Abstract
Recent advancements in the field of photoresponsive-based mercury (II) sensors have witnessed a surge in research focused on enhancing detection capabilities. Leveraging innovations in materials science, particularly with quantum dots, nanomaterials, and organic semiconductors, these sensors exhibit improved selectivity and sensitivity. Beyond traditional applications, such as environmental monitoring, the integration of photoresponsive principles with emerging technologies like the internet of things (IoT) and wearable promises real-time and remote mercury (II) ion detection. The on-going efforts also explore multifunctional sensors and miniaturization for on-site applications, addressing current challenges and paving the way for broader commercialization. This dynamic landscape underscores the potential for these sensors to play a crucial role in ensuring the effective monitoring and management of mercury (II) levels in diverse settings.
Keywords: Azobenzene, Coumarin, Fluorescein, Pyrene, Rhodamine, Quinoline
Graphical abstract
1. Introduction
Mercury contamination remains a formidable global concern, posing serious threats to ecosystems and human health due to its persistence, toxicity, and ability to biomagnify in the food chain [1]. As anthropogenic activities continue to release mercury into the environment, effective monitoring and detection strategies are paramount for timely intervention and mitigation. In response to this imperative, recent years have witnessed a remarkable surge in research dedicated to the development of advanced sensing technologies, with a particular focus on photoresponsive-based mercury (II) sensors [2,3]. This comprehensive introduction navigates through the multifaceted landscape of mercury pollution, elucidating the environmental ramifications, traditional detection methodologies, and the evolving role of photoresponsive -based sensors in addressing the shortcomings of conventional approaches. The pervasive impact of mercury contamination spans terrestrial, aquatic, and atmospheric domains, making it a truly global environmental challenge [[4], [5], [6], [7], [8]]. Industrial processes, coal combustion, and artisanal gold mining are prominent sources of mercury emissions, contributing to its ubiquitous presence in air, water, and soil. Once released, mercury undergoes complex biogeochemical transformations, ultimately leading to the formation of methylmercury, a highly toxic compound that accumulates in organisms, posing severe health risks to both wildlife and human populations. Understanding the intricate pathways through which mercury cycles in the environment is essential for devising targeted and effective monitoring strategies [[9], [10], [11], [12]].
Traditional methods for detecting mercury ions, including atomic absorption spectrometry and fluorescence-based techniques, have played crucial roles in understanding mercury distribution. However, the procedures often suffer from limitations such as high detection limits, complex sample preparation, and limited real-time capabilities. The urgency to overcome these challenges has fueled the exploration of alternative sensing technologies, propelling photoresponsive -based sensors to the forefront of mercury detection research. The unique capability of these sensors to convert light signals into electrical responses offers a paradigm shift in the quest for enhanced sensitivity, selectivity, and real-time monitoring [[13], [14], [15]]. The heart of this paradigm shift lies in the innovative use of materials. Quantum dots, nanomaterials, and organic semiconductors have emerged as key players in amplifying the performance of photoresponsive -based mercury sensors. Quantum dots, with their tunable electronic properties and high surface area, provide a platform for precise and efficient detection. Nanomaterials, on the other hand, offer increased sensitivity through their unique structural characteristics. Organic semiconductors contribute to the development of flexible and cost-effective sensor platforms, extending the reach of these technologies to diverse applications and environments [16,17].
The versatility of photoresponsive-based sensors goes beyond mere detection. The integration of these sensors with the internet of things (IoT) and wearable devices introduces a dynamic dimension to environmental monitoring. This amalgamation allows for real-time, remote, and continuous monitoring, transforming the static nature of traditional sampling methods [18]. The implications of this integration extend into various sectors, from industrial processes to ecological studies, promising a comprehensive approach to mercury ion detection. As we delve deeper into the recent trends of photoresponsive-based mercury sensors, it becomes evident that these innovations not only address current detection challenges but also lay the groundwork for future advancements [19,20]. The following pages will unravel the intricate developments in sensor design, material synthesis, and the evolving applications of photoresponsive-based mercury (II) sensors (1). Through this exploration, we aim to elucidate the potential pathways that may shape the future of mercury sensing technologies and their broader implications for environmental sustainability and public health. This study looks at current developments in the synthesis, a design concept with photophysical characteristics, sensing mechanism, and analyte identification investigations with many parameters of photoresponsive-based mercury (II) sensors with a focus on biological applications (Fig. 1).
Fig. 1.
Schematic representation of photoresponsive-based mercury (II) sensor.
2. Azobenzene-based mercury (Hg2+) sensor
The azobenzene derivative with 1,3,4-triazole units as the basis for a highly sensitive fluorescent probe (2) for the selective detection of mercury has been devised, synthesized, and tested for its ability to recognize cations by UV–vis fluorescence. In DMF aqueous environments, the fluorescent probe shows a very selective response of fluorescence increase toward mercury (Fig. 2a) [21]. In addition, the two novel very specific colorimetric chemosensors (3) for Hg2+ have been developed and synthesized. They are based on azobenzene and highly selective Hg2+-promoted deprotection of a dithioacetal. The sensor's color changes from pale yellow to deep red, which is readily visible to the naked eye in the presence of as little as 20 mm Hg2+. Intramolecular charge transfer (ICT) is the fundamental signaling process. The Hg2+ selectivity of the sensors is good for several typical alkaline substances, alkaline earth, and transition metal ions. Moreover, they don't require any extra equipment to be used for in-field measurements using a dipstick method (Fig. 2b) [22].
Fig. 2.
a). Fluorescence analysis of Azobenzene-Based Fluorescent Mercury Sensor (2). b). Absorbance analysis of Azobenzene-Based Colorimetric Chemosensors (3).
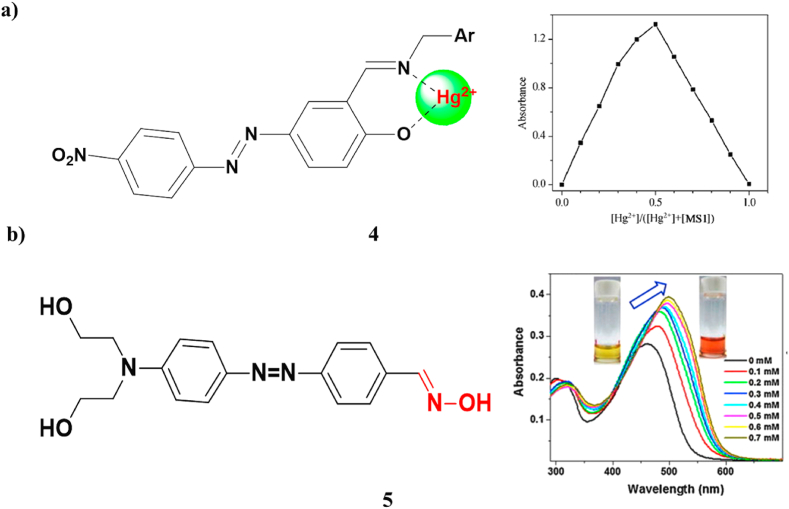
The development and characterization of a novel family of azobenzene dyes (4) based on the internal charge transfer (ICT) mechanism allowed for the colorimetric and ratiometric detection of Hg2+. In an aqueous methanol solution, the molecules containing azo and imino group functions may work in concert with Hg2+ to produce a significant blue shift from 453 to 363 nm, which corresponds to a noticeable shift in hue from orange to light yellow. This effect can be used for Hg2+ detection with the naked eye. UV–Vis spectroscopy was used to examine the sensors' sensitivity, specificity, and binding mode to Hg2+ (Fig. 3a) [23]. A molecular probe (5) was created for the dual detection of Hg2+ and F-ions, utilizing the recently discovered aldoxime chemistry. The azobenzene-containing probe (Azo-2) produced from aldoxime was created and examined. In a DMSO/H2O combination, it demonstrated remarkable sensitivity and selectivity towards ions containing mercury via an Hg2+ ion-catalyzed dehydration process at pH = 5. With the addition of Hg2+ ions, the probe showed a 70 nm red shift in its wavelength of absorption maximum along with a light yellow to dark violet color shift in the solution. It's interesting to note that Azo-2 also showed strong sensitivity and selectivity for F− ions in a DMSO solution. This was made possible by the deprotonation of oxime-OH caused by F−ions and the ensuing creation of an energetically preferred HF2− species. Visible detection of the F−ion was aided by the solution's color changing from pale yellow to a deep red. NMR spectroscopy using 1H- and 19F produced confirmation of the oxime-fluoride interaction and the production of HF2. The whole process is reversible, as demonstrated by the reaction of the oxime anion produced by F− in a DMSO solution with water molecules to revert to the originally protonated oxime (Fig. 3b) [24].
Fig. 3.
a). Ratiometric study of azobenzene dye colorimetric and chemosensors (4). b). Structure of Aldoxime-functionalized azobenzene-based molecular probe Detection of Mercury and Fluoride ions (5).
A thioacetal chemosensor (6) was created by synthesizing an azobenzene and carbazole moiety at the same time. Hg2+ was shown to be able to specifically elicit changes in both color and fluorescence among other metal ions. 1H NMR measurements validated the mechanism of Hg2+-promoted cleavage of thioacetal. The yellow CH3CN/H2O solution turned into a colorless solution when Hg2+ was added. This precipitated as a mercuric salt and left behind an azobenzene-containing thioglycolamide (Fig. 4a) [25]. A novel set of benzoyl-thiourea derivatives (7) was synthesized, and their chemo-dosimetric responses to metal cations were examined in room-temperature aqueous solutions. Only Hg2+ ions showed reactions to permanent color changes in receptors among the several metal cations that were studied, as well as characteristic blue shifts in UV/vis spectra. The Hg2+ ion may be monitored using the receptors in a water-based solution having a pH range of 4–9 (Fig. 4b) [26].
Fig. 4.
a). Photophysical study of thioacetals based fluorogenic chemosensor for Hg(II) (6). b). Absorption study of chemodosimetric detection of Hg(II) (7).
The azo chromophore and phenylthiourea construction optical probes (8) were created and used for CN- and Hg2+ detection with the naked-eye. The produced probes responded differently to CN− and Hg2+ ions in terms of colorimetry. 1H NMR titration measurements were used to study the mechanism of the probe's binding interaction between CN‾ and Hg2+ ions. The chemosensor's limit of detection and Job's plot analysis indicated that the stoichiometric ratios for Hg2+ and CN− ions were respectively 1:1 (LOD = 4.89 μM and 1:2 (LOD = 2.23 μM) (Fig. 5a) [27]. The developed and synthesized a novel straightforward fluorescent probe BAA (9) for Hg2+ in aqueous solution. By using fluorescence spectra, may be utilized to selectively identify Hg2+ in an aqueous solution over other common metal ions. The confirmation of the BAA's sensing mechanism towards Hg2+ was obtained using titration spectra, ESI-MS, and DFT calculations. With Hg2+, the probe BAA showed a 1:1 binding mode. There was a reversible interaction between BAA and Hg2+. In addition, the BAA had a quick reaction to Hg2+ and could detect Hg2+ throughout a broad pH range (3-10). Ultimately, the probe was also effectively used to image Hg2+ ions in MCF-7 live cells and detect Hg2+ in the water from the lake samples (Fig. 5b) [28].
Fig. 5.
a). Absorption study of azo-benzylidene-thiourea chemosensor (8). b). HOMO-LUMO energy gap study of fluorescent probe for Hg2+ (9).
Azobenzene-based mercury (II) sensors operate through the reversible photoisomerization of azobenzene groups, which alters the optical properties of the sensor upon mercury (II) ion binding. The binding induces a change in the azobenzene's geometric configuration from trans to cis, resulting in a detectable shift in UV–Vis absorbance or fluorescence. This mechanism offers high sensitivity and selectivity due to the strong interaction between mercury ions and azobenzene. The sensors have notable advantages, including their high sensitivity, low detection limits, and potential for integration into IoT and wearable devices for real-time monitoring. However, challenges include stability issues and the need for complex fabrication processes. Case studies, such as the integration of azobenzene sensors in wearable health monitoring devices, highlight their potential in environmental and personal health applications, demonstrating their effectiveness in detecting trace mercury levels and contributing to enhanced safety and environmental monitoring.
3. Coumarin-based mercury (Hg2+) sensor
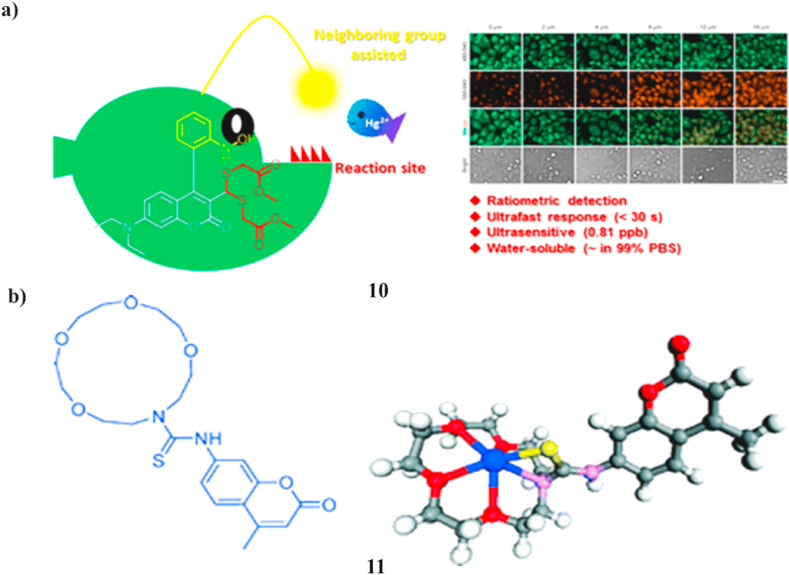
A new ultrasensitive ratiometric fluorescence probe has been created to detect mercury ions through the particular desulfurization of dithioacetal that is activated by mercury. In an aqueous solution, this probe (10) demonstrates an effective color response and fluorescence in response to Hg2+. It is superior to most reported ones due to its quick response kinetics, low limit of detection (∼0.81 ppb), high selectivity, and visible light emission (450–650 nm). The application of it to ratiometrically Hg2+ pictures in living HeLa cells has been executed easily (Fig. 6a) [29]. A quick and high-yield synthetic process was used to develop and create a water-soluble, fluorescent ion probe (11) with excellent selectivity for Hg2+ ions. The probe is a member of the turn-on sensor family. It operates by the process of photoinduced electron transfer, producing a dye that may be used as a tool for the identification and measurement of mercury in biological and environmental samples (Fig. 6b) [30].
Fig. 6.
a).Live cell imaging studies of Coumarin-Based Hg2+ Fluorescent Probe (10). b). PET study of coumarin-based fluorescent sensor (11).
Triazolyl coumarin derivatives (12) were synthesized as fluorescence sensors to investigate their selectivity and binding ability toward metal ions. These derivatives included spacer groups between the coumarin and triazole groups and ones without. With fluorescence intensification, it demonstrated a remarkable selectivity toward Hg2+ in polar protic solvents MeOH–CHCl3. Moreover, it was shown to bind two Hg2+ at a high concentration (>12.5 mM) of Hg(ClO4)2. It displayed a binding stoichiometry concerning a single Hg2+. Based on the findings of the ligands' 1H NMR titration, IR, and fluorescence sensing (Fig. 7a) [31]. The development of a novel colorimetric and fluorescent chemosensor (13) based on 7- (diethylamino)-3-(pyrimidin-4-yl)-2H-chromen-2-one (PYC) has been proposed and made feasible the detection of Hg2+ in the presence of other competing metals in mixed aquatic environments. When exposed to Hg2+, the PYC's color changes from green to red to a yellow-green color to light orange in fluorescence. The absorption spectra also exhibit a redshift of around 80 nm in wavelength. Test strips 15 based on PYC were created to serve as practical and effective Hg2+ test kits (Fig. 7b) [32].
Fig. 7.
a). Mechanism of triazolyl coumarins based Hg2+chemosensors (12). b). ICT study of Coumarinyl-appended Pyrimidine based Colorimetric Hg2+ Sensor (13).
A novel colorimetric and ratiometric fluorescent chemo-dosimeter (14) based on intramolecular charge transfer (ICT) has been logically conceived and established for the detection of Hg2+. The probe functions through the particular desulfurization process of thiocoumarin to coumarin, which is encouraged by mercury. It demonstrates a low LOD of 1.85 ppb and great selectivity and sensitivity in a nearly pure water solution. Moreover, it was effectively employed for Hg2+ fluorescence imaging in living cells (Fig. 8a) [33]. The method of 7-hydroxycoumarin phototautomerism in an aqueous solution served as the foundation for the creation of a novel luminous polymer (15) for Hg2+ detection. The developed method showed higher sensitivity and selectivity towards Hg2+ in acetonitrile/water combination compared to several other metal ions with significant suppression of the principal emission band in fluorescence. It was shown that the 7-hydroxycoumarin photo-tautomers chemical equilibrium is changed in the presence of Hg2+ by the excited state of the intramolecular proton exchange (ESIPT) mechanism, which results in a ratiometric fluorescence response after stepwise addition of Hg2+. A comparison of the initial alkynylated coumarin's 1H NMR spectra in polar and non-polar aprotic solvents explained the happening of the ESIPT process. Further confirmation of this came from the 1H NMR of 4 obtained by adding Hg2+ gradually in polarised aprotic solvent. Titration experiments revealed a higher sensitivity with a threshold for detection at the nanomolar level (50 nM) as well as a faster and reversible reaction. The created systems practical uses were examined using actual samples, yielding positive outcomes for both freshwater and lakeside samples (Fig. 8b) [34].
Fig. 8.
a). Live-Cell Imaging of Thiocoumarin-Based Colorimetric Hg2+ sensor (14). b). Mechanism of coumarin-triazole polymer-based ESIPT-based Hg2+ detection (15).
The design and manufacture of fluorescence-based devices for in-field Hg2+ detection and screening for ecological and industrial samples remains a tough problem, even though fluorescent sensing molecules have been suggested and investigated to indicate Hg2+ ion binding. Three novel coumarin-based fluorescent chemosensors (16) with mixed thia/aza macrocyclic frameworks as receptor units have been synthesized and characterized. By revealing an OFF-ON selective response to Hg2+ ions in MeCN/H2O, these probes made it possible to image this metal ion in Cos-7 cells when performed in vitro. When ligands are reinforced on polyvinyl chloride (PVC)-based polymeric membranes or incorporated into silica core–polyethylene glycol (PEG) shell nanoparticles, they may also detect Hg2+ ions in pure water with selectivity. Specifically, an optical detecting array based on the computer's screen photo-assisted method (CSPT) was created, utilizing the fluorescence characteristics of ligands. The device responds quantitatively to Hg2+ ions in samples of natural water when the ligand is distributed across PVC membranes (Fig. 9a) [35]. The Cu(I) catalyzed Huisgen cycloaddition to create a triazole-bridged coumarin conjugated quinoline sensor (17) that showed excellent selectivity for hazardous Hg2+. Remarkably, a time-resolved fluorescence analysis has shown that no indication of the transfer of energy from the quinoline molecule to coumarin has been discovered. TDDFT simulations corroborate the potential binding mechanism of this sensor to Hg2+, which has been determined by NMR analysis, steady-state and time-resolved fluorescence spectroscopy, and other methods. Because the sensor is non-toxic and permeable to cell membranes, it can be used for intracellular Hg2+ detection (Fig. 9b) [36].
Fig. 9.
a). Fluorescence studies of Coumarin-Based Luminous Hg2+Chemosensors (16). b). FRET studies of triazole bridged coumarin-hydroxyquinoline based Hg2+ sensor (17).
Novel reactive fluorescent probes (18) towards ions composed of mercury were created by utilizing the well-known coumarin chromophore and the unique Hg2+-promoted deprotection process of dithioacetals. It showed impressive fluorescence quenching with the incorporation of mercury ions, which helped to provide a strong signal output throughout the sensing process. However, its fluorescence (60 nm) and absorption (60 nm) spectra both displayed a notable red shift with a detection limit of 90 nM. It responded preferentially to Hg2+ under this particular chemical reaction, and changes in the apparent absorption and emission spectra could only be caused by the addition of Hg2+. Furthermore, it was effectively used in ratiometric fluorescent techniques for microscopic imaging to detect Hg2+ in HeLa cells (Fig. 10a) [37]. A perimidine moiety was included in the design and synthesis of a reaction-based fluorescent probe PIC (19) intended for Hg2+ detection. At physiological pH, it can preferentially separate Hg2+ with a 42-fold fluorescence amplification from the remaining metal ions. The upper detection limit of Hg2+ with this probe is 1.08 μM. It may be used to detect Hg2+ in blue-emitting cells in real-time (Fig. 10b) [38].
Fig. 10.
a). Bioimaging studies of Thioacetalized coumarin-based fluorescent sensor (18). b). Zebrafish studies of Coumarin-based Hg2+ fluorescent sensor (19).
A novel coumarin-based N-[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl] was successfully synthesized for the first time. The fluorescent monomer of acrylamide (OCTAA) was achieved. When metal ions are present, the fluorescence characteristics of the OCTAA monomer (20) are quenched. The polymerization was verified by the use of 13C NMR and FTIR spectroscopy. The purpose of the investigation using X-ray photoelectron spectroscopy is to assess the functional groups engaged in the complexation process with mercury. IIP's morphology was examined using the FE-SEM. The BET surface area of NIP and IIP was found to be 48.1 m2g −1 and 76.90 m2g −1 in nitrogen adsorption-desorption studies. With a response spectrum that is linear ranging from 0.05 to 1.2 μmol L−1 and a limit of detection of 0.020 μmol L−1, the IIP fluorescence was recorded at 450 nm. Mercury was effectively detected from a genuine water sample using the fluorescent Hg(II)-IIP (Fig. 11a) [39]. A novel fluorescence sensor (21, PPC-S) for Hg2+ that relies on the coumarin chromophore based on pyrazolo[3,4-b]pyridine aggregation-induced emission (AIE) (PPC–O). PPC-S that is AIE inactive after the desulfurization process with Hg2+ may be converted to PPC-O that is AIE active. This PPC-O can then be used for the fluorescence turn-on detection of Hg2+ in aqueous solutions with appropriate selectivity and sensitivity (Fig. 11b) [40].
Fig. 11.
a). Nanomolar study of Coumarin-based Fluorescent Hg(II) sensor (20). b). AIE activity of pyrazolo[3,4-b]pyridine-based coumarin chromophores (21).
Mercury has long been recognized to have detrimental effects on biological health, which is why the creation of fluorescent sensors (22) for Hg2+ has received a lot of interest. Using time-dependent density functional theory, the optical characteristics of two recently synthesized Hg2+ chemical sensors based on the coumarin-rhodamine system were thoroughly examined. It is demonstrated that Probes are efficient ratiometric fluorescent Hg2+ probes that identify Hg2+ via bond energy transfer processes and Förster resonance energy transfer, respectively. Thus, it is shown how the spacer group between the acceptor and the donor affects the probe's ability to sense. In particular, these two probes' two-photon absorption characteristics are computed. Significant two-photon responses are seen by the probes in the near-infrared light spectrum. However, the presence of Hg2+ only significantly increases Probe's maximum two-photon absorption cross-section, suggesting that Probe may be used as a two-photon stimulated fluorescent probe for Hg2+. Theoretical research might establish a connection between the optical characteristics and probe structure, offering insights into the creation of effective two-photon fluorescence sensors for in vivo biological imaging of Hg2+ (Fig. 12) [41].
Fig. 12.
Theoretical Studies of Coumarin-Rhodamine based Hg2+ Probes (22).
Coumarin-based mercury (II) sensors operate primarily through fluorescence quenching or colorimetric changes upon mercury ion binding, exploiting the chelating properties of coumarin derivatives. The sensing mechanism involves mercury (II) ions coordinating with the coumarin-based ligands, leading to a measurable decrease in fluorescence or a color shift due to altered electronic states. These sensors are advantageous due to their high sensitivity, rapid response, and ease of functionalization. However, they face limitations such as potential interference from other metal ions and limited stability under harsh conditions. In real-world applications, such as IoT and wearable devices, coumarin-based sensors have been integrated into water quality monitoring systems and environmental sensing devices, demonstrating their practical utility and effectiveness in continuous, remote, and real-time detection.
4. Fluorescein-based mercury (Hg2+) sensor
Two rhodamine B fluorophores are linked to a fluorescein-based fluorescent chemosensor and "naked-eye" indicator (23) via hydrazide moieties. It was created and manufactured to detect Hg2+ with extreme sensitivity and selectivity. The fluorescein energy donor and the ring-opened rhodamine B receivers were connected to the sensor system via the FRET mechanism. Both fluorescence amplification and a chromogenic shift (from monochrome to pink) were indicative of the binding to Hg2+. It is demonstrated that the sensor can distinguish between different foreign metal ions, with a detection limit of 2.02 × 10−8 M for Hg2+ (Fig. 13a) [42]. The synthesis of a fluorescein hydrazone-based conjugate (24) involves the coupling of fluorescein hydrazide with 2-(Pyridin-2- ylmethoxy)-napthlene-1-carbaldehyde. It may be characterized using a variety of spectroscopic methods, including IR, 1H, 13C NMR, and ESI-MS. In comparison to other metal ions, it demonstrated a high degree of sensitivity and selectivity towards hazardous Hg2+ ions in the semi-aqueous medium. Notably, the emission of fluorescence centered at 520 nm was found to be significantly enhanced due to the ring opening of the spirolactam molecule in the fluorescein structure brought on by Hg2+. The binding constant of Hg2+ was determined to be (0.43 ± 0.04) x 104 M−1 with detection limits of 1.24 μM. The 1:1 binding of Hg2+ was established using Job's technique and validated by ESI-MS tests. Due to a larger binding force among Hg2+ and S2−, S2− grabbed the Hg2+ in the complex, quenching the intensity of the fluorescence. DFT experiments established the putative coordinated environment in the Hg+ complex. Instruments with logic gate functions can make use of the fluorescence "OFF-ON-OFF" mode, which was studied in the event of Hg2+ and S2−. Additionally, they show little cytotoxicity and biocompatibility, making them appropriate for fluorescent cell visualization of Hg2+ ions in living HepG2 cells (Fig. 13b) [43].
Fig. 13.
a). FRET studies of fluorescent Hg2+-sensor (23). b). Cell-Imaging studies of fluorescein-based Hg2+chemosensor (24).
The fluorescein-based fluorescent Hg2+ sensor (25) that uses EDPMA as a receptor was created and used to successfully image Hg2+ in live cells. It shows good selectivity and reactivity for detecting Hg2+ with a 51-fold increase in aqueous solution, and it displays a characteristic fluorescein emission band at 539 nm (Fig. 14a) [44]. The synthesis of a new symmetrical diarylethene derivatives (26) with a fluorescein unit and its potential use as a fluorescent probe for the identification of metal ions in biological, industrial, and environmental materials were investigated. The 1:1 binding stoichiometry showed both extremely sensitive and selective colorimetric and fluorescence reactions toward Hg2+. When Hg2+ was present, the derivative's fluorescence intensity increased noticeably, blue-shifting from 550 nm to 504 nm. Furthermore, it demonstrated several regulated transition patterns in response to chemical and light stimuli. A combinational logic gate function was built employing the intensity of fluorescence at 504 nm as the output and the light and chemical stimulation as the inputs, based on the fluorescence switching behaviors that were "OFF-ON-OFF" (Fig. 14b) [45].
Fig. 14.
a). Living cell imaging of Fluorescein-based fluorescent sensor (25). b). Photophysical study of fluorescein-linked diarylethene Hg2+ chemosensor (26).
The fluorescence probe fluorescein hydrazide (27) was created specifically for the fluorescence assay method of mercury sensing. When Hg2+ ions were present, the probe was extremely luminescent and green in color, but it was colorless and non-fluorescent otherwise. In pH 12 buffer conditions, it is very selective to Hg2+ ions. During the fluorimetric examination of the Hg2+ ions, no other ions caused any interference. The excitation wavelength for the fluorescence test was 502 nm, while the emission wavelength was 520 nm. The amount of Hg2+ ions present in the test solution is proportionate to the emission wavelength, which is measured at 520 nm. Lead acid battery samples were successfully subjected to mercury speciation using the established fluorescence test procedure, which employed fluorescein hydrazide as the fluorescent probe (Fig. 15a) [46]. A novel fluorescent sensor (28) was created and manufactured as a fluoro-ionophore for the ocular and visual-eye detection of Hg2+ in a buffered aqueous solution. It is based on the fluorescein dithia-cyclic skeleton. Alkylation, ester hydrolysis, imine production, imine reduction, and Kornblum oxidation were used in its preparation. It was demonstrated that the sensor could distinguish between different interfering metal ions and offered highly sensitive and selective ON-OFF fluorescence detection toward Hg2+. In addition, they demonstrated chromogenic alteration upon adhering to Hg2+, acting as a "visual-eye" indication that was discernible by a discernible shift in the hue of the solution from yellow to orange in color. The sensor's Hg2+ detection limit was 7.38 × 10−9, or 1.48 ppb, which was less than the amount that the US Environmental Protection Agency allowed to be present in drinking water (Fig. 15b) [47].
Fig. 15.
a). Fluorescence study of fluorescein hydrazide based Mercury sensor (27). b). Biostudy of dithia-cyclic fluorescein Hg2+-chemosensor (28).
A red-emitting sensor for mercuric ions is given together with its synthesis, photophysical characteristics, and Hg(II) binding. A thioether-rich metal-binding unit is used by Mercury Sensor (29, MS5), which is based on the seminaphthofluorescein chromophore. This sensor allows for the detection of Hg(II) in an aqueous solution in both turn-on and single-excitation dual-emission ratiometric modes. It has a Hg(II)-specific fluorescence response and is selective for Hg(II) over alkali and alkaline earth metals, mainly divalent first-row transition metal ions. Recycling-friendly MS5 binds mercury (II) reversibly. The more modest threshold for detection of 50 nM is achieved by using a 500 nM probe, while the EC50 for 1 μM MS5 is 910 nM. Additionally presented are X-ray crystallographic experiments utilizing a salicylaldehyde-based structure model for MS5. Research on naturally occurring water samples that have been tainted with mercuric salts shows that MS5 is capable of quickly identifying Hg(II) in these complicated solutions, indicating its possible use in field applications (Fig. 16a) [48]. The cation-sensing characteristics of a straightforward colorimetric and turn-on fluorescence receptor FT (30) were developed and examined. In a DMSO/H2O solution, receptor FT bound to Hg2+ and changed color selectively. The FT- Hg2+ complex's association constant was determined to be 3.03 × 109 M−1, and the Hg2+ detection limit was discovered to be 0.24 ppm (Fig. 16b) [49].
Fig. 16.
a). Photophysical properties of seminaphthofluorescein mercury sensor (29). b). Photophysical study of thiophene-based fluorescein-hydrazone derivative for Hg2+Chemosensor (30).
Fluorescein-based mercury (II) sensors operate on a fluorescence quenching mechanism, where the presence of Hg2⁺ ions leads to a significant decrease in fluorescence emission due to the interaction between the ions and the fluorescein dye. This quenching effect results from the formation of a complex that disrupts the dye's fluorescence properties. Advantages include high sensitivity and selectivity towards mercury ions, along with a relatively straightforward sensor design. However, limitations involve potential interference from other metal ions and limited stability under diverse environmental conditions. In real-world applications, such as IoT-enabled water quality monitoring and wearable devices for environmental safety, these sensors provide valuable, real-time detection of mercury contamination, enhancing both public health and safety management. Case studies highlight their use in environmental monitoring systems, demonstrating their effectiveness in detecting trace levels of mercury in industrial and natural waters.
5. Pyrene based mercury (Hg2+) sensor
A sensor for Mercury ions (Hg2+), utilizing a bottom gate top contact organic thin film transistor (OTFT), was successfully fabricated. Pyrene, including a thiol group with a significant affinity for Hg2+ ions, was used to functionalize the OTFT channel region. The OTFT sensor (31) exhibited a charge mobility of 0.28 cm2 V−1 s −1, a threshold voltage of −22.3 V, and an on-to-off ratio of 103. Compared to the other two valence metal ions, the sensor exhibits strong selectivity for Hg2+ ions. When being subjected to various concentrations of Hg2+ ions, ranging from 1 mM to 0.01 μM, the OTFT sensor demonstrated a high sensitivity to Hg2+ ions, as evidenced by an increase in drain current. In addition, the practical significance of the OTFT sensor was showcased by its capacity to detect the presence of 25 μM Hg2+ ion in tap, drinking, and ocean samples (Fig. 17a) [50]. The Hg2+ was detected by synthesizing a new long-wavelength turn-on fluorescence chemosensor (32, CS) based on pyrene. It could efficiently detect Hg2+ and generate the turn-on fluorescence emission at 607 nm under the presence of other metallic ions. In addition, there was a red shift in the absorption spectra. However, the naked eye was able to immediately witness the solution's color change from yellow to orange. The job plot, the results from electrospray ionization mass spectrometry, microscopy with scanning electrons, and calculations using density functional theory all supported the connection between CS and Hg2+. It was discovered that adding I− to the CS and Hg2+ solution might cause the fluorescence of CS to be reversible. CS demonstrated excellent sensitivity and great selectivity for Hg2+, with a 36 nm detection limit. In addition, CS might be used to detect Hg2+ using silica gel plates and test strips (Fig. 17b) [51].
Fig. 17.
a). Aquatic environments study of Pyrene-SH functionalized Hg2+ sensor (31). b). Photophysical study of pyrene Hg2+ Fluorescent Chemosensors (32).
A novel derivative of bispyrenyl azadiene (33) has been produced that exhibits cation recognition towards distinct cations. Hg2+ ions are strongly preferred by the ligand over other cations. The concurrent presence of Hg2+ ions was associated with an "OFF-ON" type of fluorescence activity. Furthermore, an ion-selective electrode (ISE) is created, and it exhibits superior selectivity for Hg2+ compared to all other tested cations. There is a 7.08 × 10−6 M lower limit of detection (Fig. 18a) [52]. Fluorescent chemosensor hybrid material (34, Py-SBA-15) was synthesized to detect Hg2+ ions in water by functionalizing mesoporous silica (SBA-15) with a modified fluorescent chromophore, 1-(4-hydroxyphenyl)-4-pyrenyl-2,3-diaza-1,3-butadiene (Py-OH). The mesoporous hybrid material was prepared using Py-OH as the precursor, which was covalently bonded to the coupling agent 3-(triethoxysilyl)propylisocyanate (TESPIC). A mesoporous hybrid substance was prepared using Py-OH covalently bonded to 3-(triethoxysilyl)propylisocyanate (TESPIC) as a substrate. Py-SBA-15's XRD measurements and TEM pictures attest to the preservation of the hexagonally structured mesoporous structure following integration. Due to a significant increase in fluorescence caused by the incorporation of mercury ions during the suspension process of Py-SBA-15. In the presence of other metal ions, it displays a remarkable preference for Hg2+ ions. Py-SBA-15 fluorescence intensity and Hg2+ concentration in the solution are positively correlated (R2 = 0.9989) with a reasonable detection limit of 1.7 × 10−7 g mL−1. More notably, it has a strong regeneration ability because the covalent bonding technique significantly reduces the dissolution impact of the sensing molecules. According to the results of this research, this hybrid material has the possible to be a useful fluorescence chemosensor for identifying Hg2+ ions (Fig. 18b) [53].
Fig. 18.
a). Photograph of emission changes of bispyrenyl derivative mercury chemosensor (33). b). Py-SBA-15 sensor mechanism for Hg2+ ions (34).
The purpose of a ratiometric fluorescence sensor (35) was to identify biothiols in water and other biological materials, such as serum and urine. Biothiols affected the complexation-induced association and reaggregation of mercury ions and pyrene-thymine ions, which in turn regulated the variations in the monomer-excimer emission of the sensor (Fig. 19a). [54]. A new type of "turn on" fluorescent chemosensor based on pyrene (36), showed a significant increase in fluorescence intensity when Hg2+ ions were present and an exceptional selectivity towards Hg2+ ions throughout a wide range of metal ions in aqueous acetonitrile. It exhibits a 12-fold increase in fluorescence towards Hg2+ demonstrating its selectivity and sensitivity. The addition of Hg2+ ions to the probe caused an apparent color change that was discernible to the naked eye when exposed to UV light. Its in vitro sensitivity to Hg2+ in candida albicans cells was determined using confocal microscopy (Fig. 19b) [55].
Fig. 19.
a). Fluorescence studies of pyrene-biothiols based Hg2+ sensor (35). b). Confocal microscopy studies of pyrene-based Hg2+ chemosensor (36).
A one-pot procedure was used to create the pyrene-based free thiol-containing Schiff base derivative (37). It is used in conjunction with live cell imaging as a fluorescence turn-on probe for Hg2+ ion detection. It demonstrated the fluorescence turn-on response to the presence of Hg2+ ions by excimer PT1-PT1* production and chelation-enhanced fluorescence (CHEF). The sensing complex's 2:1 stoichiometry was determined using UV–Vis absorption titrations and the resultant plot. Furthermore, the sensor complex's binding locations were confirmed through the ESI-mass analysis, the fluorescence reversal of PT1+Hg2+ upon the addition of Hg2+ ions and EDTA, and the 1H NMR titrations. The concentrations of the Hg2+ complex's limit of detection (LOD) and association constant (Ka) were determined using standard deviation and quadratic fits, as well as using the Benesi-Hildebrand 15 plot, accordingly. For the PT1+Hg2+ sensor system, additional investigations were conducted on the quantum yield (Φ), pH effect, time-resolved photoluminescence (TRPL) decay constant (τ) variations, and density functional theory (DFT). Crucially, the utility of PT1 as a fluorescent probe to detect Hg2+ in living cells was demonstrated by stereo fluorescence microscopy imaging in HeLa cells (Fig. 20a) [56]. A number of cyclotriphosphazene-based pyrene-functionalized chemosensors (38) with numerous binding sites were created and synthesized in a short amount of time using straightforward reaction methods, yielding excellent results. UV/Vis and fluorescence spectroscopies were used to examine their sensing behaviors towards Hg2+ ions. It showed clear selectivity and sensitivity at detection limits of up to 50 nM as being sufficient when compared to other existing techniques for the identification of mercury ions. A visible, proportional, and "TURN-ON" emission enhancement was apparent to the naked eye as a result of their combined effects of chelation-enhanced fluorescence (CHEF), C=N isomerization, and intramolecular pyrene excimer formation due to the non-covalent π-π and CH-π stacking interactions. They can also be used to detect Hg2+ in live cells because they were shown to have no lethal effect on HeLa cancer cells. Comparing the Hg2+sensing capabilities of chemosensors with three binding sites, it was shown that they were substantially more sensitive and capable of imaging mercury (II) than the comparable mono- and two-site ones. The findings show that molecular chemosensors growing ability to bind metal has increased their fluorescence detection sensitivity. This finding may be applied to different molecular systems to develop creative ways to boost chemosensor efficiency (Fig. 20b) [57].
Fig. 20.
a). Live cell application of Pyrene-based Schiff base probe fluorescent Hg2+ ions (37). b). Live cell imaging of pyrenyl-cyclotriphosphazenes based Hg(II) chemosensors (38).
UV–vis, fluorescence, and NMR spectroscopy have been used to develop, synthesize, and test new terphenyl-based reversible receptors (39) using pyrene and quinoline as the fluorophores for cation recognition towards different cations. The constructed receptors demonstrated the "Off-On" fluorescence signalling behavior for Hg2+ ions in THF and mixed aqueous environments, which is extremely sensitive and selective (Fig. 21a) [58]. A fluorogenic pyrene-amino mercaptothiadiazole (40, PYAMT) probe is used to determine Hg2+ in water samples with bioimaging applications in live cells. This method is very selective and sensitive. It showed three maxima of emission at 378, 388, and 397 nm (λex = 348 nm) and a trio of peaks of absorbance at 333, 348, and 394 nm. When 2.5 μM Hg2+ ion was added to CH3CN: H2O, it exhibited strong fluorescent quenching (96 %) with I/I0 = 0.051, although its fluorescence did not change when other metal ions were present. The Heavy atom action of the Hg2+ ion followed by electron transfer is responsible for the quenching phenomena. With a threshold for detection of as few as 0.35 nM (S/N = 3), the fluorescence intensity dropped linearly against an expansive range between 100 nM and 2.5 μM Hg2+ (R2 = 0.9937). PYAMT-Hg2+ interaction equilibrium ratio is demonstrated to be 1:1 through DFT and fluorescence studies. For an OFF-ON process, the detector can detect cysteine reversibly which has a high association constant with the Hg2+ ion, approximately 9.08 × 105 M−1. Lastly, the suggested approach is effectively used to identify Hg2+ ions in real water samples and in bioimaging investigations involving living cells ((Fig. 21b) [59].
Fig. 21.
a). Fluorescence response ofTerphenyl derivatives of fluorescent sensors (39). b). Bioimaging studies of pyrene-amino mercaptothiadiazole based Hg2+ sensor (40).
A new fluorescent probe made from pyrene-based products (41) having aggregation-induced emission (AIE) features was created. It was identified by NMR, SEM, UV–vis, fluorescence, and other methods. When compared to other metal ions in H2O/DMF solvent, it showed great sensitivity and selectivity to Hg2+ with a detection limit of 4.2 × 10−7 M. The probe- Hg2+ combination produced with a 2:1 synthesis having an additional dose of Hg2+. Also, the probe showed high output of fluorescence and extremely minimal cytotoxicity in living cells. This demonstrated the probe's prospective applications for detecting Hg2+ in both environmental and living organisms (Fig. 22a) [60]. By post-functionalizing a macroporous polyacrylamide-based cryogel by adding a pyrene component a virtually recoverable fluorescent ion-sensing cryogel (42, PAAm-Pyr) was created. The sensor has remarkable selectivity for Hg2+ even in the presence of opposing metal ions in water that is purified, while it can detect Hg2+ concentrations as low as 2 ppb in aqueous solutions. The created sensor may be cleaned with pure water and used repeatedly (Fig. 22b) [61].
Fig. 22.
a). AIE study of pyrene-based Hg2+ fluorescent probe (41). b). Fluorescence Quenching of Pyrene-Polyacrylamide-Based Mercury (II) Sensor (42).
Fluorescence quenching was observed in three novel pyrene derivatives (43) that included two triazole units when exposed to Hg2+ ions. It was discovered that the chemosensor-Hg2+ combinations had attachment constants (Ka) of 1.68 × 103 M−1, 1.57 × 103 M−1, and 1.52 × 103 M−1 in that order. Additionally, studies using fluorescence microscopy demonstrated the efficacy of a fluorescent probe in identifying Hg2+ within live cells (Fig. 23a) [62]. A new calixarene derivative (44) with two pyrene and rhodamine fluorophores bound in the 1,3-alternate conformation was created as a Hg2+ ion-selective sensor. The sensor depends on FRET coupling ring-opened rhodamine absorbance following the association of the Hg2+ ion to pyrene excimer emissions. By using FRET with an excitation at 343 nm, adding a bit of Hg2+ to a combined solution of sensor resulted in noticeably increased fluorescence at about 576 nm. They discovered that intramolecular π-π interactions produce stronger FRET bands than between molecules π-π interactions (Fig. 23b) [63].
Fig. 23.
a). Fluorescence study of pyrene-triazole based fluorescent sensors (43). b). FRET study of Pyrene Excimer-Based Calix arene Chemosensor (44).
A new pyrene derivative with an azadiene group was developed as a ratiometric chemosensor (45) for mercury in a solution of aqueous acetonitrile. The fluoroionophores "OFF-ON" type signalling behavior is caused by conformational changes brought on by metal ions, which increase the emission of strong pyrene excimer from weak pyrene monomer (Fig. 24) [64].
Fig. 24.
Ratiometric study of Azadiene-Pyrene based Hg2+Chemosensor (45).
Pyrene-based mercury (II) sensors utilize the unique photophysical properties of pyrene, which exhibit significant fluorescence changes upon interaction with mercury ions. The sensing mechanism often involves the quenching of pyrene fluorescence due to the formation of a complex between pyrene derivatives and mercury ions. This interaction results in a measurable reduction in fluorescence intensity, enabling sensitive detection. Advantages include high sensitivity, selectivity, and potential for real-time monitoring. Limitations involve photobleaching and environmental stability. In practical applications, such sensors are integrated into IoT and wearable devices for environmental monitoring and health diagnostics. For instance, pyrene-based sensors have been deployed in wearable devices for detecting mercury contamination in water and air, demonstrating their effectiveness in real-world scenarios.
6. Quinoline based mercury (Hg2+) sensor
A novel quinoline-based chemosensor (46) has been created and produced. Its ability to bind metal ions has been shown in both aqueous and organic solutions. However, chemosensors identify Hg2+ ions (Ka = 2.15 × 104 M−1) by displaying a ratiometric shift in CHCl3/CH3OH emission. In comparison with the other metal ions analyzed, it demonstrates a higher selectivity for Hg2+ ions (Ka = 9.20 × 103 M−1) (Fig. 25a) [65]. A new non-sulfur mercury fluorescent chemosensor (47) has been devised and manufactured. It uses the quinoline and benzimidazole groups as the fluorescence signal groups. Fluorescence spectroscopic variations in the H2O/DMSO solution allowed the binding site to recognize the Hg2+ cation with excellent sensitivity as well as specificity. The fluorescence colors of the solution-containing sensor changed dramatically from blue to colorless shortly after Hg2+ was added to the aqueous media, whereas other cations did not cause significant color changes. Further investigation further shows that the sensor's detection limit for the fluorescence response to Hg2+ is 9.56 × 10−9 M, well below the EPA's allowable amount of mercury in drinking water of 0.01 M. Ligand-based test strips were created to serve as practical and effective Hg2+ test kits. Therefore, the probe should be useful for detecting mercury in an aquatic environment (Fig. 25b) [66].
Fig. 25.
a). Ratiometric sensing of quinoline-based Hg2+chemosensor (46). b). Fluorescence spectra of benzimidazole-quinoline based Hg2+chemosensor (47).
Synthesis and characterization of a water-soluble fluorescence sensor based on 2-(2-(8-hydroxyquinolin)-yl)benzimidazole (48) have been completed. In buffered aqueous solution, it exhibits extremely selective and sensitive detection of Hg2+ with a fluorescence "ON-OFF" response. The nitrogen and oxygen molecules of 8-hydroxyquinoline and the imine N atom of the benzimidazole unit bind Hg2+ through a 1:1 binding stoichiometry, according to the X-ray structural analysis of the Hg2+ complex. Quantum chemistry analyses qualitatively examine the workings of stopping fluorescence and demonstrate that ligand-to-metal charge transfer (LMCT) in the state of excitation is the origin of the phenomena (Fig. 26a) [67]. The purpose of this study was to design and synthesize a new phenothiazine-based derivative (49). As a result of the development of a 1:1 metal-ligand complex, this molecule showed considerable fluorescence quenching following the introduction of Hg2+. Its exceptional sensitivity towards Hg (II) was accompanied by a fast reaction time and a good selectivity for Hg2+ over other transition metal ions. Concurrently, the experiment on cell imaging showcased the significance of the sensor in fluorescently seeing Hg2+ within biological systems (Fig. 26b) [68].
Fig. 26.
a). Ligand-tometal charge transfer study of quinoline-benzimidazole based Hg2+ fluorescent sensor (48). b). Living cells study of phenothiazine-based fluorescent sensor (49).
A chemosensor derived from benzothiazoles (50) has been developed by using the excited state proton transfer (ESIPT) mechanism. It differentiates Hg2+ from a range of physiologically significant metal ions and hazardous heavy metal ions by causing a fluorescence turn-on response only in the presence of Hg2+ ions. With a detection limit as low as 0.11 μM, it demonstrates excellent sensitivity. Calculations based on theories employing the theory of density functions, ESI-MS spectral analyses, and 1H NMR titrations all indicate the metal binding. In HeLa cells, it displays the permeability and effectiveness of their cell membrane for Hg2+ detections (Fig. 27a) [69]. A new colorimetric and fluorescent "ON-OFF" chemosensor 1O (51) based on a photochromic diarylethene with a quinoline unit was developed and synthesized. In acetonitrile, the chemosensor 1O showed sensitivity and selective Hg2+ ion detection even in an atmosphere of competing metal ions. The detector 1O's stoichiometric ratio for Hg2+ was found to be 1:1, and the probe 1O's detection limit for Hg2+ was computed to be 56.3 nM. Additionally, a molecular logic circuit exhibiting metal-responsive behavior and UV/vis light-responsiveness was successfully built with 4 inputs along with one output. The binding behavior between 1O and Hg2+ is confirmed by 1H NMR titration tests, Job's plot analysis, and ESI-MS spectroscopy (Fig. 27b) [70].
Fig. 27.
a). ESIPT study of HBT-quinoline conjugate-based Hg2+ sensor (50). b). Emission spectra of diarylethene-quinoline based chemosensor (51).
A new quinoline-based ratiometric fluorescence probe (52) known as the "naked-eye" was developed. The Hg2+ ion-containing probe has a 2:1 reactive stoichiometry. With a 410-fold rise in absorbance concentration ratio (A402/A340) in the presence of water throughout a wide pH value range, it demonstrated a high degree of selectivity towards Hg2+ ions compared to other metal ions. It also showed a resonant shifting of color from colorless to pale yellow that was noticeable to the naked eye (Fig. 28a) [71]. The click method has been used to create a number of innovative C-glycosyl triazolyl quinoline-based fluorescence sensors (53). It was discovered that new sensors have strong Hg2+ selectivity over a wide range of other metal ions as well. In order to improve the fluorescent sensors' water-soluble properties and expand their range of applications for Hg(II) detection in biological structures with water solubility, the glucose structure was introduced. Several spectroscopic approaches have been used to characterize the binding mode of triazolyl quinoline with Hg2+, which has been identified as the mechanism behind the sensors' chemodosimetric behaviour (Fig. 28b) [72].
Fig. 28.
a). Fluorescence titration of Naked-eye’ quinoline-based ‘reactive’ Hg2+ sensor (52). b). UV–Vis spectra of C-glycosyl triazolyl quinoline-based fluorescent sensors (53).
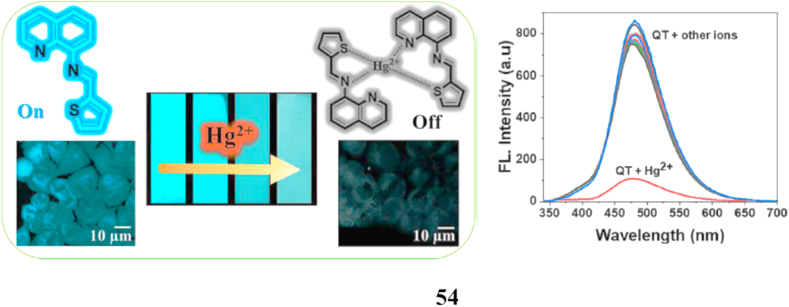
A new Schiff base probe (54, QT) containing thiophene-2-carboxaldehyde (T) and 8-aminoquinoline (Q) moieties has been synthesized. When QT becomes exposed to Hg2+, chelation enhances its fluorescence quenching because the nitrogen and sulfur atoms of QT coordinate, making QT an easy "turn-off" sensor. UV–visible absorption and emission spectral studies, 1H NMR titration, and calculation using density functional theory all supported the development of the chelation complex. According to these investigations, the probe shows excellent sensitivity and selectivity towards Hg2+ when other common metal ions are present. The low detection limit of 23.4 nM was established, and a 2:1 stoichiometry between QT and Hg2+ was verified by a job plot. Moreover, confocal fluorescence microscopy was used to assess the potential usefulness of QT as a sensor for Hg2+ions in human HeLa cells, and Whatman filter paper strips were used to evaluate the sensor's appropriateness for field application using environmental samples (Fig. 29) [73].
Fig. 29.
Confocal fluorescence study of quinoline-thiophene based chemosensor (54).
Quinoline-based mercury (II) sensors utilize a fluorescence quenching mechanism for mercury detection, where the binding of mercury ions to the quinoline moiety alters its photophysical properties, resulting in a measurable decrease in fluorescence intensity. This high sensitivity to mercury ions is advantageous for trace detection. Quinoline sensors offer benefits such as high selectivity, low detection limits, and potential for integration into IoT and wearable devices due to their compact size and efficient sensing capabilities. However, limitations include potential interference from other metal ions and stability issues. Real-world applications include environmental monitoring and health diagnostics, as demonstrated by their use in wearable devices for detecting mercury exposure in occupational settings.
7. Rhodamine based mercury (Hg2+) sensors
A new rhodamine hydrazone derivative (55) featuring thiol and carboxylic acid groups is developed as a selective fluorescent and colorimetric chemosensor for Hg2+. The substantial fluorescence amplification and colorimetric transformation upon the addition of Hg2+ are made possible by the ring-opening mechanism of spirolactam. Confocal laser scanning microscopy was used to analyze one of the chemosensors in a microchannel after it had been combined with an object containing Hg2+. The fluorescence brightness of both chemosensors showed a linear response (r2 = μ 0.95) in the range of 1 nM when plotted against the log concentration of Hg2+, the corresponding detection limits were 1 nM and 4.2 nM (Fig. 30a) [74]. Rhodamine B was combined with the 8-hydroxyquinoline group to provide a unique fluorescent probe (56). The results demonstrated highly responsive and specific fluorescence emission above 500 nm and Hg2+ amplified absorbance in an aqueous solution with a broad pH range of 4–9. The finding that mercury ions align reversibly to the opening spirolactam ring led to the formation of a 1:1 metal-ligand complex. It was also used to confirm its usefulness as a fluorescent probe for monitoring Hg2+ in living cells using in vivo imaging in HeLa cells (Fig. 30b) [75].
Fig. 30.
a). Microfluidic system of Rhodamine-hydrazone based Hg2+chemosensors (55). b). Live cell imaging study of Rhodamine-hydroxyquinoline based Hg2+chemosensor (56).
A rhodamine-based novel and highly sensitive colorimetric off-on fluorescent chemosensor (57) for Hg2+ ions is formulated and synthesized, utilizing the renowned thiospirolactam rhodamine chromophore and furfural hydrazone as signal-reporting groups. Investigated are the sensor's Hg2+-binding characteristics and photophysical characterization in a neutral DMF aqueous solution. A specific metal ion-induced reversible ring-opening mechanism of the rhodamine spirolactam is required for the chemosensor signal to change. The chemosensor reacts instantly and reversibly to Hg2+ ions. Compared with other metal ions, it effectively displays a significant "turn-on" reaction toward Hg2+. Further proving the sensor's usefulness for real-world applications in biological and environmental structures, it is also employed for in vivo imaging in Rat Schwann cells to verify that the probe may be used as a fluorescent probe for monitoring Hg2+ in living cells with satisfactory results (Fig. 31a) [76]. The design and description of a fluorescent turn-on chemosensor (58, RN2) for Hg2+ based on rhodamine B thiohydrazide was done. It demonstrated exceptionally high selectivity and sensitivity to Hg2+ in comparison to other metal ions. The Hg2+-induced breaking of the spiro ring in rhodamine B resulted in selective "OFF-ON" type fluorescence amplification and a noticeable color shift in the RN2. Biological imaging experiments showed the said probe was fluorescent and able to be employed as a cell-permeable probe for Hg2+ monitoring in living cells. It was also effective to use the constructed chemosensor to identify Hg2+ in water samples obtained from the environment (Fig. 31b) [77].
Fig. 31.
a). Live cell imaging of Rho6G-thiospirolactam based chemosensor (57). b). Bioimaging study of RhB thiohydrazide based Hg2+chemosensor (58).
Rapid colorimetric and fluorometric sensing is demonstrated by a rhodamine-based sensor (59) with dual ion detection features following 1:1 binding with Hg2+. The Hg2+-induced opening of the spirolactam ring in the rhodamine structure is responsible for the solution's apparent color shift from colorless to pink and the fluorescence color shift from dark to orange. Fluorescence methods yielded a detection limit of 44 nM, whereas absorbance yielded a value of 32 nM. Moreover, the resultant complex Hg2+ may be used as an I− reversible flux sensor. The addition of I− strengthened the force that links Hg2+ and I− by grabbing the Hg2+ in the complex, which decreased the amount of fluorescence. A molecular device based on the "OFF-ON-OFF" characteristic was constructed with a "INHIBIT" logic gate function (Fig. 32a) [78]. A chemosensor (60) using rhodamine-B and diphenylselenium (RhoSe) has been created. UV/Vis and fluorescence spectroscopy have been used to study its reaction to different metal ions. In CH3OH/H2O solutions, it responds to Hg2+ more so than other metal ions. The RhoSe solution exhibits a noticeable color shift from colorless to pink upon the addition of Hg2+, as well as a notable 48-fold increase in fluorescence. Caused by Hg2+ binding, the ring-opening of the spirolactam in the rhodamine fluorophore is responsible for the color shift and increased fluorescence. The binding ratio of RhoSe-Hg2+ was determined using a work plot, and it was discovered to be 1:1. The pH range of 4.0–10 proved the most effective for Hg2+ detection. Significantly, the inclusion of Na2S allowed for the observation of the RhoSe-Hg2+ complex's reversibility. For practical purposes, the Hg2+ in a water/methanol solution was detected using the strip technique. Furthermore, confocal fluorescence microscopy shows that RhoSe is a useful fluorescent probe for Hg2+ detection in vitro and in vivo (Fig. 32b) [79].
Fig. 32.
a). Fluorescence analysis of a Hg2+ sensor based on rhodamine B (59). b). Zebrafish study of RhoSe-based chemosensor (60).
Rhodamine 6G served as the basis for the design, synthesis, and characterization of a fluorescent and colorimetric chemosensor (61). It successfully identified Hg2+ based on a two-step reaction. Through the use of Fourier-transform infrared spectroscopy, electrospray ionization-mass spectrometry, ultraviolet–visible spectrophotometry, fluorescence spectroscopy, and frontier molecule orbital simulations, the interaction between the chemosensor and Hg2+ was verified. Additionally, it was added to silica gel plates and test strips, which showed excellent Hg2+ selectivity and sensitivity (Fig. 33a) [80]. Three brand-new Hg2+ fluorescence sensors (62) based on rhodamine were created and manufactured. It exhibited exceptional fluorescence amplification and demonstrated good selectivity and sensitivity to Hg2+ even in semi-aqueous solutions with a pH close to neutral. The thiourea-unit numbers rose for these three sensors, expanding their linear operating range and raising their sensitivity. Additionally, the sensors demonstrated outstanding resistance to interference from several metal ions that are relevant to both the environment and biology. The sensors' good practicability was demonstrated by the pond and tap water test. A novel mechanism of interaction between Hg2+ and the sensor was suggested by the number of bound Hg2+ matching that of the thiourea units and the irreversible recognition process (Fig. 33b) [81].
Fig. 33.
a). Fluorescent study of Rho6G-based Hg2+Chemosensor (61). b). FL intensity of RhB thiourea-based fluorescent sensors (62).
The high selectivity for Hg2+ detection was achieved by designing and synthesizing the rhodamine-based fluorescent chemosensor (63). Characterization was conducted through IR, 1H NMR, and HRMS spectroscopies. In an ethanol/water solution, it demonstrated clear fluorescence and colorimetric shifts toward Hg2+, which led to the formation of Hg2+complex with the Hg2+induced ring opening of the spirolactam ring in rhodamine. By its spectral response with Hg2+ions as well as TBAI (tetrabutylammonium iodide) titration studies, the chemosensor's reversibility was confirmed (Fig. 34a) [82]. A rhodamine B group linkage introduced as a reversible switch into squaraine-diamine dyads is an efficient method based on coordination-induced signalling. A new optical squaraine-bis(rhodamine-B) chemosensor (64, SRB) was designed and synthesized, and its best possible usage was investigated. The goal of the probe was to identify Hg2+ ions using an "OFF-ON" fluorescence system. In the presence of other metal ions, it demonstrated a high selectivity toward Hg2+. The complex known as SRB-Hg2+ was studied through the use of UV–vis and fluorescence spectroscopy in acetonitrile since it demonstrated a high degree of selectivity towards Hg2+ when exposed to different metal ions. The probe's "OFF-ON" fluorescence and color signal shift are due to a domino effect triggered by Hg2+, which uses rhodamine spirolactam in its open-ring form to restore the conjugated mechanism of the rhodamine skeleton. Employing a job plot calculation, optical titration, and FT-IR, the mechanism for the opening of the rhodamine spirolactam ring generated by Hg2+ binding and the 1:1 stoichiometric structure of SRB and Hg2+ were confirmed. After that, CN− in acetonitrile was detected using an SRB- Hg2+complex chemosensor in the presence of several anions. Additionally, when Hg2+ was added, this sensor showed extremely sensitive and selective identification of cyanide ions, changing color to revert to colorless in the identical solution. The application of SRB with the PEGDMA polymer to detect Hg2+ ions was ultimately successful, and the results were examined using SEM and fluorescent confocal laser scanning microscopy (CLSM) (Fig. 34b) [83].
Fig. 34.
a). Absorption properties of RhB-based Hg2+chemosensor (63). b). Cell imaging study of squaraine-bis(rhodamine-B) based chemosensor (64).
The High selectivity and sensitivity to Hg2+ are achieved in the effective design and synthesis of a pair of 1,8-naphthalimide-Rhodamine based chemosensors (65). When it interacts with Hg2+ in pure CH3CN, it changes color from yellow to orange and displays a typical fluorescence resonance power transfer signal from 1,8-naphthalimide to Rhodamine 6G. However, RB-NA in CH3CN/HEPES buffer is limited to its ability to function as a photoinduced electron transfer-off process in 1,8-naphthalimide, with an increase in fluorescence intensity centered at 525 nm upon the addition of Hg2+. But in the pure CH3CN system, the Hg2+ can open the spirolactam ring of Rhodamine B in RB-NA and block the PET process in 1,8-naphthalimide, which results in the formation of a new emission band at 575 nm and an intensity of fluorescence enhancement at 515 nm, along with a noticeable shift in color from yellow to pink (Fig. 35a) [84]. The rhodamine-based colorimetric and fluorescent sensor (66) for Hg2+ showed remarkable selectivity and sensitivity, as evidenced by the growing absorption peak at 565 nm and a 32-fold fluorescence amplification at 586 nm with notable color changes. Between 0 and 90 μM, the absorbance and fluorescence maxima increased linearly in proportion to the Hg2+ concentration. The limits of the colorimetric and fluorescence detection methods were 6.36 μM and 60.78 nM, respectively. It showed good interference immunity and minimal cytotoxicity, and it could operate in a nearly neutral pH range of 6.01–8.57. It was possible to construct a 1:2 sensor-Hg2+ complex with a binding constant of 2.89 × 108 M−2. A brand-new sensing method was suggested. It was effectively used for living cell imaging and real sample assay. Moreover, the sensor for signaling Hg2+ in 100 % aqueous solution may be supported in inexpensive cellulose discs (Fig. 35b) [85].
Fig. 35.
a). UV study of Naphthalimide-Rhodamine based Hg2+ chemosensors (65). b). Living cell study of RhB-thiol based Hg2+ fluorescent sensor (66).
A new fluorescent chemosensor (67) based on rhodamine 6G for Hg2+ has been synthesized and validated using NMR and LC-MS methods. In the mixed organic aqueous phase, the fluorescent chemosensor exhibits a strong preference and sensitivity for Hg2+ over other metal ions. After adding Hg2+, there was a noticeable increase in fluorescence and ring-opening of a rhodamine spiro-cyclic structure. The PS- Hg2+ complexes' stoichiometric proportion was found to be 1:1 using the 1H NMR experiment and Job's plot. For PS-Hg2+ complexation, the predicted binding constant and limit of detection (LOD) are 6 × 104 M−1 and 3.0375 × 10−8 M (30.37 nM), respectively. Hg2+ binding in chemosensor PS resulted in an intense fluorescence increase that was most noticeable between pH values of 6 and 8. With the use of real water samples and a spike and recovery technique, the Hg2+ metal ion was efficiently identified. Furthermore, the low-cytotoxic probe PS was employed in a cell scanning experiment employing MDA-MB-231 and A375 breast cancer cells to detect Hg2+ intracellularly using the MTT assay method (Fig. 36a) [86]. To identify metal ions, novel urea-group containing rhodamine compounds (68) have been produced. The dimeric system probe demonstrated a selective fluorescence amplification and colorimetric shift with the addition of Hg2+. This probe exploited the ring-opened amide (fluorescent) to spirolactam (nonfluorescent) mechanism. A value of 3.2 × 105 M−1 was determined for the probe's association constant with Hg2+ (Fig. 36b) [87].
Fig. 36.
a). MTT assay method of Rho6G-based Hg2+ chemosensor (67). b). Photophysical study of RhB-urea based Hg2+ chemosensors (68).
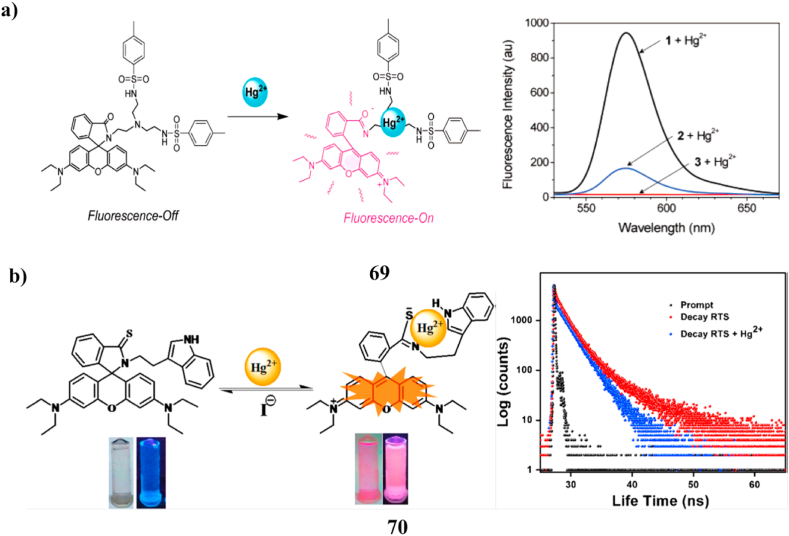
A tren-based tripodal chemosensor (69) with two tosyl groups and rhodamine was created, and UV/vis and fluorescence spectroscopies were used to study the sensor's behavior toward metal ions. The Hg2+ ion produced a visible color change and significantly increased fluorescence when added to a CH3CN solution containing the probe producing very minor color/spectral modification. These findings combined to provide a Hg2+ selective fluorescent chemosensor (OFF-ON) (Fig. 37a) [88]. A new fluorescent and chromogenic chemosensor (70, RTS) based on the rhodamine-tryptamine combination for the detection of Hg2+ in water. A deep pink coloring and a vivid orange fluorescence were seen in an aqueous methanol solution of RTS following the progressive addition of Hg2+. The probe showed a significant degree of selectivity towards Hg2+ as compared to other competing metal ions. Mass spectroscopy and Job's plot analysis were used to determine the 1:1 binding stoichiometry between RTS and Hg2+. According to preliminary research, the synthesized probe RTS was very non-toxic and was able to distinguish between intercellular Hg2+ ions by successfully passing via the cell exteriors of model cell systems, such as human neuroblastoma (SHSY5Y) cells and cervical cells (HeLa). By using fluorescence titration, the limit of detection (LOD) was determined to be 2.1 nM. Furthermore, it has been shown that synthetic chemosensors have potential utility for identifying Hg2+ ions in ambient fluid samples (Fig. 37b) [89].
Fig. 37.
a). Fluorescence study of Rhodamine-tosyl groups based Hg2+Chemosensor (69). b). Biological applications of tryptamine-appended rhodamine-based Hg2+ chemosensor (70).
A novel fluorescent with colorimetric chemosensor (71) utilizing rhodamine 6G as its basis and an N-methyl imidazole nucleus has been developed to enable the selective identification of Hg2+ ions. The fluorescence spectral examination and UV–Vis data showed that the receptor is sensitive to and selective for Hg2+, with no discernible interference from other rival metal ions. It was observed that the addition of Hg2+ to the receptor caused a rapid color shift from colorless to pink. The sensitivity of several cations to Hg2+-inducing fluorescence was examined. Job's graphic provided evidence for the stoichiometric balance of 1:1 involving the receptor and Hg2+. The spirolactam ring-opening process was identified as the cause of the color shift and the fluorescence response that occurred with the presence of the Hg2+ ion. 1H NMR and mass spectrum analysis were used to validate the likely manner of interaction between the receptor and Hg2+. Its test strips made of electrospun nanofiber were effectively used to identify Hg2+ ions in an aqueous environment (Fig. 38a) [90]. A thiospirolactam RhB ligand for an efficient colorimetric OFF-ON sensor (72) for mercury (II) ions in neutral aqueous conditions is derived from the modified p-tert-butylcalix [4] due to its inherent amphiphilic characteristic. It has been examined using electronic spectroscopy methods. The thiospirocyclic moiety of this ligand allows it to distinguish between different alkali, alkaline earth, and transition metal ions and chronic toxic Hg2+ ions. The ligand exhibits a Hg2+ ion detection limit of 9.65 × 10−9 M and an association constant of 3.63 × 105 M−1. Additionally, this ligand can penetrate HeLa cells, which may be useful in detecting Hg2+ ions in biological systems through the use of fluorescence imaging (Fig. 38b) [91].
Fig. 38.
a). Absorption study of RhB based Hg2+ Chemosensor (71). b). Live Cell Imaging study of p-tert-Butylcalix [4]arene Thiospirolactam RhB Based Mercury Sensor (72).
Rhodamine-based mercury (II) sensors utilize fluorescence quenching or fluorescence resonance energy transfer (FRET) mechanisms for detection. Upon mercury (II) ion binding, the rhodamine moiety experiences a change in its electronic environment, leading to a measurable decrease in fluorescence intensity or altered emission wavelength. This response facilitates highly sensitive mercury detection. Advantages include high sensitivity, low detection limits, and rapid response times, making these sensors suitable for real-time monitoring in IoT and wearable devices. Limitations involve potential photobleaching and interference from other ions. For instance, rhodamine-based sensors have been employed in wearable devices for environmental monitoring, offering real-time feedback on mercury exposure levels, and demonstrating their practical application in ensuring environmental safety.
A summary of sensors, principles, Detection Mechanism, Advantages, Disadvantages, and Biomaterial applications is found in Table 1 below.
Table 1.
A structured overview of the synthesized sensors, including their performance, detection mechanism, selectivity, limit of detection (LOD), advantages and disadvantages, and biomaterial applications, is provided in this table.
| Compounds | Principle | Performance | Detection Mechanism | Selectivity | Limit of Detection (LOD) | Advantages | Disadvantages | Biomaterial Applications |
|---|---|---|---|---|---|---|---|---|
| Azobenzene | Photoisomerization changes upon binding with Hg(II) | High sensitivity, quick response | Optical signal change | Moderate, influenced by pH | μM to nM range | Reversible, easy synthesis | Limited selectivity, possible interference | Imaging and sensing in biological systems, drug delivery |
| Coumarin | Fluorescence quenching or enhancement upon binding with Hg(II) | High sensitivity, rapid response | Fluorescence emission change | Good, often tailored for Hg(II) | nM to pM range | High quantum yield, tunable properties | Photobleaching, solvent-dependent fluorescence | Bioimaging, fluorescent probes in living cells |
| Fluorescein | Fluorescence intensity changes upon interaction with Hg(II) | Excellent sensitivity, quick detection | Fluorescence intensity change | High, can be modified for selectivity | pM range | Strong fluorescence, high molar absorptivity | Photobleaching, pH sensitivity | Cellular imaging, pH sensors, molecular probes |
| Pyrene | Fluorescence quenching or enhancement upon binding with Hg(II) | High sensitivity, good performance | Fluorescence emission change | Good, selective for Hg(II) | nM to pM range | High fluorescence, long lifetime | Limited water solubility, potential toxicity | Biosensors, environmental monitoring, cell imaging |
| Quinoline | Fluorescence change or chelation induced by Hg(II) | High sensitivity, rapid response | Fluorescence signal change | Good, can be modified for selectivity | nM range | Versatile, easy functionalization | Photostability issues, solvent effects | Bioimaging, fluorescent probes, metal ion detection in cells |
| Rhodamine | Fluorescence “off-on” change due to Hg(II) induced spirocyclic ring opening | Excellent sensitivity, fast response | Fluorescence “off-on” signal change | High, specific for Hg(II) | pM to nM range | High fluorescence quantum yield, water-soluble | Photobleaching, possible interference | Cellular imaging, tracking of metal ions in biological systems |
8. Conclusion
Recent advancements in photoresponsive-based mercury (II) sensors represent a pivotal step forward in addressing the pressing need for efficient and reliable detection methods. These sensors utilize materials such as azobenzene, coumarin, fluorescein, pyrene, quinoline, and rhodamine, which exhibit changes in optical properties upon interaction with mercury ions, allowing for high sensitivity and selectivity. The integration of cutting-edge materials and emerging technologies like IoT and wearables has endowed these sensors with enhanced capabilities, enabling real-time and remote monitoring. This has broadened their applications beyond traditional environmental monitoring to include bioimaging and personal exposure assessment. For example, coumarin-based sensors offer high quantum yield and rapid response, while fluorescein-based sensors provide strong fluorescence and low detection limits, ideal for cellular imaging. Despite their potential, challenges such as improving multifunctionality, refining selectivity, and enhancing on-site portability remain. Addressing issues like photobleaching, solvent dependency, and potential interference is crucial for further development. Nevertheless, the transformative potential of these sensors in revolutionizing environmental monitoring and public health is undeniable. As research continues, advancements promise to refine our understanding of mercury contamination and catalyze tangible improvements in pollution management and preventative strategies, paving the way for more effective and sustainable solutions.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Mani Rajasekar: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision. Chiterasu Narendran: Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jennita Mary: Methodology, Investigation, Funding acquisition, Formal analysis. Sivakumar Meenambigai: Software, Resources, Project administration, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
M. R. thanks Prof. T. Sasiprabha, Vice-Chancellor, Sathyabama Institute of Science and Technology (Deemed to be University), for her encouragement.
Biographies

Dr. M. Rajasekar is currently working as Scientist-C/Assistant Professor (Research) of Organic Chemistry in the Centre for Molecular and Nanomedical Sciences, International Research Centre, Sathyabama Institute of Science and Technology (Deemed to be University), Chennai since 2018. He was born in Agatheripattu village, Tiruvannamalai district in Tamil Nadu, India, and his main research interest is in the area of synthetic organic chemistry. He received his B.Sc degree from the Department of Chemistry, Arignar Anna Govt. Arts College, Cheyyar, affiliated to the University of Madras in 2005 and M.Sc Degree from the Department of Chemistry, C. Abdul Hakeem College of Arts and Science, Vellore, affiliated to Thiruvalluvar University in 2007. He received his M. Phil degree from the Department of Chemistry, The New College, affiliated to the University of Madras, Chennai, in 2008. He worked as a Visiting Lecturer at the Department of Chemistry, Anna University, Chennai from June 26, 2008 to December 30, 2008 and a Ph.D. degree from the Department of Organic Chemistry, the University of Madras in 2015. He worked as a National Postdoctoral (DST-SERB) at Sathyabama University, Chennai from 2016 to 2018. He received his B.Ed (2021) and M.Ed (2023) degrees from Arunachala College of Education, affiliated with Tamil Nadu Teachers Education University, Chennai. He was awarded NET, SLET, SET, NPDF, DSKPDF, Nehru PDF, Young Scientist, RSC Advances panel reviewer, and National Educational Star Award by “The Glorious Organization for Accelerated to Literacy (GOAL)”, New Delhi. Additionally, he has been granted five patents, published twelve books and has authored two monographs.

Narendran C was born in Tamil Nadu, India in 1998. He received his Master's in Medical Biotechnology and Clinical Research from Sathyabama Institute of Science and Technology (Deemed to be University), Chennai in 2022 and he also received his Bachelor's in Microbiology from Kumararani Meena Muthiah College of Arts and Science (Affiliated to Madras University), Chennai in 2020. He also received his Diploma in Medical Laboratory Technology from Naga Institute of Paramedical, Chennai in 2019. He has done a project on Anti-bacterial activity of cinnamon oil on clinical pathogens and also in the Isolation and characterization of Beta-lactamase inhibitors from marine Actinomycetes. His current research interests are biosensors and their applications.

Ms. Jennita Mary was born in 2002, Tamil Nadu, India. She is currently pursuing her undergraduate degree in the School of Bio and Chemical Engineering, Department of Biotechnology, Sathyabama Institute of Science and Technology (Deemed to be University), Chennai, Tamil Nadu, India. She has developed two working products during her 3rd year of her UG Program in the Medical field. She has also published a review paper in the field of Nanotechnology. Currently, she's doing her project under the supervision of Dr. M. Rajasekar. Her research interest generally includes biosensors, protecting the marine ecosystem and the organic field of cosmetics.

Ms. Meenambigai. S, was born in 2002 in Tamil Nadu, India. She is presently pursuing her undergraduate studies at the School of Bio and Chemical Engineering within the Department of Biotechnology at Sathyabama Institute of Science and Technology, a deemed university located in Chennai, Tamil Nadu, India. During her third year, she engaged in research involving leaf disease detection using CNN (Industry 4.0) and also gained valuable experience through an internship in the field of bioinformatics. Currently, she is actively involved in a research project under the guidance of Dr. M. Rajasekar. Her research pursuits predominantly revolve around the intriguing realm of cosmetics-based products and metal sensor significance.
References
- 1.Dai D., Yang J., Wang Y., Yang Y.W. Recent progress in functional materials for selective detection and removal of mercury (II) ions. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 2.Qu J., Zhang X., Zhou W., Yao R., Zhang X., Jing S. Carbon dots/Ruthenium (III) nanocomposites for FRET fluorescence detection and removal of mercury (II) via assembling into nanofibers. Talanta. 2024;268 doi: 10.1016/j.talanta.2023.125322. [DOI] [PubMed] [Google Scholar]
- 3.Lim J.W., Kim T.Y., Woo M.A. Trends in sensor development toward next-generation point-of-care testing for mercury. Biosens. Bioelectron. 2021;183 doi: 10.1016/j.bios.2021.113228. [DOI] [PubMed] [Google Scholar]
- 4.Tang K., Chen Y., Tang S., Wu X., Zhao P., Fu J., Lei H., Yang Z., Zhang Z. A smartphone-assisted down/up-conversion dual-mode ratiometric fluorescence sensor for visual detection of mercury ions and L-penicillamine. Sci Total Env. 2023;856 doi: 10.1016/j.scitotenv.2022.159073. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D., Zhang Y., Lu W., Le X., Li P., Huang L., Zhang J., Yang J., Serpe M.J., Chen D., Chen T. Fluorescent hydrogel-coated paper/textile as flexible chemosensor for visual and wearable mercury(II) detection. Adv. Mater. Technol. 2019;4 [Google Scholar]
- 6.Wang L., Ma Y., Lin W. A coumarin-based fluorescent probe for highly selective detection of hazardous mercury ions in living organisms. J. Hazard Mater. 2024;461 doi: 10.1016/j.jhazmat.2023.132604. [DOI] [PubMed] [Google Scholar]
- 7.Li M., Zhang S., Zhang P., Qin K., Chen Q., Cao Q., Zhang Y., Zhang J., Yuan C., Xiao H. Dansyl-labelled cellulose as dual-functional adsorbents for elimination and detection of mercury in aqueous solution via aggregation-induced emission. J. Environ. Manag. 2023;338 doi: 10.1016/j.jenvman.2023.117773. [DOI] [PubMed] [Google Scholar]
- 8.Mansha M., Ali S., Baig N., Khan S.A. Naphthalene-based silica nanoparticles as a highly sensitive fluorescent chemosensor for mercury detection in real seawater. J. Mol. Liq. 2023;374 [Google Scholar]
- 9.Rajasekar M. Recent development in fluorescein derivatives. J. Mol. Struct. 2021;1224 [Google Scholar]
- 10.Rajasekar M. Recent Trends in Rhodamine derivatives as fluorescent probes for biomaterial applications. J. Mol. Struct. 2021;1235 [Google Scholar]
- 11.Rajasekar M., Agash S.G., Narendran C., Rajasekar K. Recent trends in fluorescent-based copper (II) chemosensors and their biomaterial applications. Inorg. Chem. Commun. 2023;151 [Google Scholar]
- 12.Preman N.K., Jain S., Antony A., Shetty D.M., Fathima N., Prasad K.S., Johnson R.P. Stimuli-responsive copolymer-mediated synthesis of gold nanoparticles for nanozyme-based colorimetric detection of mercury (II) ions. ACS Appl. Polym. Mater. 2023;5:6377–6389. [Google Scholar]
- 13.Wu S., Yang Y., Cheng Y., Wang S., Zhou Z., Zhang P., Zhu X., Wang B., Zhang H., Xie S., Zeng Z. Fluorogenic detection of mercury ion in aqueous environment using hydrogel‐based AIE sensing films. Aggregate. 2023;3:287. [Google Scholar]
- 14.Soni R., Sharma D.S., Rana D., Singh N. Gupta, Structural designs of functional metal organic frameworks for the detection of mercury in contaminated water sources. Coord. Chem. Rev. 2023;494 [Google Scholar]
- 15.Tan T., Zhang C., Han Y., Chu R., Xi W., Chen X., Sun J., Huang H., Hu Y., Huang X. Fine-tuning bromide AIE probes for Hg2+ detection in mitochondria with wash-free staining. J. Hazard Mater. 2024;464 doi: 10.1016/j.jhazmat.2023.132999. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan U., Manickam S., Iyer S.K. Turn-off fluorescence of imidazole-based sensor probe for mercury ions. Sens. Diagn. 2024;3:87–94. [Google Scholar]
- 17.Zhang D., Yao Y., Wu J., Protsak I., Lu W., He X., Xiao S., Zhong M., Chen T., Yang J. Super hydrophilic semi-IPN fluorescent poly(N-(2-hydroxyethyl)acrylamide) hydrogel for ultrafast, selective, and long-term effective mercury(II) detection in a bacteria-laden system. ACS Appl. Bio Mater. 2019;2:906–915. doi: 10.1021/acsabm.8b00761. [DOI] [PubMed] [Google Scholar]
- 18.Bi X., Liu X., Luo L., Liu S., He Y., Zhang L., Li L., You T. Isolation of sensing units and adsorption groups based on MOF-on-MOF hierarchical structure for both highly sensitive detection and removal of Hg2+ Inorg. Chem. 2024;63:2224–2233. doi: 10.1021/acs.inorgchem.3c04177. [DOI] [PubMed] [Google Scholar]
- 19.Han X., Wang Y., Wang T., Zhang J., Xie J., Han J., Zhao X. Ultra-sensitive mercury detection from surface wrapping of carbon dots reduced and decorated silver nanoparticles on SERS fiber probes. J. Alloys Compd. 2024;976 [Google Scholar]
- 20.Zhang, Liang M., Shao C., Li Z., Cao X., Wang Y., Wu Y., Lu S. Visual detection and sensing of mercury ions and glutathione using fluorescent copper nanoclusters. ACS Appl. Bio Mater. 2023;6:1283–1293. doi: 10.1021/acsabm.3c00031. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y., Song J., Wang W. A novel azobenzene-based fluorescent sensor for selective detection of mercury ion. Lett. Org. Chem. 2011;8:749–751. [Google Scholar]
- 22.Cheng X., Li Q., Li C., Qin J., Li Z. Azobenzene-based colorimetric chemosensors for rapid naked-eye detection of mercury(II) Chem. Eur J. 2011;17:7276–7281. doi: 10.1002/chem.201003275. [DOI] [PubMed] [Google Scholar]
- 23.Mina G., Wei X., Jianhua G. Mingyun S., Gui Y. New azobenzene dye colorimetric and RatiometricChemosensors for mercury(II) ion. Chin. J. Chem. 2009;27:1773–1776. [Google Scholar]
- 24.Lee A. Balamurugan H. Single molecular probe for multiple analyte sensing: efficient and selective detection of mercury and fluoride ions. Sens Actuators B Chem. 2015;216:80–85. [Google Scholar]
- 25.Yu Z., Tian Z., Li Z., Luo Z., Li Y., Li Y., Ren J. A new chromogenic and fluorogenic chemosensor for Hg(II) with high selectivity based on the Hg2+ -promoted deprotection of thioacetals. Sens Actuators B Chem. 2016;223:172–177. [Google Scholar]
- 26.Lee M., Cho B., Yoon J., Kim J. Selectively chemodosimetric detection of Hg(II) in aqueous media. Org. Lett. 2007;9:4515–4518. doi: 10.1021/ol7020115. [DOI] [PubMed] [Google Scholar]
- 27.Pirdehi H.H., Mahmoodi N.O., Nadamani M.P., Taheri A. Novel synthesized azo-benzylidene-thiourea as dual naked-eye chemosensor for selective detection of Hg2+ and CN‾ ions. J. Photochem. Photobiol., A: Chem. 2020;391 [Google Scholar]
- 28.Yuan Y., Guo L., Chen Z., Zhu Y., Feng L., Hu W., Tian M., Wang H., Feng F. A novel quick and highly selective “turn-on” fluorescent probe for Hg2+ and its application. Microchem. J. 2019;147:615–621. [Google Scholar]
- 29.Ding Y., Pan Y., Han Y. A coumarin-based fluorescent probe for ratiometric monitoring of Hg2+ in live-cells. Ind. Eng. Chem. Res. 2019;58:7786–7793. [Google Scholar]
- 30.Voutsadaki S., Tsikalas G., Klontzas E., Froudakis G., Katerinopoulos H. A ‘‘turn-on’’ coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media. Chem. Commun. 2010;46:3292–3294. doi: 10.1039/b926384e. [DOI] [PubMed] [Google Scholar]
- 31.Ho I., Lai T., Wu R., Tsai M., Wu C., Lee G., Chung W. Design and synthesis of triazolyl coumarins as Hg2+ selective fluorescent chemosensors. Analyst. 2012;137:5770–5776. doi: 10.1039/c2an36076d. [DOI] [PubMed] [Google Scholar]
- 32.Goswami S., Das A., Maity S. ‘PET’ vs. ‘Push-Pull’ induced ICT: a remarkable Coumarinyl-appended Pyrimidine based Naked Eye Colorimetric and Fluorimetric Sensor for detection of Hg2+ ion in aqueous media with test trips. Dalton Trans. 2013;42:16259–16263. doi: 10.1039/c3dt52252k. [DOI] [PubMed] [Google Scholar]
- 33.Qin S., Chen B., Huang J., Han Y. A thiocoumarin-based colorimetric and ratiometric fluorescent probe for Hg2+ in aqueous solution and its application in live-cell imaging. New J. Chem. 2018;42:12766–12772. [Google Scholar]
- 34.Ngororabanga J., Tshentu Z., Mama N. A highly selective and sensitive ESIPT-based coumarin-triazole polymer for the ratiometric detection of Hg2+ New. J. Chem. 2019;43:12168–12177. [Google Scholar]
- 35.Bazzicalupi C., Caltagirone C., Cao Z., Chen Q., Natale C., Garau A., Lippolis V., Lvova L., Liu H., Lundstrçm I., Mostallino M., Nieddu M., Paolesse R., Prodi L., Sgarzi M., Zaccheroni N. Multimodal use of new coumarin-based fluorescent chemosensors: towards highly selective optical sensors for Hg2+ probing. Chem. Eur J. 2013;19:14639–14653. doi: 10.1002/chem.201302090. [DOI] [PubMed] [Google Scholar]
- 36.Mondal S., Patra N., Nayek H., Hira S., Chatterjee S., Dey S. Unusual absence of FRET in triazole bridged coumarin-hydroxyquinoline, an active sensor for Hg2+ detection. Photochem.Photobiol. Sci. 2020;19:1211–1221. doi: 10.1039/d0pp00140f. [DOI] [PubMed] [Google Scholar]
- 37.Cheng X., Qu S., Xiao L., Li W., He P. Thioacetalized coumarin-based fluorescent probe for mercury(II): ratiometric response, high selectivity and successful bioimaging application. J. Photochem. Photobiol. Chem. 2018;364:503–509. [Google Scholar]
- 38.Chen C., Vijay N., Thirumalaivasan N., Velmathi S., Wu S. Coumarin-based Hg2+ fluorescent probe: fluorescence turn-on detection for Hg2+ bioimaging in living cells and zebrafish. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019;219:135–140. doi: 10.1016/j.saa.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Hande P., Samui A., Kulkarni P. Selective nanomolar detection of mercury using coumarin based fluorescent Hg(II)-Ion imprinted polymer. Sens Actuators B Chem. 2017;246:597–605. [Google Scholar]
- 40.Chen J., Liu W., Wang Y., Zhang H., Wu J., Xu H., Ju W., Wang P. Turn-on fluorescence sensor based on the aggregation of pyrazolo[3,4-b]pyridine-based coumarin chromophores induced by Hg2+ Tetrahedron Lett. 2013;54:6447–6449. [Google Scholar]
- 41.Zhang Y., Leng J. Theoretical studies on two-photon fluorescent Hg2+ probes based on the coumarin-rhodamine system. Sensors. 2017;17:1672. doi: 10.3390/s17071672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanichacheva N., Hanmeng O., Kraithong S., Sukrat K. Dual optical Hg2+-selective sensing through FRET system of fluorescein and rhodamine B fluorophores. J. Photochem. Photobiol. Chem. 2014;278:75–81. [Google Scholar]
- 43.Mohammad H., Islam A., Prodhan C., Ali M. A fluorescein-based chemosensor for “turn-on” detection of Hg2+ and resultant complex as a fluorescent sensor for S2- in semi aqueous medium with cell-Imaging application: experimental and Computational studies. New J. Chem. 2019;3:5297–5307. [Google Scholar]
- 44.Liu D., Wang Y., Wang R., Wang B., Chang H., Chen J., Yang G., He H. Fluorescein-based fluorescent sensor with high selectivity for mercury and its imaging in living cells. Inorg. Chem. Commun. 2018;89:46–50. [Google Scholar]
- 45.Kang H., Xu H., Fan C., Liu G., Pu S. A new sensitive symmetric fluorescein-linked diarylethene chemosensor for Hg2+ detection. J. Photochem. Photobiol., A: Chem. 2018;364:465–470. [Google Scholar]
- 46.Sureshkumar K., Ramakrishnappa T., Pandurangappa M. Mercury speciation at trace level using fluorescein hydrazide as fluorescent probe. Mater. Today Proc. 2022;49:576–582. [Google Scholar]
- 47.Piyanuch P., Watpathomsub S., Lee V., Nienaber H., Wanichacheva N. Highly sensitive and selective Hg2+-chemosensor based on dithia-cyclic fluorescein for optical and visual-eye detections in aqueous buffer solution. Sens Actuators B Chem. 2016;224:201–208. [Google Scholar]
- 48.Nolan E., Lippard S. Turn-on and ratiometric mercury sensing in water with a red-emitting probe. J. Am. Chem. Soc. 2007;129:5910–5918. doi: 10.1021/ja068879r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiou Y., Wan C., Tai A. A selective colorimetric and turn-on fluorescent chemosensor for Hg2+ in aqueous solution. J. Fluoresc. 2017;27:317–322. doi: 10.1007/s10895-016-1960-7. [DOI] [PubMed] [Google Scholar]
- 50.Rullyani C., Shellaiah M., Rameshc M., Lina H., Chu C. Pyrene-SH functionalized OTFT for detection of Hg2+ ions in aquatic environments. Org. Electron. 2019;69:275–280. [Google Scholar]
- 51.Bai C., Xu P., Zhang J., Qiao R., Chen M., Mei M., Wei B., Wang C., Zhang L., Chen S. Long-wavelength fluorescent chemosensors for Hg2+ based on pyrene. ACS Omega. 2019;11:14621–14625. doi: 10.1021/acsomega.9b02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar M., Dhir A., Bhalla V., Sharma R., Puri R., Mahajan R. Highly effective chemosensor for mercury ions based on bispyrenyl derivative. Analyst. 2010;135:1600–1605. doi: 10.1039/b922072k. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Li B., Zhang L., Liu L., Zuo Q., Li P. A highly selective regenerable optical sensor for detection of mercury(II) ion in water using organic–inorganic hybrid nanomaterials containing pyrene. New J. Chem. 2010;34:1946–1953. [Google Scholar]
- 54.Ma B., Zeng F., Lia X., Wu S. A facile approach for sensitive, reversible and ratiometric detection of biothiols based on thymine-mediated excimer–monomer transformation. Chem. Commun. 2012;48:6007–6009. doi: 10.1039/c2cc32064a. [DOI] [PubMed] [Google Scholar]
- 55.Sivaraman Gandhi, Anand Thangaraj, Chellappa Duraisamy. Development of a pyrene based ‘‘turn on’’ fluorescent chemosensor for Hg2+ RSC Adv. 2012;2:10605–10609. [Google Scholar]
- 56.Shellaiah M., Rajan Y., Balu P., Murugan A. A Pyrene based schiff base probe for selective fluorescent turn-on detection of Hg2+ ions with live cell application. New J. Chem. 2015;39:2523–2531. [Google Scholar]
- 57.Tumay S., Uslua A., Alidagi H., Kazan H., Bayraktar C., Yolaçan T., Durmuş M., Yeşilot S. A systematic series of fluorescence chemosensors with multiple binding sites for Hg(II) based on pyrenyl-functionalized cyclotriphosphazenes and their application in live cell imaging. New.J. Chem. 2018;42:14219–14228. [Google Scholar]
- 58.Bhalla V., Tejpal R., Kumar M., Sethi A. Terphenyl derivatives as “turn on” fluorescent sensors for mercury. Inorg. Chem. 2009;48:11677–11684. doi: 10.1021/ic9016933. [DOI] [PubMed] [Google Scholar]
- 59.Rani B., John S. Fluorogenic mercury ion sensor based on pyrene-amino mercaptothiadiazole unit. J. Hazard Mater. 2018;343:98–106. doi: 10.1016/j.jhazmat.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y., Wen X., Fan Z. An AIE active pyrene based fluorescent probe for selective sensing Hg2+ and imaging in live cells. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019;223 doi: 10.1016/j.saa.2019.117315. [DOI] [PubMed] [Google Scholar]
- 61.Sahin Z., Alimli D., Tonta M., Kose M., Yilmaz F. Highly sensitive and reusable mercury (II) sensor based on fluorescence quenching of pyrene moiety in polyacrylamide-based cryogel. Sens Actuators B Chem. 2017;242:362–368. [Google Scholar]
- 62.Wang H., Wu S. Highly selective fluorescent sensors for mercury(II) ions and their applications in living cell imaging. Tetrahedron. 2013;69:1965–1969. [Google Scholar]
- 63.Lee Y., Lee M., Zhang J., Kim J. Pyrene excimer-based Calix arene FRET chemosensor for mercury(II) J. Org. Chem. 2010;75:7159–7165. doi: 10.1021/jo101113f. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y., Zhu C., Gao X., You X., Yao C. Hg2+-Selective ratiometric and “OFF-ON” chemosensor based on the azadiene-pyrene derivative. Org. Lett. 2010;12:2566–2569. doi: 10.1021/ol1007636. [DOI] [PubMed] [Google Scholar]
- 65.Tarafdar K. Ghosh D. A new quinoline-based chemosensor in ratiometric sensing of Hg2+ ions. Supramol. Chem. 2013;25:127–132. [Google Scholar]
- 66.Hu J., Li J., Qi J., Chen J. Highly selective and effective mercury(II) fluorescent sensors. New J. Chem. 2015;39:843–848. [Google Scholar]
- 67.Zhong K., Zhou X., Hou R., Zhou P., Hou S., Bian Y., Zhang G., Tang L., Shang X. A water-soluble highly sensitive and selective fluorescent sensor for Hg2+ based on 2-(2-(8- hydroxyquinolin)-yl)benzimidazole via ligand-tometal charge transfer (LMCT) RSC Adv. 2014;4:16612–16617. [Google Scholar]
- 68.Zhou C., Song Y., Li Y. A novel highly sensitive and selective fluorescent sensor for imaging mercury(II) in living cells. RSC Adv. 2014;4:33614–33618. doi: 10.1007/s10895-014-1419-7. [DOI] [PubMed] [Google Scholar]
- 69.Sahana S., Mishra G., Sivakumar S., Bharadwaj P. 2-(2’-Hydroxyphenyl)-benzothiazole (HBT)-quinoline conjugate: highly specific fluorescent probe for Hg2+based on ESIPT and its application in bioimaging. Dalton Trans. 2015;44:20139–20146. doi: 10.1039/c5dt03719k. [DOI] [PubMed] [Google Scholar]
- 70.Guo S., Fan C., Liu G., Pu S. A colorimetric and fluorescent chemosensor for Hg2+ based on a photochromic diarylethene with a quinoline unit. RSC Adv. 2018;8:39854–39864. doi: 10.1039/c8ra08358d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y., Yan Y., Chen S., Gao Z., Xu H. Naked-eye’ quinoline-based ‘reactive’ sensor for recognition of Hg2+ ion in aqueous solution. Bioorg. Med. Chem. Lett. 2014;24:5373–5376. doi: 10.1016/j.bmcl.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 72.Wang L., Jin J., Zhao L., Shen H., Shen C., Zhang P. Synthesis of C-glycosyl triazolyl quinoline-based fluorescent sensors for the detection of mercury ions. Carbohydr. Res. 2016;433:41–46. doi: 10.1016/j.carres.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Musikavanhu B., Muthusamy S., Zhu D., Xue Z., Yu Q., Chiyumba C., Mack J., Nyokong T., Wang S., Zhao L. A simple quinoline-thiophene Schiff base turn-off chemosensor for Hg2+ detection: spectroscopy, sensing properties and applications. Spectrochim. Acta Mol. Biomol. Spectrosc. 2022;264 doi: 10.1016/j.saa.2021.120338. [DOI] [PubMed] [Google Scholar]
- 74.Kim H., Nam S., Swamy K., Jin Y., Chen X., Kim Y., Kim S., Park S., Yoon J. Rhodamine hydrazone derivatives as Hg2+ selective fluorescent and colorimetric chemosensors and their applications to bioimaging and microfluidic system. Analyst. 2011;136:1339–1343. doi: 10.1039/c0an00804d. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Y., Sun Y., Lv X., Liu Y., Chen M., Guo W. Rhodamine-based chemosensor for Hg2+ in aqueous solution with a broad pH range and its application in live cell imaging. Org. Biomol. Chem. 2010;8:4143–4147. doi: 10.1039/c0ob00013b. [DOI] [PubMed] [Google Scholar]
- 76.Wang H., Li Y., Xu S., Li Y., Zhou C., Fei X., Sun L., Zhang C., Li Y., Yang Q., Xu X. Rhodamine-based highly sensitive colorimetric off-on fluorescent chemosensor for Hg2+ in aqueous solution and for live cell imaging. Org. Biomol. Chem. 2011;9:2850–2855. doi: 10.1039/c0ob01032d. [DOI] [PubMed] [Google Scholar]
- 77.Wang L., Zheng B., Zhao Y., Du J., Xiao D. A Hg2+ selective fluorescent chemosensor based on rhodamine B thiohydrazide and its application in bioimaging. Anal. Methods. 2012;4:2369–2374. [Google Scholar]
- 78.Dinga H., Zheng C., Lia B., Liu G., Pub S., Jiaa D., Zhou Y. A rhodamine–based sensor for Hg2+ and resultant complex as a fluorescence sensor for I– RSC. Adv. 2016;6:80723–80728. [Google Scholar]
- 79.Venkatesan P., Thirumalivasan N., Wu S. A rhodamine-based chemosensor with diphenylselenium for highly selective fluorescence turn-on detection of Hg2+ in vitro and in vivo. RSC Adv. 2017;7:21733–21739. [Google Scholar]
- 80.Bai C., Wang W., Zhang J., Wang C., Qiao R., Wei B., Zhang L., Chen S., Yang S. A fluorescent and colorimetric chemosensor for Hg2+ based on rhodamine 6G with a two-step reaction mechanism. Front. Chem. 2020;8:14. doi: 10.3389/fchem.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong M., Liu A., Xu Y., Xu D. Synthesis and properties of three novel rhodamine-based fluorescent sensors for Hg2+ Chin. Chem. Lett. 2016;27:989–992. [Google Scholar]
- 82.Liu W., Chen J., Xu L., Wu J., Xu H., Zhang H., Wang P. Reversible “off–on” fluorescent chemosensor for Hg2+ based on rhodamine derivative. Spectrochim. Acta Mol. Biomol. Spectrosc. 2012;85:38–42. doi: 10.1016/j.saa.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 83.Lee S., Rao B., Son Y. A highly selective fluorescent chemosensor for Hg2+ based on a squaraine–bis(rhodamine-B) derivative: Part II. Sens. Actuators B Chem. 2015;210:519–532. [Google Scholar]
- 84.Fang Y., Zhou Y., Lia J., Ruia Q., Yao C. Naphthalimide-Rhodamine based chemosensors for colorimetric and fluorescent sensing Hg2+ through different signalingmechanisms in corresponding solvent systems. Sens. Actuators B Chem. 2015;215:350–359. [Google Scholar]
- 85.Hong M., Lu X., Chen Y., Xu D. A novel rhodamine-based colorimetric and fluorescent sensor for Hg2+ in water matrix and living cell. Sens. Actuators B Chem. 2016;232:28–36. [Google Scholar]
- 86.Vanjare B., Mahajan P., Ryoo H., Dige N., Choi N., Han Y., Kim S., Kim C., Lee K. Novel rhodamine based chemosensor for detection of Hg2+: nanomolar detection, real water sample analysis, and intracellular cell imaging. Sens. Actuators B Chem. 2021;330 [Google Scholar]
- 87.Soh J., Swamy K., Kim S., Kim S., Lee S., Yoon J. Rhodamine urea derivatives as fluorescent chemosensors for Hg2+ Tetrahedron Lett. 2007;48:5966–5969. [Google Scholar]
- 88.Lee M., Wu J., Lee J., Jung J., Kim J. Highly sensitive and selective chemosensor for Hg2+ based on the rhodamine fluorophore. Org. Lett. 2007;9:2501–2504. doi: 10.1021/ol0708931. [DOI] [PubMed] [Google Scholar]
- 89.Hazra S., Bodhak C., Chowdhury S., Sanyal D., Mandal S., Chattopadhyay K., Pramanik A. A novel tryptamine-appended rhodamine-based chemosensor for selective detection of Hg2+ present in aqueous medium and its biological applications. Anal. Bioanal. Chem. 2019;411:1143–1157. doi: 10.1007/s00216-018-1546-0. [DOI] [PubMed] [Google Scholar]
- 90.Rao P., Saritha B., Rao S. Colorimetric and turn-on fluorescence chemosensor for Hg2+ ion detection in aqueous media. J. Fluoresc. 2019;29:353–360. doi: 10.1007/s10895-018-02342-4. [DOI] [PubMed] [Google Scholar]
- 91.Anandababu A., Anandan S., Ashokkumar M. A simple discriminating p-tert-Butylcalix[4]arene thiospirolactam rhodamine B based colorimetric and fluorescence sensor for mercury ion and live cell imaging applications. ChemistrySelect. 2018;3:4413–4420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.