Abstract
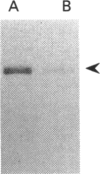
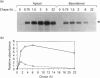
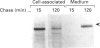
LLC-PK1 cells were transfected with a cDNA encoding rabbit neutral endopeptidase (NEP; EC 3.4.24.11), an abundant enzyme of the kidney proximal brush border. Clones of cells expressing high levels of the protein were isolated. Selective biotinylation and radioimmunolabelling were used to determine that 85-95% of NEP was localized in the apical domain of filter-grown LLC-PK1 cells. Pulse-chase and selective biotinylation studies revealed that the majority (85%) of newly made NEP was directly targeted to the apical membrane. However, a soluble form of NEP was found to be secreted in approximately equal amounts from both sides of the monolayer when expressed in LLC-PK1 cells. Transfected pro-opiomelanocortin, a pituitary hormone precursor, was secreted almost exclusively into the basolateral medium, suggesting that the bulk flow is to the basolateral membrane. This behaviour contrasts with that observed in MDCK cells, where both the transmembrane and secreted forms of NEP are directly targeted to the apical membrane and where the secretion of pro-opiomelanocortin is unpolarized.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Hubbard A. L. Plasma membrane protein sorting in epithelial cells: do secretory pathways hold the key? Trends Biochem Sci. 1988 May;13(5):181–184. doi: 10.1016/0968-0004(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Canipari R., Zurzolo C., Polistina C., Garbi C., Aloj L., Calì G., Gentile R., Nitsch L. Polarized secretion of plasminogen activators by epithelial cell monolayers. Biochim Biophys Acta. 1992 Dec 15;1175(1):1–6. doi: 10.1016/0167-4889(92)90002-s. [DOI] [PubMed] [Google Scholar]
- Chevrier D., Lemieux E., Fyfe M., Labonté N., Zollinger M., Bennett H. P., Crine P. Production of monoclonal antibodies against the N-terminal glycopeptide of porcine pro-opiomelanocortin: their use for solid-phase radioimmunoassay, western blotting, and immunogold cytochemistry. Biochem Cell Biol. 1991 Jan;69(1):58–65. doi: 10.1139/o91-008. [DOI] [PubMed] [Google Scholar]
- Chevrier D., Vézina C., Bastille J., Linard C., Sonenberg N., Boileau G. Higher order structures of the 5'-proximal region decrease the efficiency of translation of the porcine pro-opiomelanocortin mRNA. J Biol Chem. 1988 Jan 15;263(2):902–910. [PubMed] [Google Scholar]
- Corbeil D., Boileau G., Lemay G., Crine P. Expression and polarized apical secretion in Madin-Darby canine kidney cells of a recombinant soluble form of neutral endopeptidase lacking the cytosolic and transmembrane domains. J Biol Chem. 1992 Feb 5;267(4):2798–2801. [PubMed] [Google Scholar]
- Crine P., LeGrimellec C., Lemieux E., Labonté L., Fortin S., Blachier A., Aubry M. The production and characterization of a monoclonal antibody specific for the 94,000 dalton enkephalin-degrading peptidase from rabbit kidney brush border. Biochem Biophys Res Commun. 1985 Aug 30;131(1):255–261. doi: 10.1016/0006-291x(85)91796-6. [DOI] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Sales V., Nault C., Beaumont A., Roques B., Crine P., Boileau G. Exploration of the catalytic site of endopeptidase 24.11 by site-directed mutagenesis. Histidine residues 583 and 587 are essential for catalysis. FEBS Lett. 1988 Apr 11;231(1):54–58. doi: 10.1016/0014-5793(88)80701-4. [DOI] [PubMed] [Google Scholar]
- Fossiez F., Lemay G., Labonté N., Parmentier-Lesage F., Boileau G., Crine P. Secretion of a functional soluble form of neutral endopeptidase-24.11 from a baculovirus-infected insect cell line. Biochem J. 1992 May 15;284(Pt 1):53–59. doi: 10.1042/bj2840053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. A., Beaudry G., Rizzolo L., Colman A., Rindler M., Adesnik M., Sabatini D. D. Secretion of endogenous and exogenous proteins from polarized MDCK cell monolayers. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2100–2104. doi: 10.1073/pnas.83.7.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstraunthaler G., Pfaller W., Kotanko P. Biochemical characterization of renal epithelial cell cultures (LLC-PK1 and MDCK). Am J Physiol. 1985 Apr;248(4 Pt 2):F536–F544. doi: 10.1152/ajprenal.1985.248.4.F536. [DOI] [PubMed] [Google Scholar]
- Jalal F., Lemay G., Zollinger M., Berteloot A., Boileau G., Crine P. Neutral endopeptidase, a major brush border protein of the kidney proximal nephron, is directly targeted to the apical domain when expressed in Madin-Darby canine kidney cells. J Biol Chem. 1991 Oct 15;266(29):19826–19832. [PubMed] [Google Scholar]
- Kenny A. J. Regulatory peptide metabolism at cell surfaces: the key role of endopeptidase-24.11. Biomed Biochim Acta. 1986;45(11-12):1503–1513. [PubMed] [Google Scholar]
- Landry C., Santagata P., Bawab W., Fournié-Zaluski M. C., Roques B. P., Vinay P., Crine P. Characterization of neutral endopeptidase 24.11 in dog glomeruli. Biochem J. 1993 May 1;291(Pt 3):773–779. doi: 10.1042/bj2910773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990 Oct;111(4):1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay G., Waksman G., Roques B. P., Crine P., Boileau G. Fusion of a cleavable signal peptide to the ectodomain of neutral endopeptidase (EC 3.4.24.11) results in the secretion of an active enzyme in COS-1 cells. J Biol Chem. 1989 Sep 15;264(26):15620–15623. [PubMed] [Google Scholar]
- Lorkowski G., Zijderhand-Bleekemolen J. E., Erdös E. G., von Figura K., Hasilik A. Neutral endopeptidase-24.11 (enkephalinase). Biosynthesis and localization in human fibroblasts. Biochem J. 1987 Dec 1;248(2):345–350. doi: 10.1042/bj2480345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Wong S. H., Tang B. L., Hong W. J. Involvement of both vectorial and transcytotic pathways in the preferential apical cell surface localization of rat dipeptidyl peptidase IV in transfected LLC-PK1 cells. J Biol Chem. 1991 Oct 15;266(29):19710–19716. [PubMed] [Google Scholar]
- Low S. H., Wong S. H., Tang B. L., Subramaniam V. N., Hong W. J. Apical cell surface expression of rat dipeptidyl peptidase IV in transfected Madin-Darby canine kidney cells. J Biol Chem. 1991 Jul 15;266(20):13391–13396. [PubMed] [Google Scholar]
- Massey D., Feracci H., Gorvel J. P., Rigal A., Soulié J. M., Maroux S. Evidence for the transit of aminopeptidase N through the basolateral membrane before it reaches the brush border of enterocytes. J Membr Biol. 1987;96(1):19–25. doi: 10.1007/BF01869331. [DOI] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell. 1990 Feb 9;60(3):429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Heath L. N. Growth, enzyme activity, sugar transport, and hormone supplement responses in cells cloned from a pig kidney cell line LLC-PK1. J Cell Physiol. 1989 Jun;139(3):538–549. doi: 10.1002/jcp.1041390313. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Breitfeld P., Harris J. M. An anchor-minus form of the polymeric immunoglobulin receptor is secreted predominantly apically in Madin-Darby canine kidney cells. J Cell Biol. 1987 Nov;105(5):2031–2036. doi: 10.1083/jcb.105.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I. R., Le Bivic A., Fambrough D., Rodriguez-Boulan E. An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol. 1991 Dec;115(6):1573–1584. doi: 10.1083/jcb.115.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R., Herz R. E. Polarized insertion of an intracellular glycoprotein pool into the apical membrane of MDCK cells. Am J Physiol. 1990 Mar;258(3 Pt 1):C390–C398. doi: 10.1152/ajpcell.1990.258.3.C390. [DOI] [PubMed] [Google Scholar]
- Rabito C. A., Karish M. V. Polarized amino acid transport by an epithelial cell line of renal origin (LLC-PK1). The basolateral systems. J Biol Chem. 1982 Jun 25;257(12):6802–6808. [PubMed] [Google Scholar]
- Ragno P., Estreicher A., Gos A., Wohlwend A., Belin D., Vassalli J. D. Polarized secretion of urokinase-type plasminogen activator by epithelial cells. Exp Cell Res. 1992 Nov;203(1):236–243. doi: 10.1016/0014-4827(92)90060-l. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Traber M. G. A specific sorting signal is not required for the polarized secretion of newly synthesized proteins from cultured intestinal epithelial cells. J Cell Biol. 1988 Aug;107(2):471–479. doi: 10.1083/jcb.107.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C., Ausiello D. A. Characterization of (8-lysine) vasopressin binding sites on a pig kidney cell line (LLC-PK1). Evidence for hormone-induced receptor transition. J Biol Chem. 1981 Apr 10;256(7):3415–3422. [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Schulz W. W., Hagler H. K., Buja L. M., Erdös E. G. Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest. 1988 Dec;59(6):789–797. [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Vogel L. K., Spiess M., Sjöström H., Norén O. Evidence for an apical sorting signal on the ectodomain of human aminopeptidase N. J Biol Chem. 1992 Feb 5;267(4):2794–2797. [PubMed] [Google Scholar]
- Weisz O. A., Machamer C. E., Hubbard A. L. Rat liver dipeptidylpeptidase IV contains competing apical and basolateral targeting information. J Biol Chem. 1992 Nov 5;267(31):22282–22288. [PubMed] [Google Scholar]
- Wessels H. P., Hansen G. H., Fuhrer C., Look A. T., Sjöström H., Norén O., Spiess M. Aminopeptidase N is directly sorted to the apical domain in MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 2):2923–2930. doi: 10.1083/jcb.111.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., Le Bivic A., Quaroni A., Nitsch L., Rodriguez-Boulan E. Modulation of transcytotic and direct targeting pathways in a polarized thyroid cell line. EMBO J. 1992 Jun;11(6):2337–2344. doi: 10.1002/j.1460-2075.1992.tb05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]