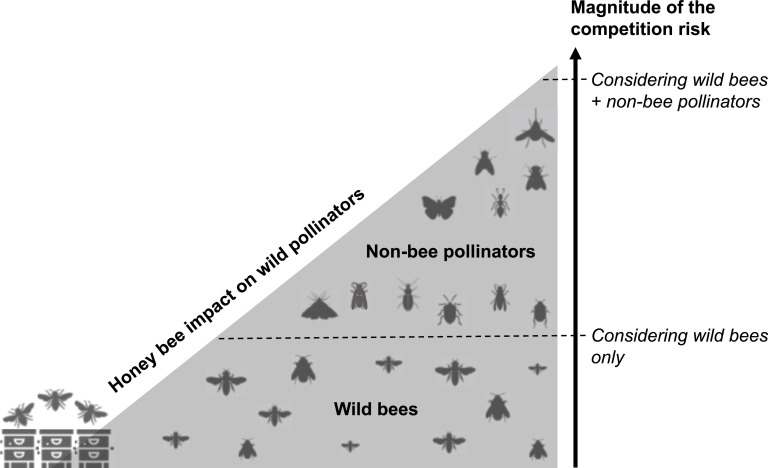
One hypothesis of the context-dependency of the competition risk among pollinators could be the diversity of the pollinator community considered in the studies. Indeed, increasing number of studies demonstrates the critical role of Diptera, Lepidoptera, Coleoptera, Hemiptera and non-bee Hymenoptera in the pollination of wild plants and crops (Rader et al., 2016, 2020; Doyle et al., 2020; Requier et al., 2023). Pollinator diversity, including non-bee species such as ants, beetles, bugs, butterflies, flies, moths, and wasps, contributes to crop pollination even in the presence of honey bees (Garibaldi et al., 2013; Orford et al., 2015; Rader et al., 2016, 2020; Schurr et al. 2021). Overall, it is estimated that non-bee pollinators perform 25–50% of the flower visits in global important crops (Rader et al., 2016). Thus, we explored whether the competition studies actually considered this pollinator diversity. Importantly, we found that 82% (n = 79 articles) of the studies assessing the risk of competition among pollinators focused only on the impact of honey bees on wild bees, i.e. focused on the risk of competition between Apis and non-Apis bees. Among the 17 studies (18%) that extended their analyses to other insect groups (Table 1), we found that Diptera were systematically considered (see also Sladonja et al., 2023), followed by Lepidoptera and Coleoptera (Table 1). Other insect groups, such as Hemiptera and Neuroptera, were considered in an even more marginal way. Very interestingly, we found that 88% of these studies (n = 15 of the 17 articles) reported a negative effect of the presence of honey bees on these wild (non-bee) pollinators, instead of 76% (n = 60 of the 79 articles) for the articles considering only bees in the competition risk (Fig. 3). This result suggests that focusing on the effect of honey bees on wild bees could underestimate the risk of competition (Fig. 4).
Table 1.
Summary of the species studied in the articles resulting from the synthesis of the literature and taking wild pollinators into account more broadly (n = 17).
| Diptera | Lepidoptera | Coleoptera | Hymenoptera (other than bees) | Hemiptera | Other (e.g. Araneae, Neuroptera, Thysanopter) | |
|---|---|---|---|---|---|---|
| Horskins and Turner (1999) | X | X | X | X | X | X |
| Kato and Kawakita (2004) | X | X | X | X | X | |
| Levitt et al. (2013) | X | X | X | X | X | X |
| Polatto and Chaud-Netto (2013) | X | X | X | X | ||
| Hung et al. (2019) | X | X | X | X | ||
| Aizen and Feinsinger (1994) | X | X | X | |||
| Alomar et al. (2018) | X | X | X | X | ||
| Badano and Vergara (2011) | X | X | X | |||
| Smith-Ramírez et al. (2014) | X | X | X | |||
| Mallick and Driessen (2009) | X | X | X | |||
| Ropars et al. (2019) | X | X | X | |||
| Valido et al. (2019) | X | X | X | |||
| Conner and Neumeier (1995) | X | |||||
| Jeavons et al. (2020) | X | |||||
| Lindstrom et al. (2016) | X | |||||
| Magrach et al. (2017) | X | |||||
| Tepedino et al. (2007) | X |
Fig. 3.
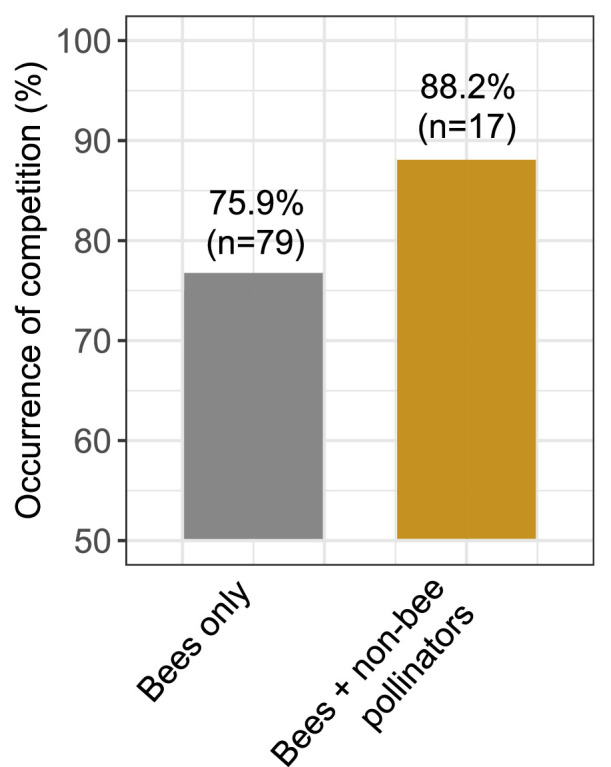
Frequency of occurrence of competition (%) reported considering only wild bees (n = 79 articles) or wild bees + non-bee pollinators (n = 17 articles).
Fig. 4.
Conceptual illustration of the potential underestimation of the competition risk among pollinators by neglecting non-bee pollinators. Icons used from www.freepik.com.
4. Neglecting pollinator diversity would underestimate competition risk among pollinators
A large number of studies assessing the risk of competition among pollinators currently omit pollinator diversity in their entirety by solely focusing on the interaction between honey bees and wild bees. Although bees contribute critically to pollination in agriculture (Requier et al., 2023), the rest of the pollinators have an essential role in plant-pollinator interaction networks in nature. Diptera, for example, are the second most important pollinators and visit about 72% of crops (Rader et al., 2016, 2020). It is also not surprising to note that it is the most studied taxon within the 16 articles extending their fields of research to pollinators other than Apoidea. Among them, hoverflies are one of the main taxa visiting plants for floral rewards in nectar and pollen (Doyle et al., 2020). Adult hoverflies indeed require significant supplies of energy through nectar for flight or for laying eggs (Schneider, 1948). During their sexual maturation, females also need to consume large amounts of protein and amino acids, and thus consume substantial amounts of pollen. Lepidoptera (butterflies) are also a known example of a nectar-consuming pollinator. We found that 59% (n = 10) of the article considering non-bee pollinators in the competition risk from honey bees included Lepidoptera. Competition with honey bees for floral resources should therefore impact all wild pollinators and not considering them would underestimate this competition especially since some recent studies have shown that this impact differs from one species to another, probably related to their morphological characteristics (Henry and Rodet, 2018; Leguizamón et al., 2021; Lázaro et al., 2021; Ropars et al. 2022).
On the other hand, to improve our understanding of these risks of competition, taking into account the complex networks of interactions between plants and pollinators constitutes a significant path for improvement. While plant-bee networks are largely explored, there is a lack of knowledge about floral diet of Diptera, Lepidoptera and Coleoptera taxa (Orford et al., 2015; Klecka et al., 2018; Howlett et al., 2021). Therefore, specialization of these taxa on plant species is still unknown. Some bees, for example, are monolectic and specialize in pollen from specific plants, as opposed to oligolectic bees that forage on several genera of plants or polylectic bees that forage on several families of plant (Wojcik et al., 2018). This is also reported for hoverflies, which sometimes select their floral resources (Gilbert 1981; Lucas et al., 2018b). Competition risk is consequently stronger on specialist species which cannot modify their floral diet when honey bees focused on their host plant species. Moreover, the dominance of certain pollinator species within network pollination strongly influences the access of floral resources for other pollinators (Hung et al., 2019; Weekers et al., 2022). The composition and diversity of pollinator communities are affected by the dominant species, increasing competition for non-dominant and rare species (see Kunte, 2008 among butterflies; Gilbert and Owen, 1990 among hoverflies and Morse, 1981 between bumblebees and hoverflies). Finally, aggressive or territorial behavior of some pollinator species could also increase the risk of competition and the partitioning of floral resources (e.g. Fitzpatrick and Wellington 1983). Thus, the higher abundance and diversity of wild pollinators with potentially more specialist species could explain why we observed a higher frequency of competition risk when considering together wild bees and other wild pollinator species (Fig. 4). Developing models taking these specificities into account would refine our understanding of these risks and inform more appropriate management practices.
5. The context-dependency of competition risks among pollinators
Although focusing on the risk of competition between honey bees and wild bees could underestimate the real risk of competition among pollinators, other factors should be considered as context-dependent variables. For instance, the landscape composition and configuration may directly affect the risk of competition and even explain the lack of competition in some studies (see Herbertsson et al., 2016; Leguizamón et al., 2021). First, landscape composition and configuration have direct effects on the availability of flower resources, and thus the intensity of the resource limitation that drives competition risk (Herbertsson et al., 2016; St. Clair et al., 2022). Moreover, some landscape characteristics critically affects on the abundance and diversity of wild pollinators, which may have restricted foraging range (e.g. for bees, Kendall et al., 2022). The presence of semi-natural habitats in the landscape drives the availability of nesting sites for many wild pollinator species (Proesmans et al., 2019; Eeraerts et al., 2022), and thus directly affects the risk of competition.
In agricultural landscapes, food shortage may exacerbate competition and could occur early in the season when the demand for floral resources is high compared to their availability (Timberlake et al., 2019), between flowering periods of mass-flowering crops (Requier et al., 2015; Timberlake et al., 2019), or at the end of the season when flowers are scarce while floral resources demands is still high (Timberlake et al., 2019). These periods therefore appear to be particularly critical in the event of competition with honey bees. However, the amount of resources available to pollinators is not assessed in most of the studies, although it may constitute a context influencing competition and despite recent advances in the estimation of floral resources at the landscape scale (Timberlake et al., 2019; Alignier et al., 2023; Mouillard-Lample et al., 2023). Precisely, in order to estimate the amount of floral resources available to pollinators, databases on nectar and pollen production by plant species are currently being developed (Baude et al., 2016; Flo et al., 2018; Filipiak et al., 2022; Venjakob et al., 2022). These include measurements of nectar volume and sugar concentration from a single sample per day and the quantity of pollen grains as well as analysis of protein content. While the amount of pollen is more consistent per flower, nectar production is variable and recent studies emphasize the importance to consider the kinetic of nectar production. Frequent visits may stimulate the flower to replenish its nectar leading to potential current underestimation of nectar production in the studies with a high density of pollinators (Carisio et al., 2022).
6. Towards inclusive studies of the risk of competition among pollinators
Our review shows that the risk of competition between honey bees and wild pollinators is frequently observed. However, most of the studies tend to underestimate the magnitude of the risk by focusing on the interaction between bees (Apis vs. non-Apis) rather than considering the full diversity of wild pollinators, which includes non-bee species such as ants, beetles, bugs, butterflies, flies, moths, and wasps (Rader et al., 2016, 2020; Doyle et al., 2020; Requier et al., 2023). Currently, some beekeepers are banished from natural areas due to suspicion of competition from their honey bee colonies on wild pollinators and recommendations of conservation plans foster this practice (Geldmann and González-Varo, 2018). Nevertheless, the magnitude of the risk of competition shows potential context-dependency due to the diversity of pollinators considered, the landscape and the methods used to assess the risk. A conflict is therefore crystallizing between beekeepers and managers of natural areas due to considerable uncertainty about the management measures to be recommended. Standardization of the assessment of the risk of competition and the integration of non-bee pollinators into scientific studies are urgently required. There is a critical need (i) to investigate the entire pollinator community in competition assessment, with the inclusion of non-bee pollinators; (ii) to set methodological standards and metrics to measure the impact of honey bees and the response of wild pollinators, especially for considering the number of colonies at a given site, (iii) to assess the flower resource availability in order to estimate the intensity of resource limitation; (iv) to consider the landscape composition and configuration that could modulate the risk of competition. Given the diversity of pollinators that are likely to be affected by this competition, it is important to remain vigilant in order to better adapt conservation practices, in collaboration with all the stakeholders involved.
CRediT authorship contribution statement
Fabrice Requier: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition. Myriam Abdelli: Data curation, Formal analysis, Investigation. Mathilde Baude: Validation, Writing – review & editing. David Genoud: Validation, Writing – review & editing. Hadrien Gens: Validation, Writing – review & editing. Benoît Geslin: Validation, Writing – review & editing. Mickaël Henry: Validation, Writing – review & editing. Lise Ropars: Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank Léo Mouillard-Lample for constructive discussions. This work was supported by the French agency of ecological transition (ADEME); grant number 00105370 of the CO3 (CO-COnstruction de COnnaissances) program.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cris.2024.100093.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Agüero J.I., Rollin O., Torretta J.P., Aizen M.A., Requier F., Garibaldi L.A. Honeybee impact on plants and wild bees in natural habitats. Ecosistemas. 2018;27(2):60–69. doi: 10.7818/ECOS.1365. [DOI] [Google Scholar]
- Aizen M.A., Feinsinger P. Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine "Chaco Serrano”. Ecol. Soc. Am. 1994 doi: 10.2307/1941941. [DOI] [Google Scholar]
- Alger S.A., Burnham P.A., Boncristiani H.F., Brody A.K. RNA virus spillover from managed honeybees (Apis mellifera) to wild bumblebees (Bombus spp.) PLoS ONE. 2019;14:1–13. doi: 10.1371/journal.pone.0217822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger S.A., Burnham P.A., Brody A.K. Flowers as viral hot spots: honey bees (Apis mellifera) unevenly deposit viruses across plant species. PLoS ONE. 2019;14(9):1–16. doi: 10.1371/journal.pone.0221800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alignier A., Lenestour N., Jeavons E., Van Baaren J., Aviron S., Uroy L., Ricono C., Le Lann C. Floral resource maps: a tool to explain flower-visiting insect abundance at multiple spatial scales. Landsc. Ecol. 2023;38:1511–1525. doi: 10.1007/s10980-023-01643-9. [DOI] [Google Scholar]
- Alomar D., Gonzalez-Estevez M.A., Traveset A., Lazaro A. The intertwined effects of natural vegetation, local flower community, and pollinator diversity on the production of almond trees. Agric. Ecosyst. Environ. 2018;264:34–43. doi: 10.1016/j.agee.2018.05.004. [DOI] [Google Scholar]
- Badano E.I., Vergara C.H. Potential negative effects of exotic honey bees on the diversity of native pollinators and yield of highland coffee plantations. Agric. For. Entomol. 2011;13(4):365–372. doi: 10.1111/j.1461-9563.2011.00527.x. [DOI] [Google Scholar]
- Barascou L., Requier F., Sené D., Crauser D., Le Conte Y., Alaux C. Delayed effects of a single dose of a neurotoxic pesticide (sulfoxaflor) on honeybee foraging activity. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150351. [DOI] [PubMed] [Google Scholar]
- Baude M., Kunin W., Boatman N., et al. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature. 2016;530:85–88. doi: 10.1038/nature16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesmeijer J.C., Roberts S.P.M., Reemer M., Ohlemüller R., Edwards M., et al. Parallel declines in pollinators and insect pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Booth A., Sutton A., Clowes M., Martyn-St James M. 3rd ed. SAGE; Los Angeles: 2021. Systematic Approaches to a Successful Literature Review. [Google Scholar]
- Bosch J. Production of undersized offspring in a solitary bee. Anim. Behav. 2008;75:809–816. doi: 10.1016/j.anbehav.2007.06.018. [DOI] [Google Scholar]
- Bruckner S., Wilson M., Aurell D., Rennich K., vanEngelsdorp D., Steinhauer N., Williams G.R. A national survey of managed honey bee colony losses in the USA: results from the Bee Informed Partnership for 2017–18, 2018–19, and 2019–20. J. Apic. Res. 2023;62:429–443. doi: 10.1080/00218839.2022.2158586. [DOI] [Google Scholar]
- Cairns C.E., Villanueva-Gutiérrez R., Koptur S., Bray D.B. Bee populations, forest disturbance, and Africanization in Mexico. Biotropica. 2005;37:686–692. doi: 10.1111/j.1744-7429.2005.00087.x. [DOI] [Google Scholar]
- Cane J.H., Tepedino V.J. Gauging the effect of honey bee pollen collection on native bee communities. Conserv. Lett. 2017;10(2):205–210. doi: 10.1111/conl.12263. [DOI] [Google Scholar]
- Carisio L., Schurr L., Masotti V., Porporato M., Nève G., Affre L., Gachet S., Geslin B. Estimates of nectar productivity through a simulation approach differ from the nectar produced in 24 h. Funct. Ecol. 2022;36(12):3234–3247. doi: 10.1111/1365-2435.14210. [DOI] [Google Scholar]
- Carneiro L.T., Martins C.F. Africanized honey bees pollinate and preempt the pollen of Spondias mombin (Anacardiaceae) flowers. Apidologie. 2012;43:474–486. doi: 10.1007/s13592-011-0116-7. [DOI] [Google Scholar]
- Casanelles-Abella J., Fontana S., Fournier B., Frey D., Moretti M. Low resource availability drives feeding niche partitioning between wild bees and honeybees in a European city. Ecol. Appl. 2023;33:e2727. doi: 10.1002/eap.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia G., Flaminio S., Zavatta L., Ranalli R., Quaranta M., Bortolotti L., Nanetti A. Occurrence of honey bee (Apis mellifera L.) pathogens in wild pollinators in northern Italy. Front. Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.907489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner J.K., Neumeier R. Effects of black mustard population size on the taxonomic composition of pollinators. Oecologia. 1995;104:218–224. doi: 10.1007/BF00328586. [DOI] [PubMed] [Google Scholar]
- Dalmon A., Diévart V., Thomasson M., Fouque R., Vaissière B.E., Guilbaud L., Le Conte Y., Henry M. Possible spillover of pathogens between bee communities foraging on the same floral resource. Insects. 2021;12:122. doi: 10.3390/insects12020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D.J., Zheng H., Otto C.R.V. Land conversion and pesticide use degrade forage areas for honey bees in America’s beekeeping epicenter. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0251043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T., Hawkes W.L.S., Massy R., Powney G.D., Menz M.H.M., Wotton K.R. Pollination by hoverflies in the Anthropocene. Proc. R. Soc. B. 2020;287 doi: 10.1098/rspb.2020.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeraerts M., Clymans R., Van Kerckvoorde V., Beliën T. Nesting material, phenology and landscape complexity influence nesting success and parasite infestation of a trap nesting bee. Agric. Ecosyst. Environ. 2022;332 doi: 10.1016/j.agee.2022.107951. [DOI] [Google Scholar]
- Filipiak M., Walczyńska A., Denisow B., Petanidou T., Ziółkowska E. Phenology and production of pollen, nectar, and sugar in 1612 plant species from various environments. Ecology. 2022;103(7):e3705. doi: 10.1002/ecy.3705. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S., Wellington W. Contrasts in the territorial behavior of three species of hover flies (Diptera: Syrphidae) Can. Entomol. 1983;115(5):559–566. doi: 10.4039/Ent115559-5. [DOI] [Google Scholar]
- Flo V., Bosch J., Arnan X., Primante C., Martín González A.M., Barril-Graells H., Rodrigo A. Yearly fluctuations of flower landscape in a Mediterranean scrubland: consequences for floral resource availability. PLoS ONE. 2018;13(1) doi: 10.1371/journal.pone.0191268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibaldi L.A., et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339:1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- Geldmann J., González-Varo J.P. Conserving honey bees does not help wildlife: high densities of managed honey bees can harm populations of wild pollinators. Science. 2018;359:392–393. doi: 10.1126/science.aar2269. [DOI] [PubMed] [Google Scholar]
- Geslin B., Gauzens B., Baude M., Dajoz I., Fontaine C., et al. Massively introduced managed species and their consequences for plant–pollinator interactions. Adv. Ecol. Res. 2017;57:147–199. doi: 10.1016/bs.aecr.2016.10.007. [DOI] [Google Scholar]
- Geslin B., Mouillard-Lample L., Zakardjian M., Dajoz I., Flacher F., Henry M., Perrard A., Requier F., Ropars L., Schatz B., Vereecken N.J., Gauzens B. New insights on Massively Introduced Managed Species and their consequences for plant–pollinator interactions. Adv. Ecol. Res. 2023;68:63–89. doi: 10.1016/bs.aecr.2023.09.003. [DOI] [Google Scholar]
- Gilbert F.S. Foraging ecology of hoverflies: morphology of the mouthparts in relation to feeding on nectar and pollen in some common urban species. Ecol. Entomol. 1981;6(3):245–262. doi: 10.1111/j.1365-2311.1981.tb00612.x. [DOI] [Google Scholar]
- Gilbert F.S., Owen J. Size, shape, competition, and community structure in hoverflies (Diptera: Syrphidae) J. Anim. Ecol. 1990;59:21–39. doi: 10.2307/5156. [DOI] [Google Scholar]
- Goodell K. Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecologia. 2003;134:518–527. doi: 10.1007/s00442-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Goras G., Tananaki C., Dimou M., Tscheulin T., Petanidou T., Thrasyvoulou A. Impact of honey bee (Apis mellifera L.) density on wild bee foraging behaviour. J. Apic. Sci. 2016 doi: 10.1515/JAS-2016-0007. [DOI] [Google Scholar]
- Gray A., et al. Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: the combined effects of operation size, migration and queen replacement. J. Apic. Res. 2023;62:204–210. doi: 10.1080/00218839.2022.2113329. [DOI] [Google Scholar]
- Henry M., Béguin M., Requier F., Rollin O., Odoux J.F., et al. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Henry M., Cerrutti N., Aupinel P., Decourtye A., Gayrard M., et al. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proc. R. Soc. B. 2015;282 doi: 10.1098/rspb.2015.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M., Rodet G. Controlling the impact of the managed honeybee on wild bees in protected areas. Sci. Rep. 2018;8:9308. doi: 10.1038/s41598-018-27591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbertsson L., Lindström S.A.M., Rundlöf M., Bommarco R., Smith H. Competition between managed honeybees and wild bumblebees depends on landscape context. Basic Appl. Ecol. 2016;17(7):609–616. doi: 10.1016/j.baae.2016.05.001. [DOI] [Google Scholar]
- Horskins K., Turner V.B. Resource use and foraging patterns of honeybees, Apis mellifera, and native insects on flowers of Eucalyptus costata. Aust. J. Ecol. 1999;24(3):221–227. doi: 10.1046/j.1442-9993.1999.00965.x. [DOI] [Google Scholar]
- Howlett B.G., et al. Using non-bee and bee pollinator-plant species interactions to design diverse plantings benefiting crop pollination services. Adv. Ecol. Res. 2021;64:45–103. doi: 10.1016/bs.aecr.2020.11.002. [DOI] [Google Scholar]
- Hudewenz A., Klein A.M. Red mason bees cannot compete with honey bees for floral resources in a cage experiment. Ecol. Evol. 2015;5(21):5049–5056. doi: 10.1002/ece3.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K.L.J., Kingston J.M., Lee A., Holway D.A., Kohn J.R. Non-native honey bees disproportionately dominate the most abundant floral resources in a biodiversity hotspot. Proc. R. Soc. B. 2019 doi: 10.1098/rspb.2018.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki J.M., Hogendoorn K. Mounting evidence that managed and introduced bees have negative impacts on wild bees: an updated review. Curr. Res. Insect Sci. 2022 doi: 10.1016/j.cris.2022.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeavons E., van Baaren J., Le Lann C. Resource partitioning among a pollinator guild: a case study of monospecific flower crops under high honeybee pressure. Acta Oecologica. 2020;104 doi: 10.1016/j.actao.2020.103528. [DOI] [Google Scholar]
- Kato M., Kawakita A. Plant-pollinator interactions in New Caledonia influenced by introduced honey bees. Am. J. Bot. 2004;91(11):1814–1827. doi: 10.3732/ajb.91.11.1814. [DOI] [PubMed] [Google Scholar]
- Kendall L.K., Mola J.M., Portman Z.M., Cariveau D.P., Smith H.G., Bartomeus I. The potential and realized foraging movements of bees are differentially determined by body size and sociality. Ecology. 2022;103:e3809. doi: 10.1002/ecy.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka J., Hadrava J., Biella P., Akter A. Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant-pollinator network. PeerJ. 2018;6:e6025. doi: 10.7717/peerj.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh I., Lonsdorf E.V., Williams N.M., Brittain C., Isaacs R., et al. Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. U.S.A. 2016;113:140–145. doi: 10.1073/pnas.1517685113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte K. Competition and species diversity: removal of dominant species increases diversity in Costa Rican butterfly communities. Oikos. 2008;117(1):69–76. doi: 10.1111/j.2007.0030-1299.16125.x. [DOI] [Google Scholar]
- Lázaro A., et al. Impacts of beekeeping on wild bee diversity and pollination networks in the Aegean Archipelago. Ecography. 2021;44:1–3. doi: 10.1111/ecog.05553. [DOI] [Google Scholar]
- Leguizamón Y., Debandi G., Vázquez D.P. Managed honeybee hives and the diversity of wild bees in a dryland nature reserve. Apidologie (Celle) 2021;52:991–1001. doi: 10.1007/s13592-021-00882-6. [DOI] [Google Scholar]
- Levitt A., Singh L.R., Cox-Foster D.L., Rajotte E., Hoover K., Ostiguy N., Holmes E.C. Cross-species transmission of honey bee viruses in associated arthropods. Virus Res. 2013;76(1–2):232–240. doi: 10.1016/j.virusres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Lindstrom S.A.M., Herbertsson L., Rundlof M., Bommarco R., Smith H.G. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B. 2016;283:1843. doi: 10.1098/rspb.2016.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei T., Marzi G. Honey bees collecting pollen from the body surface of foraging bumble bees: a recurring behaviour. Apidologie (Celle) 2024;55(1):4. doi: 10.1007/s13592-023-01049-1. [DOI] [Google Scholar]
- Lucas A., Bodger O., Brosi B.J., et al. Floral resource partitioning by individuals within generalised hoverfly pollination networks revealed by DNA metabarcoding. Sci. Rep. 2018;8:5133. doi: 10.1038/s41598-018-23103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A., Bodger O., Brosi B.J., Ford C.R., Forman D.W., Greig C., Hegarty M., Neyland P.J., de Vere N. Generalisation and specialisation in hoverfly (Syrphidae) grassland pollen transport networks revealed by DNA metabarcoding. J. Anim. Ecol. 2018;87:1008–1021. doi: 10.1111/1365-2656.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis G., Normandin E., Ziter C.D. Decline in wild bee species richness associated with honey bee (Apis mellifera L.) abundance in an urban ecosystem. PeerJ. 2023;11:e14699. doi: 10.7717/peerj.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKell S., Elsayed H., Colla S. Assessing the impacts of urban beehives on wild bees using individual, community, and population-level metrics. Urban Ecosyst. 2023;26(5):1–15. doi: 10.1007/s11252-023-01374-4. [DOI] [Google Scholar]
- Magrach A., González-Varo J.P., Boiffier M., et al. Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nat. Ecol. Evol. 2017;1:1299–1307. doi: 10.1038/s41559-017-0249-9. [DOI] [PubMed] [Google Scholar]
- Mallick S.A., Driessen M.M. Impacts of hive honeybees on Tasmanian leatherwood Eucryphia lucida Labill. (Eucryphiaceae) Austral Ecol. 2009;34(2):185–195. doi: 10.1111/j.1442-9993.2008.01920.x. [DOI] [Google Scholar]
- Mallinger R.E., Gaines-Day H.R., Gratton C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS ONE. 2017;12(12) doi: 10.1371/journal.pone.0189268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune F., Normandin É., Mazerolle M.J., Fournier V. Response of wild bee communities to beekeeping, urbanization, and flower availability. Urban Ecosyst. 2020;23:39–54. doi: 10.1007/s11252-019-00909-y. [DOI] [Google Scholar]
- Morandin L.A., Kremen C. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecol. Appl. 2013;23:829–839. doi: 10.1890/12-1051.1. [DOI] [PubMed] [Google Scholar]
- Morse D.H. Interactions Among Syrphid Flies and Bumblebees on Flowers. Ecology. 1981;62:81–88. doi: 10.2307/1936671. [DOI] [Google Scholar]
- Mouillard-Lample L., Gonella G., Decourtye A., Henry M., Barnaud C. Competition between wild and honey bees: floral resources as a common good providing multiple ecosystem services. Ecosyst. Serv. 2023;62 doi: 10.1016/j.ecoser.2023.101538. [DOI] [Google Scholar]
- Muinde J., Katumo D.M. Beyond bees and butterflies: the role of beetles in pollination system. J. Nat. Conserv. 2024;77 doi: 10.1016/j.jnc.2023.126523. [DOI] [Google Scholar]
- Nielsen A., Dauber J., Kunin W.E., Lamborn E., Jauker B., Moora M., Potts S.G., Reitan T., Roberts D., Sober V., Settele J., Steffan-Dewenter I., Stout J.C., Tscheulin T., Vaitis M., Vivarelli D., Biesmeijer J.C., Petanidou T. Pollinator community responses to the spatial population structure of wild plants: a pan-European approach. Basic Appl. Ecol. 2012;13(6):489–499. doi: 10.1016/j.baae.2012.08.008. [DOI] [Google Scholar]
- Öckinger E., Smith H.G. Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J. Appl. Ecol. 2007;44:50–59. doi: 10.1111/j.1365-2664.2006.01250.x. [DOI] [Google Scholar]
- Orford K.A., Vaughan I.P., Memmott J. The forgotten flies: the importance of non-syrphid Diptera as pollinators. Proc. R. Soc. B. 2015;282:1805. doi: 10.1098/rspb.2014.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C.R.V., Roth C.L., Carlson B.L., Smart M.D. Proceedings of the National Academy of Sciences of the United States of America113. 2016. Land-use change reduces habitat suitability for supporting managed honey bee colonies in the Northern Great Plains; pp. 10430–10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.L., Nicholson C.C., Brennan R.M., Britzman A.T., Greer J., Hemberger J.…Williams N.M. A meta-analysis of single visit pollination effectiveness comparing honeybees and other floral visitors. Am. J. Bot. 2021;108(11):2196–2207. doi: 10.1002/ajb2.1764. [DOI] [PubMed] [Google Scholar]
- Page M.L., Williams N.M. Evidence of exploitative competition between honey bees and native bees in two California landscapes. J. Anim. Ecol. 2023;00:1–13. doi: 10.1111/1365-2656.13973. [DOI] [PubMed] [Google Scholar]
- Page M.L., Williams N.M. Honey bee introductions displace native bees and decrease pollination of a native wildflower. Ecology. 2023;104(2):e3939. doi: 10.1002/ecy.3939. [DOI] [PubMed] [Google Scholar]
- Pérez-Méndez N., Andersson G.K.S., Requier F., Hipólito-Sousa J., Aizen M., Morales C., García N., Gennari G., Garibaldi L.A. The economic cost of losing native pollinator species for orchard production. J. Appl. Ecol. 2020;57(3):599–608. doi: 10.1111/1365-2664.13561. [DOI] [Google Scholar]
- Peterson J.H., Roitberg B.D. Impacts of flight distance on sex ratio and resource allocation to offspring in the leafcutter bee, Megachile rotundata. Behav. Ecol. Sociobiol. (Print) 2006;59:589–596. doi: 10.1007/s00265-005-0085-9. [DOI] [Google Scholar]
- Phiri B.J., Fèvre D., Hidano A. Uptrend in global managed honey bee colonies and production based on a six-decade viewpoint, 1961–2017. Sci. Rep. 2022;12:21298. doi: 10.1038/s41598-022-25290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polatto L.P., Chaud-Netto J. Influence of Apis mellifera L. (Hymenoptera: Apidae) on the Use of the Most Abundant and Attractive Floral Resources in a Plant Community. Neotrop. Entomol. 2013;42:576–587. doi: 10.1007/s13744-013-0165-x. [DOI] [PubMed] [Google Scholar]
- Potts S., Imperatriz-Fonseca V., Ngo H., Aizen M.A., Biesmeijer J.C., Breeze T.D., Dicks L.V., Garibaldi L.A., Hill R., Settele J., Vanbergen A.J. Safeguarding pollinators and their values to human well-being. Nature. 2016;540:220–229. doi: 10.1038/nature20588. [DOI] [PubMed] [Google Scholar]
- Prado A., Pioz M., Vidau C., Requier F., Brunet J.L., Jury M., Le Conte Y., Alaux C. Exposure to pollen-bound pesticide mixtures induces longer-lived but less efficient honey bees. Sci. Total Environ. 2019;650:1250–1260. doi: 10.1016/j.scitotenv.2018.09.102. [DOI] [PubMed] [Google Scholar]
- Prendergast K.S., Ollerton J. Impacts of the introduced European honeybee on Australian bee-flower network properties in urban bushland remnants and residential gardens. Austral Ecol. 2022;47:35–53. doi: 10.1111/aec.13040. [DOI] [Google Scholar]
- Proesmans W., Bonte D., Smagghe G., et al. Small forest patches as pollinator habitat: oases in an agricultural desert? Landsc. Ecol. 2019;34:487–501. doi: 10.1007/s10980-019-00782-2. [DOI] [Google Scholar]
- Rader R., Batomeus I., Garibaldi L., Garratt M.P.D., Howlett B., et al. Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. U.S.A. 2016;113:146–151. doi: 10.1073/pnas.1517092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader R., Cunningham S.A., Howlett B.G., Inouye D.W. Non-Bee Insects as Visitors and Pollinators of Crops: biology, Ecology, and Management. Annu. Rev. Entomol. 2020;65:391–407. doi: 10.1146/annurev-ento-011019-025055. [DOI] [PubMed] [Google Scholar]
- Requier F., Antúnez K., Morales C.L., Aldea Sánchez P., Castilhos D., Garrido M., Giacobino A., Reynaldi F.J., Rosso Londoño J.M., Santos E., Garibaldi L.A. Trends in beekeeping and honey bee colony losses in Latin America. J. Apic. Res. 2018;57(5):657–662. doi: 10.1080/00218839.2018.1494919. [DOI] [Google Scholar]
- Requier F., Jowanowitsch K.K., Kallnik K., Steffan-Dewenter I. Limitation of complementary resources affects colony growth, foraging behavior and reproduction in bumble bees. Ecology. 2020;101(3):e02946. doi: 10.1002/ecy.2946. [DOI] [PubMed] [Google Scholar]
- Requier F., Odoux J., Tamic T., Moreau N., Henry M., Decourtye A., Bretagnolle V. Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecol. Appl. 2015;25:881–890. doi: 10.1890/14-1011.1. [DOI] [PubMed] [Google Scholar]
- Requier F., Odoux J.-F., Henry M., Bretagnolle V. The carry-over effects of pollen shortage decrease the survival of honeybee colonies in farmlands. J. Appl. Ecol. 2017;54:1161–1170. doi: 10.1111/1365-2664.12836. [DOI] [Google Scholar]
- Requier F., Pérez-Méndez N., Andersson G.K.S., Blareau E., Merle I., Garibaldi L.A. Bee and non-bee pollinator importance for local food security. Trends Ecol. Evol. 2023;38(2):196–205. doi: 10.1016/j.tree.2022.10.006. [DOI] [PubMed] [Google Scholar]
- Requier F., Sibaja Leyton M., Morales C.L., et al. First large-scale study reveals important colony losses of managed honey bees and stingless bees in Latin America. Sci. Rep. 2024;14:10079. doi: 10.1038/s41598-024-59513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropars L., Affre L., Aubert M., Fernandez C., Flacher F., Genoud D., Guiter F., Jaworski C., Lair X., Mutillod C., Nève G., Schurr L., Geslin B. Pollinator specific richness and their interactions with local plant species: 10 years of sampling in Mediterranean habitats. Environ. Entomol. 2020;49:947–955. doi: 10.1093/ee/nvaa061. [DOI] [PubMed] [Google Scholar]
- Ropars L., Affre L., Schurr L., Placher F., Genoud D., Mutilod C., Geslin B. Land cover composition, local plant community composition and honeybee colony density affect wild bee species assemblages in a Mediterranean biodiversity hot-spot. Acta Oecologica. 2020;104 doi: 10.1016/j.actao.2020.103546. [DOI] [Google Scholar]
- Ropars L., Affre L., Thébault E., Geslin B. Seasonal dynamics of competition between honey bees and wild bees in a protected Mediterranean scrubland. Oikos. 2022:e08915. doi: 10.1111/oik.08915. [DOI] [Google Scholar]
- Ropars L., Dajoz I., Fontaine C., Muratet A., Geslin B. Wild pollinator activity negatively related to honey bee colony densities in urban context. PLoS ONE. 2019;14(9) doi: 10.1371/journal.pone.0222316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubik D., Wolda H. Do competing honey bees matter? Dynamics and abundance of native bees before and after honey bee invasion. Popul. Ecol. 2001;43:53–62. doi: 10.1007/PL00012016. [DOI] [Google Scholar]
- Roubik D.W., Moreno J.E., Vergara C., Wittman D. Sporadic food competition with the African honey bee: projected impact on neotropical social bees. J. Trop. Ecol. 1986;2(2):97–111. doi: 10.1017/S0266467400000699. [DOI] [Google Scholar]
- Schneider F. Beitrag zur Kenntnis der Generationsverhaltnisse und Diapause rauberischer Schwebfliegen (Syrphldae, Dipt.) Mittl. Schweiz Ent Ges. 1948;21:249–285. [Google Scholar]
- Schurr L., Geslin B., Affre L., Gachet S., Delobeau M., Brugger M., Bourdon S., Masotti V. Landscape and local drivers affecting flying insects along fennel crops (Foeniculum vulgare, apiaceae) and implications for its yield. Insects. 2021;12(5):404. doi: 10.3390/insects12050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladonja B., Tlak Gajger I., Uzelac M., Poljuha D., Garau C., Landeka N., Barták M., Bacaro G. The impact of beehive proximity, human activity and agricultural intensity on Diptera diversity in a Mediterranean mosaic of agroecosystems, with a focus on pest species. Animals. 2023;13:1024. doi: 10.3390/ani13061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ramírez C., Ramos-Jiliberto R., Valdovinos F.S., Martínez P., Castillo J.A., Armesto J.J. Decadal trends in the pollinator assemblage of Eucryphia cordifolia in Chilean rainforests. Oecologia. 2014;176:157–169. doi: 10.1007/s00442-014-3000-0. [DOI] [PubMed] [Google Scholar]
- Sponsler D., Dominik C., Biegerl C., Honchar H., Schweiger O., Steffan-Dewenter I. High rates of nectar depletion in summer grasslands indicate competitive conditions for pollinators. Oikos. 2024:e10495. doi: 10.1111/oik.10495. [DOI] [Google Scholar]
- Sponsler D., Iverson A., Steffan-Dewenter I. Pollinator competition and the structure of floral resources. Ecography. 2023 doi: 10.1111/ecog.06651. [DOI] [Google Scholar]
- St Clair A.L., Zhang G., Dolezal A.G., O'Neal M.E., Toth A.L. Agroecosystem landscape diversity shapes wild bee communities independent of managed honey bee presence. Agric. Ecosyst. Environ. 2022;327 doi: 10.1016/j.agee.2021.107826. [DOI] [Google Scholar]
- Steffan-Dewenter I., Tscharntke T. Resource overlap and possible competition between honey bees and wild bees in central Europe. Oecologia. 2000;122:288–296. doi: 10.1007/s004420050034. [DOI] [PubMed] [Google Scholar]
- Tepedino V.J., Alston D.G., Bradley B.A., Toler T.R., Griswold T.L. Orchard pollination in Capitol Reef National Park, Utah, USA. Honey bees or native bees? Biodivers. Conserv. 2007;16:3083–3094. doi: 10.1007/s10531-007-9164-8. [DOI] [Google Scholar]
- Tepedino V.J., Torchio P.F. Temporal variability in the sex ratio of a non-social bee, Osmia lignaria propinqua: extrinsic determination or tracking of an optimum? Oikos. 1982;38:177–182. doi: 10.2307/3544017. [DOI] [Google Scholar]
- Thomson D. Competitive interactions between the invasive European honey bee and native bumble bees. Ecology. 2004;85:458–470. doi: 10.1890/02-0626. [DOI] [Google Scholar]
- Timberlake T.P., Vaughan I.P., Memmott J. Phenology of farmland floral resources reveals seasonal gaps in nectar availability for bumblebees. J. Appl. Ecol. 2019;56:1585–1596. doi: 10.1111/1365-2664.13403. [DOI] [Google Scholar]
- Torné-Noguera A., Rodrigo A., Osorio S., Bosch J. Collateral effects of beekeeping: impacts on pollen-nectar resources and wild bee communities. Basic Appl. Ecol. 2016;17(3):199–209. doi: 10.1016/j.baae.2015.11.004. [DOI] [Google Scholar]
- Travis D.J., Kohn J.R. Honeybees (Apis mellifera) decrease the fitness of plants they pollinate. Proc. R. Soc. B. 2023;290(2001) doi: 10.1098/rspb.2023.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valido A., Rodríguez-Rodríguez M.C., Jordano P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019;9:4711. doi: 10.1038/s41598-019-41271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venjakob C., Ruedenauer F.A., Klein A.M., Leonhardt S.D. Variation in nectar quality across 34 grassland plant species. Plant Biol. 2022;24(1):134–144. doi: 10.1111/plb.13343. [DOI] [PubMed] [Google Scholar]
- Weaver J.R., Ascher J.S., Mallinger R.E. Effects of short-term managed honey bee deployment in a native ecosystem on wild bee foraging and plant–pollinator networks. Insect Conserv. Divers. 2022;15:634–644. doi: 10.1111/icad.12594. [DOI] [Google Scholar]
- Web of Knowledge, 2022. Online Bibliographical Database from the Institute for Scientific Information. http://apps.webofknowledge.com/.
- Weekers Dominance of honey bees is negatively associated with wild bee diversity in commercial apple orchards regardless of management practices. Agric. Ecosyst. Environ. 2022;323 doi: 10.1016/j.agee.2021.107697. [DOI] [Google Scholar]
- Wojcik V.A., Morandin L.A., Adams L.D., Rourke K.E. Floral resource competition between honey bees and wild bees: is there clear evidence and can we guide management and conservation? Environ. Entomol. 2018;47(4):822–833. doi: 10.1093/ee/nvy077. [DOI] [PubMed] [Google Scholar]
- Zakardjian M., Jourdan H., Le Féon V., Geslin B. Burleigh Dodds Science Publishing; 2022. Assessing the impact of alien bees on native ones. Promoting pollination and pollinators in farming; pp. 225–256. [DOI] [Google Scholar]
- Zattara E., Aizen M.A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth. 2021;4(1):114–123. doi: 10.1016/j.oneear.2020.12.005. [DOI] [Google Scholar]
- Zurbuchen A., Cheesman S., Klaiber J., Müller A., Hein S., Dorn S. Long foraging distances impose high costs on offspring production in solitary bees. J. Anim. Ecol. 2010;79:674–681. doi: 10.1111/j.1365-2656.2010.01675.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.