Abstract
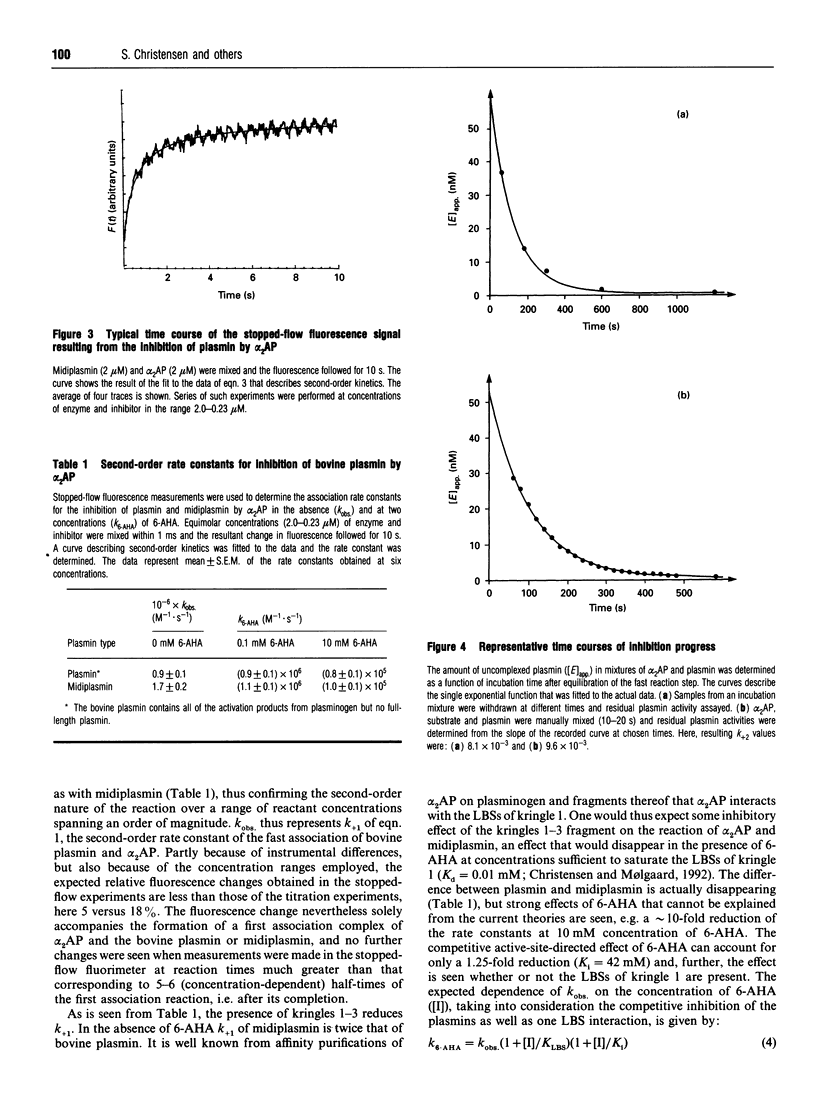
In the conversion of bovine plasminogen to bovine plasmin not only the expected urokinase-catalysed cleavage of Arg-557-Val-558, and the following autocatalytic cleavage separating the N-terminal peptide 1-77 from the heavy chain of plasmin, but also a cleavage at Arg-342-Met-343 between kringles 3 and 4 is seen. Here, kinetic studies of the interaction of bovine alpha 2-antiplasmin with bovine plasmin were performed on isolated bovine midiplasmin (lacking kringles 1-3) and on bovine plasmin containing all of the activation products from the bovine plasminogen. A series of experiments using stopped-flow fluorescence fast kinetics as well as conventional techniques suggests a reaction model in accordance with the one known for the human system. First, a tight complex (K1 in the nanomolar range) is formed in a fast reaction step; and second, a tightening of this complex occurs in a slow reaction step. The final complex is indeed so tight (Ki < or = pM), that the reaction for many practical purposes is legitimately considered irreversible. The stopped-flow method allows for the determination of reliable values of the second-order rate constant for the fast association step. At pH 7.4 and 25 degrees C, k+1 = 1.7 x 10(6) M-1 s-1 was obtained in the absence and k+1 = 0.9 x 10(6) M-1.s-1 in the presence of the kringles 1-3 domain of bovine plasmin. In contrast to this, substantial reductions of k+1 were seen in the presence of concentrations of 6-amino-hexanoic acid corresponding to lysine-binding-site interactions and far too low to be attributed to active-site interactions with the bovine plasmins (for each, Ki = 42 mM). All in all, the data indicated that the lysine-binding site(s) not of kringle 1, but of midiplasmin (those of kringles 4 and 5) are regulating the inhibition reaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anonick P. K., Gonias S. L. Soluble fibrin preparations inhibit the reaction of plasmin with alpha 2-macroglobulin. Comparison with alpha 2-antiplasmin and leupeptin. Biochem J. 1991 Apr 1;275(Pt 1):53–59. doi: 10.1042/bj2750053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert K., Johnsen A. H., Christensen U., Thorsen S. Different N-terminal forms of alpha 2-plasmin inhibitor in human plasma. Biochem J. 1993 Apr 15;291(Pt 2):623–625. doi: 10.1042/bj2910623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F. J., Powell J. R. Human plasminogen. Methods Enzymol. 1981;80(Pt 100):365–378. doi: 10.1016/s0076-6879(81)80031-6. [DOI] [PubMed] [Google Scholar]
- Christensen S., Sottrup-Jensen L. Bovine alpha 2-antiplasmin. N-terminal and reactive site sequence. FEBS Lett. 1992 Nov 2;312(1):100–104. doi: 10.1016/0014-5793(92)81419-m. [DOI] [PubMed] [Google Scholar]
- Christensen U. C-terminal lysine residues of fibrinogen fragments essential for binding to plasminogen. FEBS Lett. 1985 Mar 11;182(1):43–46. doi: 10.1016/0014-5793(85)81150-9. [DOI] [PubMed] [Google Scholar]
- Christensen U., Clemmensen I. Kinetic properties of the primary inhibitor of plasmin from human plasma. Biochem J. 1977 May 1;163(2):389–391. doi: 10.1042/bj1630389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U., Clemmensen I. Purification and reaction mechanisms of the primary inhibitor of plasmin from human plasma. Biochem J. 1978 Nov 1;175(2):635–641. doi: 10.1042/bj1750635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U. Kinetic studies of the urokinase-catalysed conversion of NH2-terminal glutamic acid plasminogen to plasmin. Biochim Biophys Acta. 1977 Apr 12;481(2):638–647. doi: 10.1016/0005-2744(77)90297-2. [DOI] [PubMed] [Google Scholar]
- Christensen U., Mølgaard L. Positive co-operative binding at two weak lysine-binding sites governs the Glu-plasminogen conformational change. Biochem J. 1992 Jul 15;285(Pt 2):419–425. doi: 10.1042/bj2850419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U., Sottrup-Jensen L., Magnusson S., Petersen T. E., Clemmensen I. Enzymic properties of the neo-plasmin-Val-422 (miniplasmin). Biochim Biophys Acta. 1979 Apr 12;567(2):472–481. doi: 10.1016/0005-2744(79)90133-5. [DOI] [PubMed] [Google Scholar]
- Christensen U. The AH-site of plasminogen and two C-terminal fragments. A weak lysine-binding site preferring ligands not carrying a free carboxylate function. Biochem J. 1984 Oct 15;223(2):413–421. doi: 10.1042/bj2230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen S. J., Bell S. M., Schaefer L. A., Elliott R. W. Characterization of the cDNA coding for mouse plasminogen and localization of the gene to mouse chromosome 17. Genomics. 1990 Sep;8(1):49–61. doi: 10.1016/0888-7543(90)90225-j. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Enghild J. J., Valnickova Z., Thøgersen I. B., Pizzo S. V., Salvesen G. An examination of the inhibitory mechanism of serpins by analysing the interaction of trypsin and chymotrypsin with alpha 2-antiplasmin. Biochem J. 1993 May 1;291(Pt 3):933–938. doi: 10.1042/bj2910933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. E., Nelles L., Lijnen H. R., Collen D. Primary structure of human alpha 2-antiplasmin, a serine protease inhibitor (serpin). J Biol Chem. 1987 Feb 5;262(4):1659–1664. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerch P. G., Rickli E. E., Lergier W., Gillessen D. Localization of individual lysine-binding regions in human plasminogen and investigations on their complex-forming properties. Eur J Biochem. 1980;107(1):7–13. doi: 10.1111/j.1432-1033.1980.tb04617.x. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., Matsuo O., Collen D. On the molecular interactions between plasminogen-staphylokinase, alpha 2-antiplasmin and fibrin. Biochim Biophys Acta. 1992 Jan 9;1118(2):144–148. doi: 10.1016/0167-4838(92)90142-z. [DOI] [PubMed] [Google Scholar]
- Llinas M., De Marco A., Hochschwender S. M., Laursen R. A. A 1H-NMR study of isolated domains from human plasminogen. Structural homology between kringles 1 and 4. Eur J Biochem. 1983 Oct 3;135(3):379–391. doi: 10.1111/j.1432-1033.1983.tb07665.x. [DOI] [PubMed] [Google Scholar]
- Longstaff C., Gaffney P. J. Serpin-serine protease binding kinetics: alpha 2-antiplasmin as a model inhibitor. Biochemistry. 1991 Jan 29;30(4):979–986. doi: 10.1021/bi00218a014. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Markus G., Priore R. L., Wissler F. C. The binding of tranexamic acid to native (Glu) and modified (Lys) human plasminogen and its effect on conformation. J Biol Chem. 1979 Feb 25;254(4):1211–1216. [PubMed] [Google Scholar]
- Marti T., Schaller J., Rickli E. E. Determination of the complete amino-acid sequence of porcine miniplasminogen. Eur J Biochem. 1985 Jun 3;149(2):279–285. doi: 10.1111/j.1432-1033.1985.tb08923.x. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Petersen T. E., Martzen M. R., Ichinose A., Davie E. W. Characterization of the gene for human plasminogen, a key proenzyme in the fibrinolytic system. J Biol Chem. 1990 Apr 15;265(11):6104–6111. [PubMed] [Google Scholar]
- Ponting C. P., Marshall J. M., Cederholm-Williams S. A. Plasminogen: a structural review. Blood Coagul Fibrinolysis. 1992 Oct;3(5):605–614. [PubMed] [Google Scholar]
- Schaller J., Moser P. W., Dannegger-Müller G. A., Rösselet S. J., Kämpfer U., Rickli E. E. Complete amino acid sequence of bovine plasminogen. Comparison with human plasminogen. Eur J Biochem. 1985 Jun 3;149(2):267–278. doi: 10.1111/j.1432-1033.1985.tb08921.x. [DOI] [PubMed] [Google Scholar]
- Schaller J., Rickli E. E. Structural aspects of the plasminogen of various species. Enzyme. 1988;40(2-3):63–69. doi: 10.1159/000469147. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Potempa J., Travis J. The use of alpha 2-antiplasmin as a model for the demonstration of complex reversibility in serpins. J Biol Chem. 1989 Aug 15;264(23):13420–13423. [PubMed] [Google Scholar]
- Suenson E., Thorsen S. Secondary-site binding of Glu-plasmin, Lys-plasmin and miniplasmin to fibrin. Biochem J. 1981 Sep 1;197(3):619–628. doi: 10.1042/bj1970619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N., Sasaki T., Iwamoto M., Abiko Y. Binding site of alpha 2-plasmin inhibitor to plasminogen. Biochim Biophys Acta. 1988 Jan 4;952(1):1–7. doi: 10.1016/0167-4838(88)90094-5. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin catalyzed by activated fibrin-stabilizing factor. J Biol Chem. 1982 Dec 25;257(24):14767–14772. [PubMed] [Google Scholar]
- Thewes T., Ramesh V., Simplaceanu E. L., Llinás M. Isolation, purification and 1H-NMR characterization of a kringle 5 domain fragment from human plasminogen. Biochim Biophys Acta. 1987 Apr 8;912(2):254–269. doi: 10.1016/0167-4838(87)90096-3. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Clemmensen I., Sottrup-Jensen L., Magnusson S. Adsorption to fibrin of native fragments of known primary structure from human plasminogen. Biochim Biophys Acta. 1981 May 29;668(3):377–387. doi: 10.1016/0005-2795(81)90171-9. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wiman B., Boman L., Collen D. On the kinetics of the reaction between human antiplasmin and a low-molecular-weight form of plasmin. Eur J Biochem. 1978 Jun 1;87(1):143–146. doi: 10.1111/j.1432-1033.1978.tb12360.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. Molecular mechanism of physiological fibrinolysis. Nature. 1978 Apr 6;272(5653):549–550. doi: 10.1038/272549a0. [DOI] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]