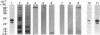Abstract
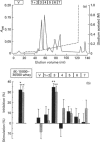
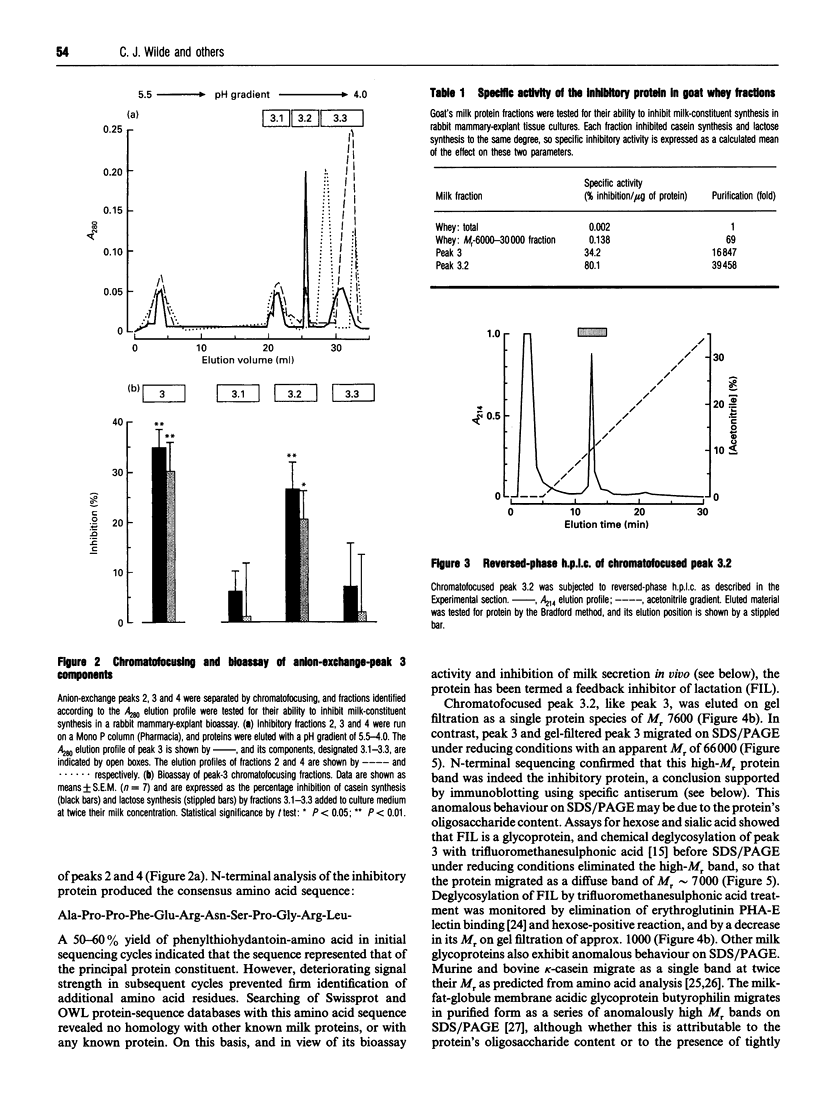
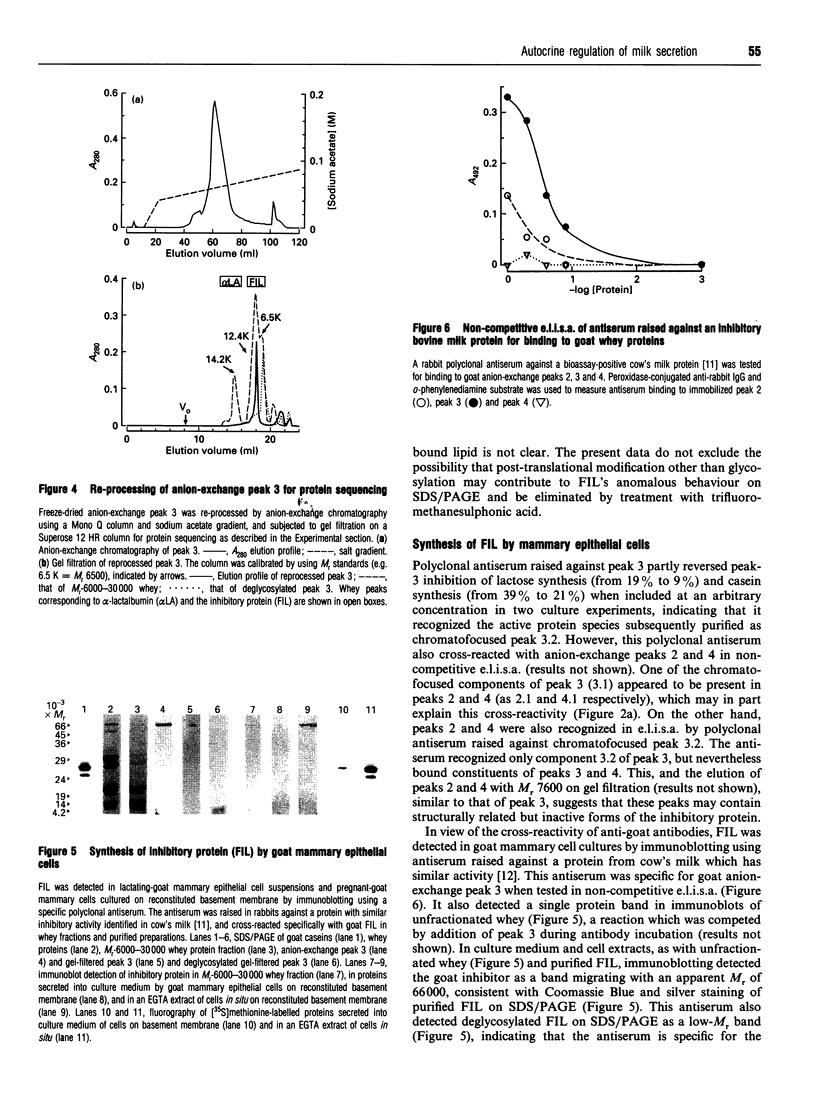
Frequency or completeness of milk removal from the lactating mammary gland regulates the rate of milk secretion by a mechanism which is local, chemical and inhibitory in nature. Screening of goat's milk proteins in rabbit mammary explant cultures identified a single whey protein of M(r) 7600 able to inhibit synthesis of milk constituents. The active whey protein, which we term FIL (Feedback inhibitor of Lactation), also decreased milk secretion temporarily when introduced into a mammary gland of lactating goats. FIL was synthesized by primary cultures of goat mammary epithelial cells, and was secreted vectorially together with other milk proteins. N-terminal amino acid sequencing indicated that it is a hitherto unknown protein. The evidence indicates that local regulation of milk secretion by milk removal is through autocrine feedback inhibition by this milk protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatchford D. R., Peaker M. Effects of frequent milking on milk secretion during lactation in the goat: relation to factors which limit the rate of secretion. Q J Exp Physiol. 1982 Apr;67(2):303–310. doi: 10.1113/expphysiol.1982.sp002638. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chu F. K. Requirements of cleavage of high mannose oligosaccharides in glycoproteins by peptide N-glycosidase F. J Biol Chem. 1986 Jan 5;261(1):172–177. [PubMed] [Google Scholar]
- Corrigan A. Z., Bilezikjian L. M., Carroll R. S., Bald L. N., Schmelzer C. H., Fendly B. M., Mason A. J., Chin W. W., Schwall R. H., Vale W. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology. 1991 Mar;128(3):1682–1684. doi: 10.1210/endo-128-3-1682. [DOI] [PubMed] [Google Scholar]
- Daly S. E., Owens R. A., Hartmann P. E. The short-term synthesis and infant-regulated removal of milk in lactating women. Exp Physiol. 1993 Mar;78(2):209–220. doi: 10.1113/expphysiol.1993.sp003681. [DOI] [PubMed] [Google Scholar]
- Dils R., Forsyth I. A. Preparation and culture of mammary gland explants. Methods Enzymol. 1981;72:724–742. doi: 10.1016/s0076-6879(81)72063-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V. Molecular weights of three mouse milk caseins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and kappa-like characteristics of a fourth casein. J Dairy Sci. 1976 Oct;59(10):1738–1745. doi: 10.3168/jds.S0022-0302(76)84431-1. [DOI] [PubMed] [Google Scholar]
- Hansen H. O., Tornehave D., Knudsen J. Synthesis of milk specific fatty acids and proteins by dispersed goat mammary-gland epithelial cells. Biochem J. 1986 Aug 15;238(1):167–172. doi: 10.1042/bj2380167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesom K. J., Souza P. F., Ilic V., Williamson D. H. Chain-length dependency of interactions of medium-chain fatty acids with glucose metabolism in acini isolated from lactating rat mammary glands. A putative feed-back to control milk lipid synthesis from glucose. Biochem J. 1992 Jan 1;281(Pt 1):273–278. doi: 10.1042/bj2810273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. J., Blatchford D. R., Peaker M. The effects of milking thrice instead of twice daily on milk secretion in the goat. Q J Exp Physiol. 1983 Oct;68(4):645–652. doi: 10.1113/expphysiol.1983.sp002754. [DOI] [PubMed] [Google Scholar]
- Henderson A. J., Peaker M. Effects of removing milk from the mammary ducts and alveoli, or of diluting stored milk, on the rate of milk secretion in the goat. Q J Exp Physiol. 1987 Jan;72(1):13–19. doi: 10.1113/expphysiol.1987.sp003039. [DOI] [PubMed] [Google Scholar]
- Henderson A. J., Peaker M. Feed-back control of milk secretion in the goat by a chemical in milk. J Physiol. 1984 Jun;351:39–45. doi: 10.1113/jphysiol.1984.sp015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyan N. K., Bahl O. P. Role of carbohydrate in human chorionic gonadotropin. Effect of deglycosylation on the subunit interaction and on its in vitro and in vivo biological properties. J Biol Chem. 1983 Jan 10;258(1):67–74. [PubMed] [Google Scholar]
- Kuhn N. J., White A. The topography of lactose synthesis. Biochem J. 1975 Apr;148(1):77–84. doi: 10.1042/bj1480077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The effects of oxytocin and milk removal on milk secretion in the goat. J Physiol. 1971 Aug;216(3):717–734. doi: 10.1113/jphysiol.1971.sp009549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Paine M. J., Desmond H. P., Theakston R. D., Crampton J. M. Gene expression in Echis carinatus (carpet viper) venom glands following milking. Toxicon. 1992 Apr;30(4):379–386. doi: 10.1016/0041-0101(92)90534-c. [DOI] [PubMed] [Google Scholar]
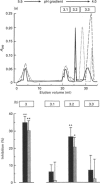
- Peaker M., Blatchford D. R. Distribution of milk in the goat mammary gland and its relation to the rate and control of milk secretion. J Dairy Res. 1988 Feb;55(1):41–48. doi: 10.1017/s0022029900025838. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Rubin K., Kjellén L., Laurent T. C., Klingeborn B. The viability of cells grown or centrifuged in a new density gradient medium, Percoll(TM). Exp Cell Res. 1977 Dec;110(2):449–457. doi: 10.1016/0014-4827(77)90311-1. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Rennison M. E., Kerr M., Addey C. V., Handel S. E., Turner M. D., Wilde C. J., Burgoyne R. D. Inhibition of constitutive protein secretion from lactating mouse mammary epithelial cells by FIL (feedback inhibitor of lactation), a secreted milk protein. J Cell Sci. 1993 Oct;106(Pt 2):641–648. doi: 10.1242/jcs.106.2.641. [DOI] [PubMed] [Google Scholar]
- Schmidt D. V., Ebner K. E. Multiple forms of pig, sheep and goat -lactalbumin. Biochim Biophys Acta. 1972 May 18;263(3):714–720. doi: 10.1016/0005-2795(72)90055-4. [DOI] [PubMed] [Google Scholar]
- Wand G. S., Takiyyuddin M., O'Connor D. T., Levine M. A. A proposed role for chromogranin A as a glucocorticoid-responsive autocrine inhibitor of proopiomelanocortin secretion. Endocrinology. 1991 Mar;128(3):1345–1351. doi: 10.1210/endo-128-3-1345. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Addey C. V., Casey M. J., Blatchford D. R., Peaker M. Feed-back inhibition of milk secretion: the effect of a fraction of goat milk on milk yield and composition. Q J Exp Physiol. 1988 May;73(3):391–397. doi: 10.1113/expphysiol.1988.sp003155. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Blatchford D. R., Knight C. H., Peaker M. Metabolic adaptations in goat mammary tissue during long-term incomplete milking. J Dairy Res. 1989 Feb;56(1):7–15. doi: 10.1017/s0022029900026169. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Blatchford D. R., Peaker M. Regulation of mouse mammary cell differentiation by extracellular milk proteins. Exp Physiol. 1991 May;76(3):379–387. doi: 10.1113/expphysiol.1991.sp003505. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Calvert D. T., Daly A., Peaker M. The effect of goat milk fractions on synthesis of milk constituents by rabbit mammary explants and on milk yield in vivo. Evidence for autocrine control of milk secretion. Biochem J. 1987 Feb 15;242(1):285–288. doi: 10.1042/bj2420285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Knight C. H. Milk yield and mammary function in goats during and after once-daily milking. J Dairy Res. 1990 Nov;57(4):441–447. doi: 10.1017/s0022029900029484. [DOI] [PubMed] [Google Scholar]
- Yao K., Ubuka T., Masuoka N., Kinuta M., Ikeda T. Direct determination of bound sialic acids in sialoglycoproteins by acidic ninhydrin reaction. Anal Biochem. 1989 Jun;179(2):332–335. doi: 10.1016/0003-2697(89)90138-3. [DOI] [PubMed] [Google Scholar]