Abstract
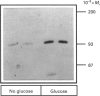
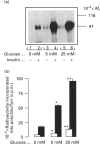
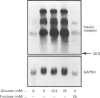
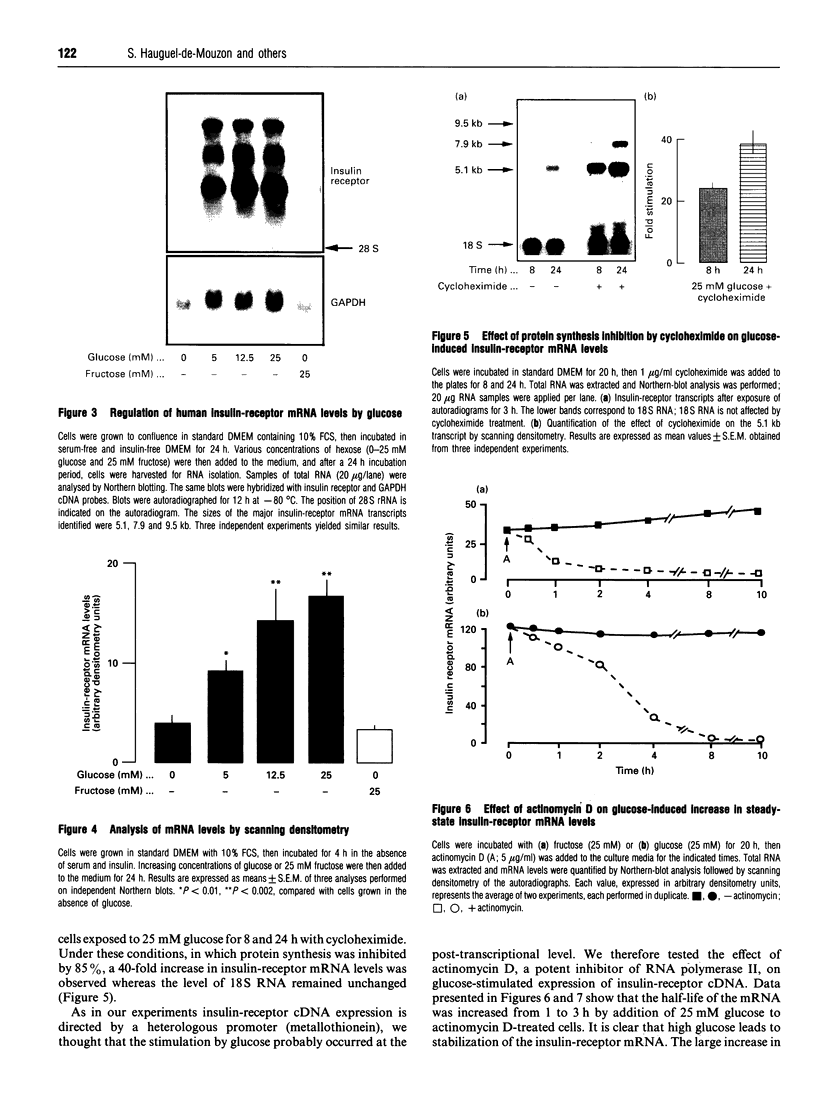
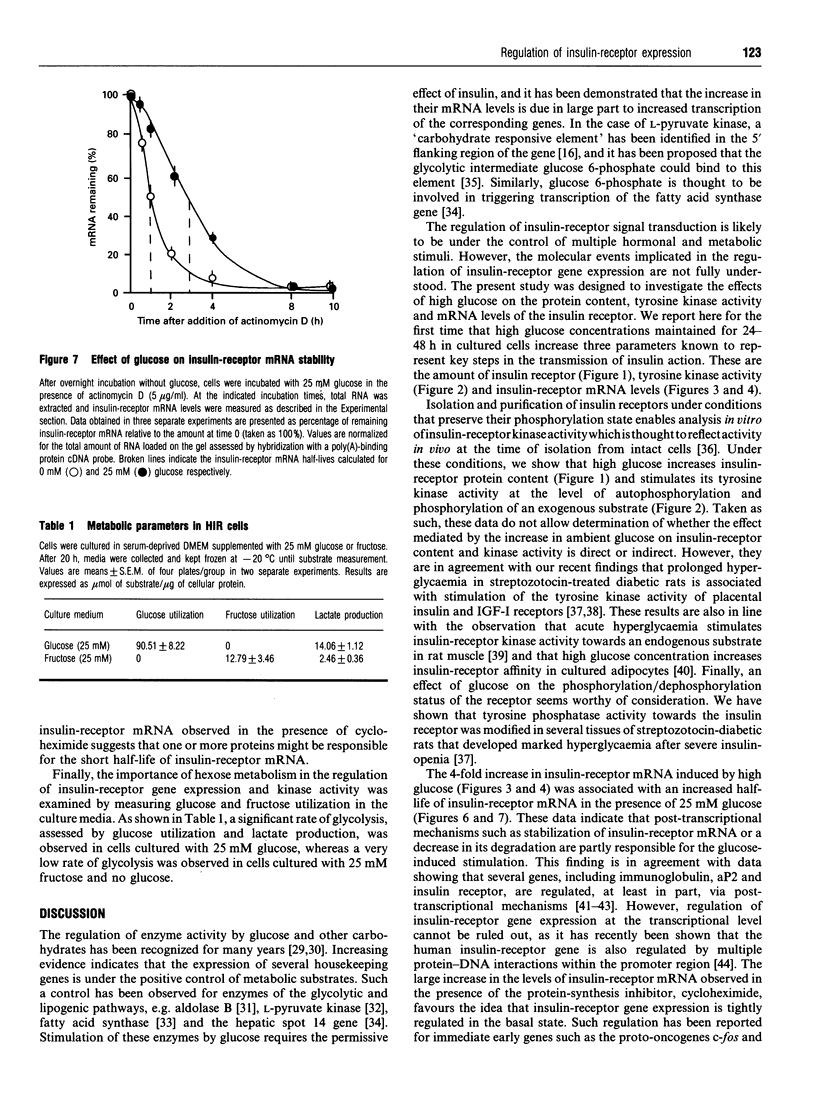
The effects of high glucose on insulin-receptor tyrosine kinase activity and gene expression were investigated in 3T3-HIR cells. Cells incubated for 48 h in the presence of 25 mM glucose showed a 5-fold increase in the amount of insulin receptors per cell, receptor autophosphorylation and phosphorylation of the exogenous substrate poly(Glu/Tyr) compared with cells grown in the absence of glucose but in the presence of 25 mM fructose. These effects were associated with a 4-fold stimulation in steady-state levels of insulin-receptor mRNA. Significant cellular glucose utilization and lactate production were observed in the presence of high glucose in the culture medium, indicating a functional glycolytic pathway in glucose-treated cells, but not in cells treated with fructose. Such a differential response to hexoses favours the hypothesis of a carbohydrate regulation via a glycolytic intermediate. This was further supported by a similar glucose-induced increase in mRNA levels of the enzyme glyceraldehyde-3-phosphate dehydrogenase. To test the hypothesis that the stimulatory effect of glucose on amount of insulin receptors and phosphorylation state could result from post-transcriptional modifications, cells exposed to glucose were incubated with actinomycin D, a potent inhibitor of gene transcription. In cells challenged with high glucose plus inhibitor, insulin-receptor mRNA half-life was increased from 1 to 3 h, indicating that posttranscriptional mechanisms are involved in these processes of glucose regulation. Inhibition of protein synthesis by cycloheximide induced an overexpression of insulin-receptor mRNA levels in the presence of glucose, suggesting that labile repressor protein(s) could be implicated in the effects of glucose. We conclude that (1) long-term culture with high glucose increases the amount of insulin receptors and their tyrosine kinase activity and (2) the glucose-induced increase in insulin-receptor mRNA levels can be accounted for, at least in part, by posttranscriptional events.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergot M. O., Diaz-Guerra M. J., Puzenat N., Raymondjean M., Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992 Apr 25;20(8):1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryer-Ash M. Regulation of rat insulin-receptor kinase by glucose in vivo. Diabetes. 1991 May;40(5):633–640. doi: 10.2337/diab.40.5.633. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Antoine B., Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989 Jul 15;264(20):11584–11590. [PubMed] [Google Scholar]
- Decaux J. F., Marcillat O., Pichard A. L., Henry J., Kahn A. Glucose-dependent and -independent effect of insulin on gene expression. J Biol Chem. 1991 Feb 25;266(6):3432–3438. [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Robinson G. S., Spiegelman B. M. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992 Mar 25;267(9):5937–5941. [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- FITCH W. M., CHAIKOFF I. L. Extent and patterns of adaptation of enzyme activities in livers of normal rats fed diets high in glucose and fructose. J Biol Chem. 1960 Mar;235:554–557. [PubMed] [Google Scholar]
- Foufelle F., Gouhot B., Pégorier J. P., Perdereau D., Girard J., Ferré P. Glucose stimulation of lipogenic enzyme gene expression in cultured white adipose tissue. A role for glucose 6-phosphate. J Biol Chem. 1992 Oct 15;267(29):20543–20546. [PubMed] [Google Scholar]
- Freidenberg G. R., Klein H. H., Cordera R., Olefsky J. M. Insulin receptor kinase activity in rat liver. Regulation by fasting and high carbohydrate feeding. J Biol Chem. 1985 Oct 15;260(23):12444–12453. [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Goto Y., Mariash C. N. Cell-specific carbohydrate metabolism regulates S14 gene transcription. Diabetes. 1992 Mar;41(3):339–346. doi: 10.2337/diab.41.3.339. [DOI] [PubMed] [Google Scholar]
- Gunn J. M., Taylor C. B. Relationships between concentration of hepatic intermediary metabolites and induction of the key glycolytic enzymes in vivo. Biochem J. 1973 Nov;136(3):455–465. doi: 10.1042/bj1360455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S., Louizeau M., Girard J. Functional alterations of type I insulin-like growth factor receptor in placenta of diabetic rats. Biochem J. 1992 Nov 15;288(Pt 1):273–279. doi: 10.1042/bj2880273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S., Peraldi P., Alengrin F., Van Obberghen E. Alteration of phosphotyrosine phosphatase activity in tissues from diabetic and pregnant rats. Endocrinology. 1993 Jan;132(1):67–74. doi: 10.1210/endo.132.1.8419148. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. Immunoglobulin mRNA stability varies during B lymphocyte differentiation. EMBO J. 1988 Apr;7(4):1041–1046. doi: 10.1002/j.1460-2075.1988.tb02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kahn B. B. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992 May;89(5):1367–1374. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. J., Sharma R. P., Sale G. J. Site-specific dephosphorylation and deactivation of the human insulin receptor tyrosine kinase by particulate and soluble phosphotyrosyl protein phosphatases. Biochem J. 1991 Apr 15;275(Pt 2):413–418. doi: 10.1042/bj2750413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. K., Tam J. W., Tsai M. J., Tsai S. Y. Identification of cis- and trans-acting factors regulating the expression of the human insulin receptor gene. J Biol Chem. 1992 Mar 5;267(7):4638–4645. [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B. Multiple mechanisms regulate c-myc gene expression during normal T cell activation. EMBO J. 1988 Sep;7(9):2787–2794. doi: 10.1002/j.1460-2075.1988.tb03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamula P. W., Wong K. Y., Maddux B. A., McDonald A. R., Goldfine I. D. Sequence and analysis of promoter region of human insulin-receptor gene. Diabetes. 1988 Sep;37(9):1241–1246. doi: 10.2337/diab.37.9.1241. [DOI] [PubMed] [Google Scholar]
- Marie S., Diaz-Guerra M. J., Miquerol L., Kahn A., Iynedjian P. B. The pyruvate kinase gene as a model for studies of glucose-dependent regulation of gene expression in the endocrine pancreatic beta-cell type. J Biol Chem. 1993 Nov 15;268(32):23881–23890. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Oemar B. S., Foellmer H. G., Hodgdon-Anandant L., Rosenzweig S. A. Regulation of insulin-like growth factor I receptors in diabetic mesangial cells. J Biol Chem. 1991 Feb 5;266(4):2369–2373. [PubMed] [Google Scholar]
- Ooi G. T., Brown D. R., Suh D. S., Tseng L. Y., Rechler M. M. Cycloheximide stabilizes insulin-like growth factor-binding protein-1 (IGFBP-1) mRNA and inhibits IGFBP-1 transcription in H4-II-E rat hepatoma cells. J Biol Chem. 1993 Aug 5;268(22):16664–16672. [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Seino S., Seino M., Bell G. I. Human insulin-receptor gene. Partial sequence and amplification of exons by polymerase chain reaction. Diabetes. 1990 Jan;39(1):123–128. doi: 10.2337/diacare.39.1.123. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Tartare S., Ballotti R., Van Obberghen E. Interaction between heterologous receptor tyrosine kinases. Hormone-stimulated insulin receptors activate unoccupied IGF-I receptors. FEBS Lett. 1991 Dec 16;295(1-3):219–222. doi: 10.1016/0014-5793(91)81422-5. [DOI] [PubMed] [Google Scholar]
- Tewari M., Tewari D. S., Taub R. Posttranscriptional mechanisms account for differences in steady state levels of insulin receptor messenger RNA in different cells. Mol Endocrinol. 1991 May;5(5):653–660. doi: 10.1210/mend-5-5-653. [DOI] [PubMed] [Google Scholar]
- Thies R. S., Molina J. M., Ciaraldi T. P., Freidenberg G. R., Olefsky J. M. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes. 1990 Feb;39(2):250–259. doi: 10.2337/diab.39.2.250. [DOI] [PubMed] [Google Scholar]
- Tozzo E., Desbuquois B. Effects of STZ-induced diabetes and fasting on insulin receptor mRNA expression and insulin receptor gene transcription in rat liver. Diabetes. 1992 Dec;41(12):1609–1616. doi: 10.2337/diab.41.12.1609. [DOI] [PubMed] [Google Scholar]
- Traxinger R. R., Marshall S. Glucose regulation of insulin receptor affinity in primary cultured adipocytes. J Biol Chem. 1990 Nov 5;265(31):18879–18883. [PubMed] [Google Scholar]
- Watarai T., Kobayashi M., Takata Y., Sasaoka T., Iwasaki M., Shigeta Y. Alteration of insulin-receptor kinase activity by high-fat feeding. Diabetes. 1988 Oct;37(10):1397–1404. doi: 10.2337/diab.37.10.1397. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]