Abstract
Background:
Excess reactive oxygen species and reactive nitrogen species are implicated in male infertility and impaired spermatogenesis.
Aim:
To investigate the effect of excess reactive nitrogen species and nitrosative stress on testicular function and the hypothalamic-pituitary-gonadal axis using the S-nitrosoglutathione reductase-null (Gsnor−/−) mouse model.
Methods:
Testis size, pup number, and epididymal sperm concentration and motility of Gsnor−/− mice were compared with those of age-matched wild-type (WT) mice. Reproductive hormones testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone were compared in Gsnor−/− and WT mice. Immunofluorescence for Gsnor−/− and WT testis was performed for 3β-hydroxysteroid dehydrogenase and luteinizing hormone receptor (LHR) and compared. Human chorionic gonadotropin and gonadotropin-releasing hormone stimulation tests were performed to assess and compare testicular and pituitary functions of Gsnor−/− and WT mice.
Outcomes:
Evaluation of fertility and reproductive hormones in Gsnor−/− vs WT mice. Response of Gsnor−/− and WT mice to human chorionic gonadotropin and gonadotropin-releasing hormone to evaluate LH and T production.
Results:
Gsnor−/− mice had smaller litters (4.2 vs 8.0 pups per litter; P < .01), smaller testes (0.08 vs 0.09 g; P < .01), and decreased epididymal sperm concentration (69 vs 98 × 106; P < .05) and motility (39% vs 65%; P < .05) compared with WT mice. Serum T (44.8 vs 292.2 ng/dL; P < .05) and LH (0.03 vs 0.74 ng/mL; P = .04) were lower in Gsnor−/− than in WT mice despite similar follicle-stimulating hormone levels (63.98 vs 77.93 ng/mL; P = .20). Immunofluorescence of Gsnor−/− and WT testes showed similar staining of 3β-hydroxysteroid dehydrogenase and LHR. Human chorionic gonadotropin stimulation of Gsnor−/− mice increased serum T (>1,680 vs >1,680 ng/dL) and gonadotropin-releasing hormone stimulation increased serum LH (6.3 vs 8.9 ng/mL; P = .20) similar to WT mice.
Clinical Translation:
These findings provide novel insight to a possible mechanism of secondary hypogonadism from increased reactive nitrogen species and excess nitrosative stress.
Strengths and Limitations:
Limitations of this study are its small samples and variability in hormone levels.
Conclusion:
Deficiency of S-nitrosoglutathione reductase results in secondary hypogonadism, suggesting that excess nitrosative stress can affect LH production from the pituitary gland.
Keywords: Reactive Nitrogen Species, Secondary Hypogonadism, Nitrosative Stress
INTRODUCTION
Male hypogonadism is defined as a combination of low serum testosterone level and associated symptoms, such decreased energy, low libido, weight gain, and loss of muscle mass.1 The diagnosis of male hypogonadism affects up to 24% of men older than 40 years.2 Hypogonadism is divided into 3 distinct classifications: primary (testicular failure), secondary (hypothalamic-pituitary failure), and compensated (eugonadism with compensatory increased pituitary hormone secretion). Secondary hypogonadism, the most common form with an estimated prevalence of 11.8%,3 results from decreased pituitary secretion of luteinizing hormone (LH) and/or follicle-stimulating hormone (FSH) or deficiencies in gonadotropin-releasing hormone (GnRH) production from the hypothalamus. Most etiologies of secondary hypogonadism remain idiopathic and associated with comorbid conditions such as excess opioid use, arthritis, diabetes, and obesity.4,5 Despite the high prevalence and association with common comorbidities, few studies have identified an etiology for secondary hypogonadism.
The current standard of care for hypogonadism, regardless of the cause, is testosterone replacement therapy. Unfortunately, testosterone replacement has side effects such as infertility, polycythemia, gynecomastia, hypertension, and possibly atherosclerosis.6 This treatment is undesirable for many men, especially those who are concerned about fertility. Identifying mechanisms of secondary hypogonadism could enable recognition of strategies to increase testosterone without administering exogenous testosterone therapy.
Reactive nitrogen species (RNS) are similar to free oxygen radicals called reactive oxygen species (ROS).7 Like ROS, RNS regulate normal cellular function at physiologic levels, but in excessive amounts can cause nitrosative stress and affect semen parameters and reproductive hormone signaling.7,8 Emerging data suggest that the nitroso-redox balance also can play a critical role in the regulation of LH signaling and therefore an imbalance can lead to secondary hypogonadism.9,10
Mice lacking S-nitrosoglutathione reductase (GSNOR), a denitrosylase that regulates S-nitrosylation, have increased levels of S-nitrosoglutathione (GSNO) and exhibit nitrosative stress.11 GSNO is in equilibrium with protein S-nitrosylation in cells, and GSNOR controls the cellular concentration of protein S-nitrosylation. GSNOR is a ubiquitous protein found in tissues such as the liver, thymus, spleen, and heart.12 Loss of GSNOR leads to increased levels of GSNO and subsequent modification of cysteine residues by S-nitrosylation, thereby affecting cellular signaling. We hypothesized that nitrosative stress in Gsnor−/− mice would affect hypothalamic-pituitary-gonadal axis function and spermatogenesis. We characterized testicular and pituitary functions of Gsnor−/− mice. We evaluated fertility, sperm parameters, testis histology, and reproductive hormone levels in Gsnor−/− mice and compared them with adult wild-type (WT) control mice.
METHODS
Mice
Male mice lacking GSNOR (Gsnor−/−) were generated as described previously and compared with age- and sex-matched WT littermates C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA).12 Pups were genotyped and sacrificed at the indicated ages. Mice were kept in a barrier-protected animal facility with 12-hour light-dark cycles. Unless otherwise stated, all experiments were carried out after 42 days of age to ensure 1 cycle of spermatogenesis was completed. All methods were performed in accordance with the approved institutional animal care and use committee protocol from the University of Miami (Miami, FL, USA).
Male Fertility Test
All mice used for breeding were at least 3 months of age. Male Gsnor−/− mice were bred with female WT mice and male WT mice were bred with female WT mice for comparison. Female mice were checked for plugs every morning. Litter size was evaluated and recorded the day after birth or at 17.5 days of gestation. Average litter sizes are presented as the number of total pups born divided by the number of litters.
Epididymal Sperm Evaluation
After weighing the testis, the epididymides were collected and used for semen analysis. Fresh epididymis was cut into small pieces and dispersed in F12 medium 200 μL (Invitrogen, Waltham, MA, USA) containing 0.1% bovine serum albumin (Invitrogen) pre-warmed to 37° C and incubated for 15 minutes to facilitate the transmigration of sperm from the epididymis. Each epididymal sperm suspension was subjected to sperm counting and sperm motility analyses by a computer-aided semen analysis (CASA) system (Microptic SL, Barcelona, Spain).
Tissue Collection
For RNA and protein isolation, WT and Gsnor−/− mice were anesthetized with isoflurane and blood was collected transcardially. Blood was allowed to clot at room temperature for 15 minutes and was centrifuged at 2,000g for 10 minutes at 4°C and the serum was stored at −80°C for future use. Tissues were removed, flash frozen in liquid nitrogen, and stored at −80°C for future use. For histology, organs from WT and Gsnor−/− mice were weighed and fixed overnight in Bouin solution at 4°C.
GSNOR Activity Assay
Gsnor−/− testis and WT liver, testis, and brain tissues were obtained for analysis. The protein concentrations of these samples were determined using a standard Bradford assay. Tissue homogenate (100 μg/mL) was incubated with Tris-HCI (2 mmol/L, pH = 8.0), ethylenediaminetetra-acetic acid (0.5 mmol/L), and reduced nicotinamide adenine dinucleotide (NADH; 200 μmol/L). The reaction was started by adding GSNO (400 μmol/L), and activity was measured as GSNO-dependent NADH consumption at absorbance of 340 nm for 10 minutes.11 All samples were maintained at 4°C. Protein samples were treated with protease inhibitor.
Testosterone, LH, and FSH Assay
Total testosterone, LH, and FSH levels were measured using the Ligand Assay & Analysis Core of the Center for Research in Reproduction at the University of Virginia (Charlottesville, VA, USA). Techniques for measurements of each hormone are described in detail at https://med.virginia.edu/research-in-reproduction/ligand-assay-analysis-core/assay-methods/. For serum testosterone, the mean intra-assay variation was 12.8% and the interassay variation was 9.3%. LH was measured using an ultrasensitive enzyme-linked immunosorbent assay. In brief, whole blood 6 μL was collected in assay buffer 54 μL for analysis. Interassay coefficients of variation were 7.3% (low quality control [QC]; 0.13 ng/mL), 5.0% (medium QC; 0.8 ng/mL), and 6.5% (high QC; 2.3 ng/mL). Functional sensitivity was 0.016 ng/mL. FSH was measured using a multiplex assay from serum. All experiments were performed during the same time of the day.
GnRH and Human Chorionic Gonadotropin Stimulation Test
GnRH was purchased from Sigma (St Louis, MO, USA) and stored at −80°C. Mice were injected intraperitoneally with GnRH at a concentration of 1.25 ng/g body weight diluted in 0.9% NaCl.13 15 minutes after injection, mice were humanely euthanized and blood was collected by cardiac puncture for analysis.
Human chorionic gonadotropin (hCG) is an LH analogue and acts on Leydig cells within the testes to stimulate testosterone production. hCG was administered intraperitoneally at a dose of 10 IU once daily for 7 days (treatment of male mice with gonadotropins to improve fertility and reproduction). After 7 days, mice were humanely sacrificed and blood was collected by cardiac puncture for analysis.
Immunofluorescence
3-μm formalin-fixed paraffin-embedded brain and hypothalamus sections from WT mice and testis cross-sections from WT and Gsnor−/− mice were deparaffinized in xylene and rehydrated with distilled water. Sections were subjected to antigen retrieval for 30 minutes at 95°C in antigen retrieval buffer (sodium citrate 10 mmol/L, 0.05% Tween-20, pH = 6.0). Then, sections were incubated in 0.3% hydrogen peroxide for 30 minutes to block endogenous peroxidase activity. Immunostaining was performed using the VECTASTAIN Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s protocol. The primary antibodies for testis immunofluorescence, 3β-hydroxysteroid dehydrogenase (3β-HSD; Santa Cruz Biotechnologies, Dallas, TX, USA) and LH receptor (LHR; Santa Cruz Biotechnologies), were diluted 1:1,000 and incubated with sections for 90 minutes at room temperature. For WT brain and hypothalamus staining, the antibody to GSNOR (Santa Cruz Biotechnologies) was diluted to 1:200 and incubated with sections for 90 minutes at room temperature. Detection was performed using the ImmPACT 3,3′-diaminobenzidine substrate (Vector Laboratories). Slides were subsequently stained with modified Mayer hematoxylin, dehydrated in an ethanol gradient, cover-slipped with Permount, and imaged.
Statistical Analysis
Data were analyzed for significance using 1-way analysis of variance with the post hoc Tukey-Kramer multiple comparisons test or the Student t-test, as indicated. All analyses were performed using GraphPad Prism 4.03 (GraphPad, South San Francisco, CA, USA), and a P value less than 0.05 was considered significant. All data are presented as mean ± standard error.
RESULTS
GSNOR Activity Is Decreased in Gsnor−/− Mice
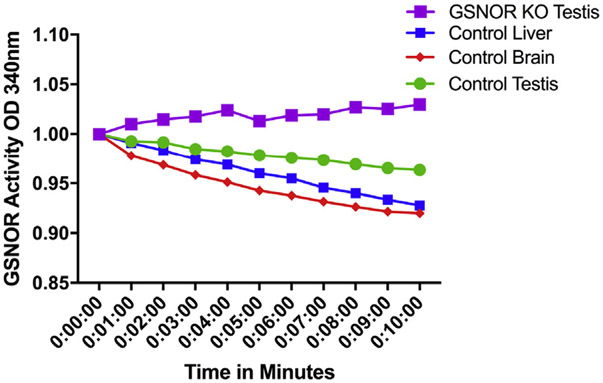
GSNOR uses NADH as a cofactor for GSNO reduction. GSNOR activity was measured by GSNO-dependent NADH consumption in testis, liver, and brain tissues. Tissue homogenate from WT mice testis and brain showed decreased levels of NADH over time, whereas NADH levels remained constant in testis tissue homogenate from Gsnor−/− mice, indicating the absence of GSNOR activity (Figure 1).
Figure 1.
Reduced nicotinamide adenine dinucleotide consumption over time of wild-type liver, testis, and brain and Gsnor−/− testis. Decreased OD of reduced nicotinamide adenine dinucleotide in wild-type liver, testis, and brain indicates the presence of GSNOR in these tissues. Reduced nicotinamide adenine dinucleotide does not decrease in Gsnor−/− mice, indicating GSNOR is not active. GSNOR = S-nitrosoglutathione reductase; KO = knockout; OD = optical density. Figure 1 is available online at www.jsm.jsexmed.org.
Gsnor−/− Mice Have Impaired Fertility
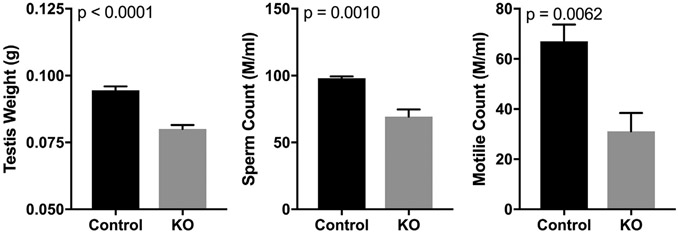
Testis weights of Gsnor−/− mice (n = 7) were decreased compared with testis weights from WT mice (n = 13; 0.08 ± 0.001 vs 0.09 ± 0.001 g; P < .01). Epididymal total sperm count and motility were significantly decreased in Gsnor−/− mice (total count = 69 ± 5 × 106, motility = 39 ± 13%) compared with WT mice (total count = 98 ± 2 × 106, motility = 65 ± 9%), indicating impaired spermatogenesis (Figure 2). We observed that the male Gsnor−/− mice were subfertile. Although 5 WT male mice bred to WT female mice over 3 months produced an average litter size of 8 ± 1 pups per litter, Gsnor−/− littermate male mice bred to WT female mice over a 1-month period showed decreased fertility and produced an average litter size of 4.2 ± 0.8 pups per litter (P < .01).14
Figure 2.
Left panel shows that Gsnor−/− mice have decreased testis weights compared with wild-type mice. Middle panel shows that Gsnor−/− mice have smaller epididymal sperm counts compared with wild-type mice. Right panel shows that Gsnor−/− mice have decreased percentage of sperm motility compared with wild-type mice. KO = knockout.
Circulating Reproductive Hormones Are Altered in Gsnor−/− Mice
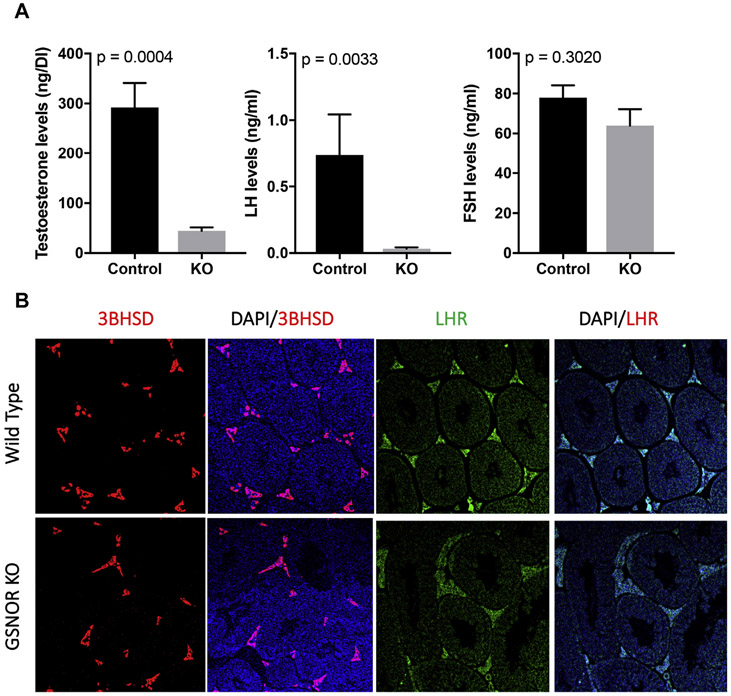
We compared levels of FSH, LH, and testosterone in Gsnor−/− mice (n = 6) and WT mice (n = 4). Unexpectedly, serum testosterone level was 6-fold lower in Gsnor−/− mice compared with WT mice (44.8 ± 5.91 vs 292.2 ± 63.3 ng/dL; P < .05). LH levels were 20-fold lower in Gsnor−/− mice (n = 11) compared with WT mice (n = 5; 0.03 ± 0.01 vs 0.74 ± 0.30 ng/mL; P = .04), whereas FSH levels were similar between Gsnor−/− and WT mice (63.98 ± 8.20 vs 77.93 ± 6.18 ng/mL; P = .20; Figure 3A). Immunofluorescence of the testis demonstrated similar expression of 3β-HSD and LHR in Gsnor−/− mice compared with WT testis, suggesting that nitrosative stress appears to affect the hypothalamic-pituitary axis without affecting testicular capacity to produce testosterone (Figure 3B).
Figure 3.
Panel A shows a comparison of reproductive hormone levels of Gsnor−/− mice with those of wild-type mice. Gsnor−/− mice have decreased testosterone and LH levels but similar FSH levels. Panel B shows immunofluorescence of similar 3BHSD and LHR immunostaining patterns in the testis of Gsnor−/− and wild-type mice. 3BHSD = 3β-hydroxysteroid dehydrogenase; DAPI = 4′,6-diamidino-2-phenylindole; FSH = follicle-stimulating hormone; GSNOR = S-nitrosoglutathione reductase; KO = knockout; LH = luteinizing hormone; LHR = luteinizing hormone receptor. Figure 3 is available online at www.jsm.jsexmed.org.
Exogenous GnRH and hCG Induce Recovery of Testosterone and LH Levels, Respectively, in Gsnor−/− Mice
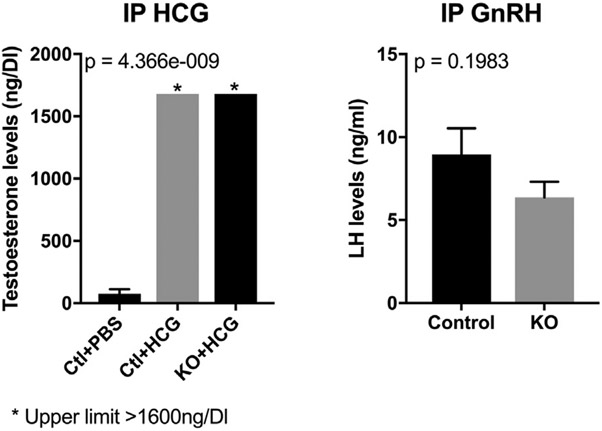
To determine whether nitrosative stress affected the pituitary gland and/or testis, we performed 2 experiments. We administered hCG 10 U or phosphate buffered saline 100 μL intraperitoneally to WT and Gsnor−/− mice (n = 3 for each condition) daily for 7 days and then measured testosterone on the 8th day. Serum testosterone levels in Gsnor−/− mice increased markedly to levels similar to those in WT mice administered hCG (>1,680 vs >1,680 ng/dL; Figure 4).
Figure 4.
Left panel shows that 15 minutes after IP injection of hCG, testosterone levels were similarly increased in Gsnor−/− and wild-type mice. Right panel shows that 15 minutes after IP administration of GnRH, LH levels were similarly increased in Gsnor−/− and wild-type mice. Ctl = control; DI = deciliter; GnRH = gonadotropin-releasing hormone; HCG = human chorionic gonadotropin; IP = intraperitoneal; KO = knockout; LH = luteinizing hormone; PBS = phosphate buffered saline.
We performed a GnRH stimulation test to determine whether the pituitary would retain the capacity to produce LH in Gsnor−/− mice. 15 minutes after intraperitoneal administration of GnRH, LH levels increased in Gsnor−/− and WT mice to similar levels (Gsnor−/− = 6.3 ± 0.9 ng/mL vs WT = 8.9 ± 0.9 ng/mL; P = .20), indicating that pituitary function in Gsnor−/− mice was intact (Figure 4). Together, this suggests a defect at the level of the hypothalamus.
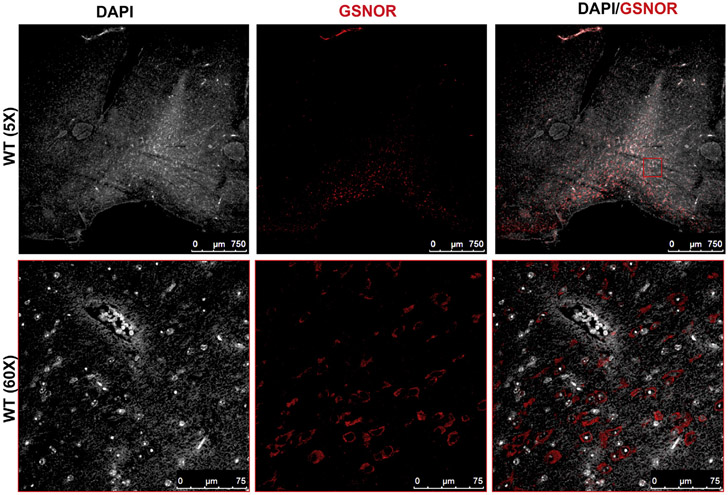
To confirm the presence of GSNOR in the hypothalamus, we preformed immunofluorescence staining of WT brain in the location of the hypothalamus. Immunostaining of the WT brain cross-section showed the presence of GSNOR and localization to the hypothalamus (Figure 5).
Figure 5.
Immunofluorescence staining of WT brains in the region of the hypothalamus shows the presence of GSNOR (red). DAPI = 4′,6-diamidino-2-phenylindole; GSNOR = S-nitrosoglutathione reductase; WT = wild-type. Figure 5 is available online at www.jsm.jsexmed.org.
DISCUSSION
Gsnor−/− mice have smaller testes, subfertility, and decreased levels of testosterone and LH, with preserved FSH. Gsnor−/− mice have intact testicular function, as demonstrated by hCG stimulation leading to increased testosterone levels, and similar patterns of immunofluorescent staining for LHR and 3β-HSD in the testes. Stimulation with GnRH led to increased LH levels in Gsnor−/− mice, suggesting inducible pituitary function. Therefore, the Gsnor−/− mouse is a model of secondary hypogonadism with likely impairment of hypothalamic function from nitrosative stress.
We investigated Gsnor−/− mice because of observed subfertility. Our initial investigation began with an evaluation of reproductive hormones. Surprisingly, the Gsnor−/− mice had nearly undetectable testosterone and LH levels with nearly normal FSH levels. Despite nearly castrate levels of testosterone and undetectable LH, the mice remained fertile, albeit less fertile than WT mice. This can be explained by the similar FSH levels between Gsnor−/− mice and WT mice. Previously published studies of differential LH and FSH expression attribute this to changes in GnRH pulse frequency.15 LH release is highly dependent on GnRH pulse frequency, with higher frequencies of pulsatile GnRH release favoring LH secretion, whereas slower frequencies of GnRH pulses favor FSH release.15,16 Furthermore, FSH expression can be constitutive and influenced by other stimulatory and inhibitory inputs such as activin and inhibin.17 The exact mechanism of how Gsnor−/− mice have very low levels of LH with normal levels of FSH will be important to evaluate mechanisms of GnRH release.
Excess nitrosative stress possibly resulting in secondary hypogonadism is an important hypothesis because of its possible implications in reproductive toxicology. Endocrine disrupting compounds, such as bisphenol A and phthalates, are found ubiquitously in food and water and are of toxicologic human health concern, especially in developing children. Mothers exposed to higher bisphenol A levels during early pregnancy and their matching term cord samples displayed increased nitrosative stress.18 Prenatal and lactational exposure to endosulfan, a compound that increases nitrosative stress, decreases LH levels and therefore has been implicated in delayed puberty.19 Therefore, Gsnor−/− mice can be used as a model to study the effects of endocrine disruptors on hypothalamic-pituitary-gonadal axis and fertility.
Gsnor−/− mice have impaired fertility and a disrupted hypothalamic-pituitary-gonadal axis likely due to nitroso-redox imbalance. RNS are generated through myriad normal cellular process and tissues.7 The largest generator of RNS is nitric oxide synthase, which is located throughout the body, including the brain. Pathologic states, such as arthritis, diabetes, cancer, and liver damage, lead to pathologic levels of RNS.7,20,21 Exogenous sources of ROS and RNS include smoking, air pollution, heavy metals, alcohol, solvents, paracetamol, radiation, and bisphenol A.17,22 Previous literature has linked RNS and ROS to impaired semen parameters. Currently, antioxidant therapy is used empirically and can improve semen parameters and pregnancy rate.22,23 Antioxidant therapy such as ascorbate can be targeted at decreasing nitrosative stress and nitroso-redox imbalance and could be used in the treatment of secondary hypogonadism.
Our study has strengths and limitations. We believe we are the 1st to report a novel model of secondary hypogonadism. Several animal models for hypogonadotropic hypogonadism (kisspeptin and the kisspeptin receptor knockout) exist but we are unaware of animal models of secondary hypogonadism with differential FSH and LH expression. Nitroso-redox imbalance in the Gsnor−/− animal model likely causes decreased LH and testosterone owing to nitrosylation of proteins involved in GnRH synthesis. Identifying the mechanism for impaired LH synthesis will enable us to identify causes for secondary hypogonadism and potentially identify therapeutic strategies for treatment of low testosterone other than exogenous testosterone therapy. Some limitations of the study include the small sample (limited by the breeding capabilities of Gsnor−/− mice) and variability in serum LH and testosterone levels in mice. Because of the lack of circulating sex hormone binding globulin, mice have highly fluctuating total testosterone serum concentrations and therefore LH levels are extremely variable.24 We accounted for this variability using an adequate sample size and drawing the blood in the morning (before 10 am). In addition, we did not investigate whether other pituitary hormones were altered in the Gsnor−/− mice. Moreover, we showed that administration of GnRH and hCG restores testosterone levels, although we did not assess for restored fertility. Future studies will include identifying markers of nitroso-redox imbalance in the hypothalamus such as 3-nitrotyrosine in Gsnor−/− mice. We also will evaluate whether exogenous agents such as GSNO when administered to WT mice can recapitulate the reproductive neuroendocrine phenotype of Gsnor−/− mice.
CONCLUSION
GSNOR deficiency results in secondary hypogonadism and impaired fertility, and this might be mediated in part by increased nitrosative stress. Our results further suggest that nitrosative stress might be affecting the hypothalamic-pituitary-gonadal axis at the level of the hypothalamus because the function of the testis and pituitary remains intact in Gsnor−/− mice.
STATEMENT OF AUTHORSHIP.
Category 1
-
(a) Conception and Design
Thomas A. Masterson; Himanshu Arora; Shathiyah Kulandavelu; Rona S. Carroll; Ursula B. Kaiser; Joshua M. Hare; Ranjith Ramasamy
-
(b) Acquisition of Data
Thomas A. Masterson; Himanshu Arora; Shathiyah Kulandavelu; Ranjith Ramasamy
-
(c) Analysis and Interpretation of Data
Thomas A. Masterson; Himanshu Arora; Shathiyah Kulandavelu; Rona S. Carroll; Ursula B. Kaiser; Sakir H. Gultekin; Joshua M. Hare; Ranjith Ramasamy
Category 2
-
(a) Drafting the Article
Thomas A. Masterson; Himanshu Arora; Shathiyah Kulandavelu; Rona S. Carroll; Ursula B. Kaiser; Sakir H. Gultekin; Joshua M. Hare; Ranjith Ramasamy
-
(b) Revising It for Intellectual Content
Thomas A. Masterson; Himanshu Arora; Shathiyah Kulandavelu; Joshua M. Hare; Ranjith Ramasamy
Category 3
-
(a) Final Approval of the Completed Article
Thomas A. Masterson; Himanshu Arora; Shathiyah Kulandavelu; Rona S. Carroll; Ursula B. Kaiser; Sakir H. Gultekin; Joshua M. Hare; Ranjith Ramasamy
Funding:
Dr Ramasamy received an American Urological Association Research Scholar Award and Stanley Glaser Award. Dr Hare received grants from the National Institutes of Health (1R01 HL137355, 1R01 HL107110, 1R01 HL134558, 5R01 CA136387, and 5UM1 HL113460) and the Soffer Family Foundation.
Footnotes
Conflicts of Interest: Dr Hare discloses a relationship with Vestion, Inc, that includes equity, board membership, and consulting; is the chief scientific officer, a compensated consultant and advisory board member for Longeveron, and holds equity in Longeveron; and is the co-inventor of intellectual property licensed to Longeveron. The other authors report no conflicts of interest.
REFERENCES
- 1.Jungwirth A, Giwercman A, Tournaye H, et al. ; European Association of Urology Working Group on Male Infertility. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol 2012;62:324–332. [DOI] [PubMed] [Google Scholar]
- 2.Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:3007–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95:1810–1818. [DOI] [PubMed] [Google Scholar]
- 4.Huhtaniemi I Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl 2014;16:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastrelli G, Carter EL, Ahern T, et al. Development of and recovery from secondary hypogonadism in aging men: prospective results from the EMAS. J Clin Endocrinol Metab 2015;100:3172–3182. [DOI] [PubMed] [Google Scholar]
- 6.Elsherbiny A, Tricomi M, Bhatt D, et al. State-of-the-art: a review of cardiovascular effects of testosterone replacement therapy in adult males. Curr Cardiol Rep 2017;19:35. [DOI] [PubMed] [Google Scholar]
- 7.Doshi SB, Khullar K, Sharma RK, et al. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol 2012;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramya T, Misro MM, Sinha D, et al. Altered levels of seminal nitric oxide, nitric oxide synthase, and enzymatic antioxidants and their association with sperm function in infertile subjects. Fertil Steril 2011;95:135–140. [DOI] [PubMed] [Google Scholar]
- 9.Ferrini M, Wang C, Swerdloff RS, et al. Aging-related increased expression of inducible nitric oxide synthase and cytotoxicity markers in rat hypothalamic regions associated with male reproductive function. Neuroendocrinology 2001;74:1–11. [DOI] [PubMed] [Google Scholar]
- 10.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med 2004;351:2112–2114. [DOI] [PubMed] [Google Scholar]
- 11.Hatzistergos KE, Paulino EC, Dulce RA, et al. S-nitrosoglutathione reductase deficiency enhances the proliferative expansion of adult heart progenitors and myocytes post myocardial infarction. J Am Heart Assoc 2015;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Yan Y, Zeng M, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 2004;116:617–628. [DOI] [PubMed] [Google Scholar]
- 13.Larder R, Clark DD, Miller NL, et al. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci 2011;31:426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulandavelu S, Kanashiro-Takeuchi RM, Balkan W, Hare JM. Ascorbate prevents hypertension, proteinuria and concentric hypertrophy in a model of preeclampsia, the S-nitrosoglutathione reductase (GSNOR) deficient mice. Circulation 2013;128:A12160. [Google Scholar]
- 15.Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol 2014;385:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson IR, Ciccone NA, Xu S, et al. GnRH pulse frequency-dependent stimulation of FSHbeta transcription is mediated via activation of PKA and CREB. Mol Endocrinol 2013;27:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma TP, Nett TM, Karsch FJ, et al. Neuroendocrine control of FSH secretion: IV. Hypothalamic control of pituitary FSH-regulatory proteins and their relationship to changes in FSH synthesis and secretion. Biol Reprod 2012;86:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veiga-Lopez A, Pennathur S, Kannan K, et al. Impact of gestational bisphenol A on oxidative stress and free fatty acids: human association and interspecies animal testing studies. Endocrinology 2015;156:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caride A, Lafuente A, Cabaleiro T. Endosulfan effects on pituitary hormone and both nitrosative and oxidative stress in pubertal male rats. Toxicol Lett 2010;197:106–112. [DOI] [PubMed] [Google Scholar]
- 20.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 21.Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 2015;30:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung JH, Seo JT. Empirical medical therapy in idiopathic male infertility: promise or panacea? Clin Exp Reprod Med 2014;41:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabaja AA, Schlegel PN. Medical treatment of male infertility. Transl Androl Urol 2014;3:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jänne M, Deol HK, Power SG, et al. Human sex hormone-binding globulin gene expression in transgenic mice. Mol Endocrinol 1998;12:123–136. [DOI] [PubMed] [Google Scholar]