Abstract
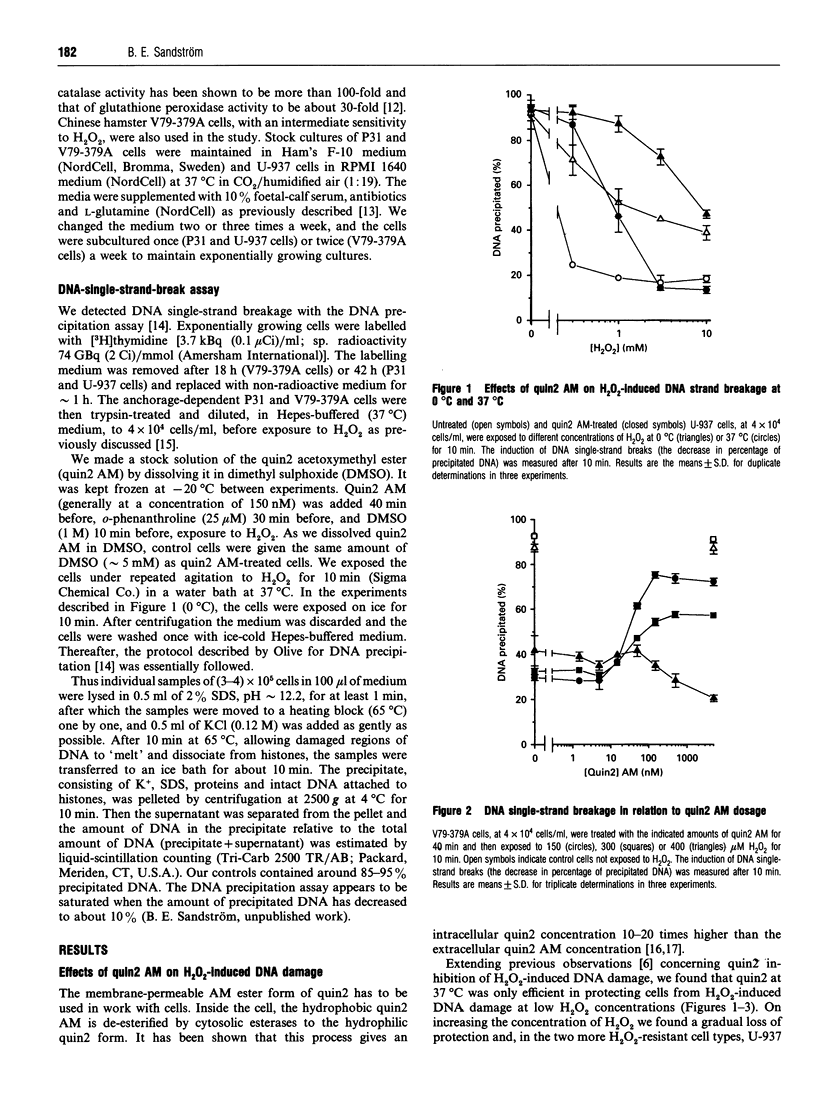
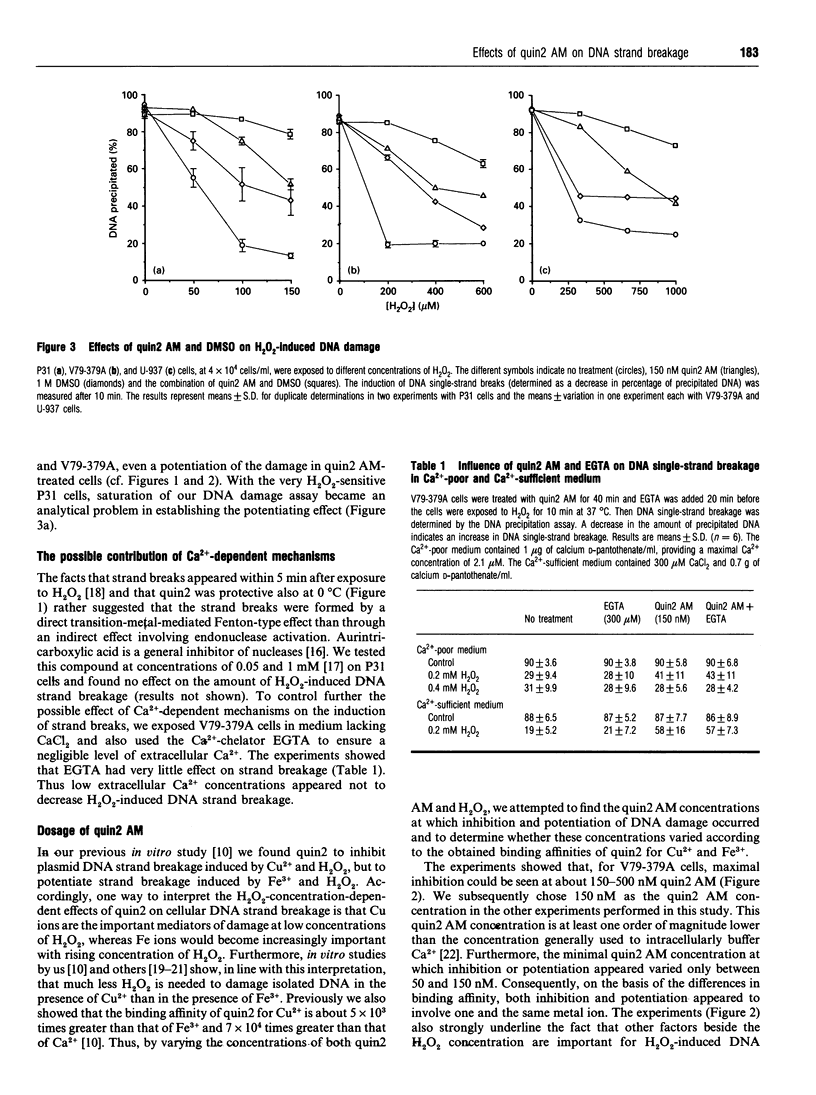
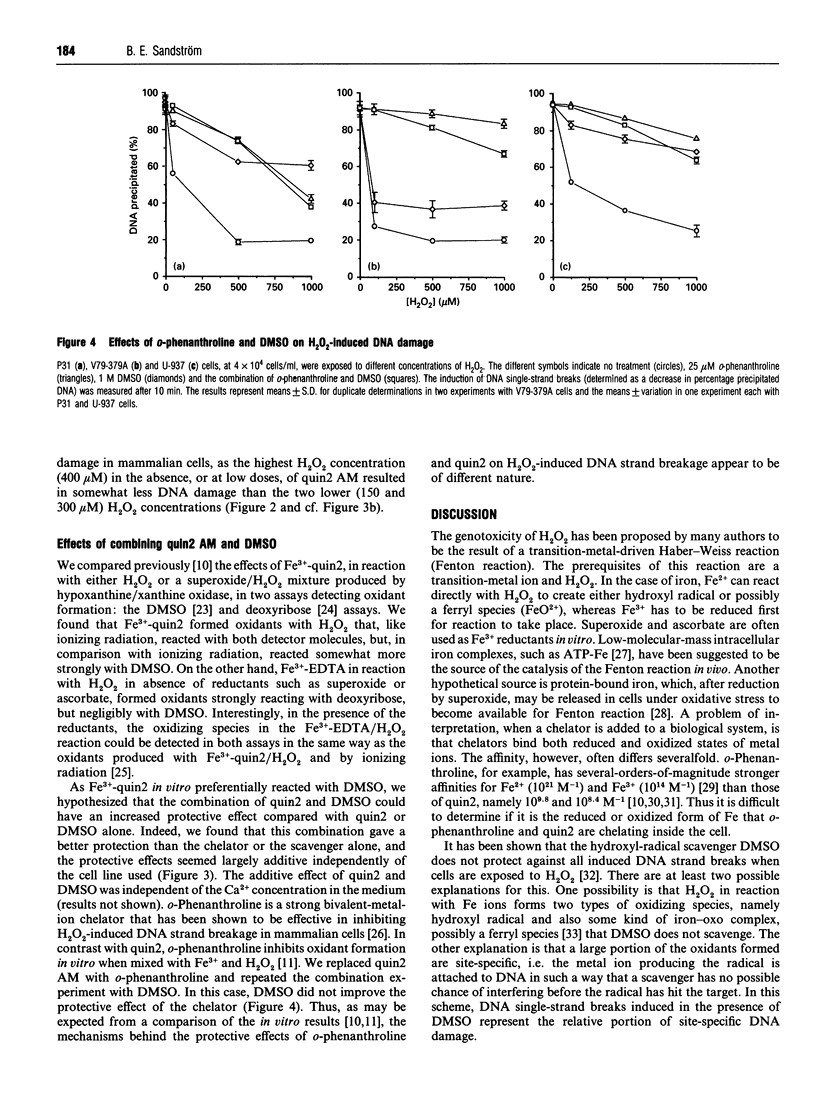
The cell-membrane-permeable calcium probe quin2 acetoxymethyl ester (quin2 AM) was ineffective, in comparison with o-phenanthroline, in protecting cells against H2O2-induced DNA single-strand breakage at H2O2 concentrations of about, and higher than, 0.5 mM. The present study shows that quin2 actually potentiated intracellular DNA damage at high H2O2 concentrations. H2O2-induced DNA breakage appeared within 5 min after exposure, and quin2 affected the induction of DNA breaks at both 0 degree C and 37 degrees C. Aurintricarboxylic acid, an endonuclease inhibitor, or a decrease in extracellular Ca2+, did not reduce DNA damage. These facts strongly suggest that the breaks were not produced by a Ca(2+)-dependent nuclease. We showed previously that, in the presence of Fe3+ and H2O2, quin2 strongly potentiated the formation of oxidizing species as well as plasmid DNA breakage, and, as could be expected for a transition-metal chelator, quin2 inhibited the Fenton reaction when Cu2+ was tested instead of Fe3+ [Sandström, Granström and Marklund (1994) Free Radicals Biol. Med. 16, 177-185]. In the present work with cultured cells, titration with quin2 AM showed that, despite the fact that Cu2+ has a three-to-four-orders-of-magnitude higher affinity for quin2 than has Fe3+, both inhibition and potentiation of H2O2-induced DNA damage occurred at quin2 AM concentrations of about 100 nM. Thus inhibition appeared not to involve Cu2+. The combination of quin2 AM and dimethyl sulphoxide (DMSO) gave an additive effect on H2O2-induced DNA damage compared with the effect of quin2 AM or DMSO alone, whereas the combination of o-phenanthroline and DMSO gave about the same effect as o-phenanthroline alone. In conclusion, our results do not support a role for Ca2+ in the inhibiting effect of quin2 on H2O2-induced DNA damage. Instead, it is likely that inhibition and potentiation by quin2 involves interaction with Fe ions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J. 1991 Feb 1;273(Pt 3):601–604. doi: 10.1042/bj2730601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs C. F., Gale M. J. Colorimetric assay for methanesulfinic acid in biological samples. Anal Biochem. 1987 May 15;163(1):67–73. doi: 10.1016/0003-2697(87)90093-5. [DOI] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., van Eijk H. G., Koster J. F. Superoxide dependent iron release from ferritin in inflammatory diseases. Free Radic Biol Med. 1988;4(3):185–198. doi: 10.1016/0891-5849(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A. The inhibition of nucleic acid-binding proteins by aurintricarboxylic acid. Biochem Biophys Res Commun. 1973 Dec 10;55(3):680–688. doi: 10.1016/0006-291x(73)91198-4. [DOI] [PubMed] [Google Scholar]
- Cantoni O., Sestili P., Cattabeni F., Bellomo G., Pou S., Cohen M., Cerutti P. Calcium chelator Quin 2 prevents hydrogen-peroxide-induced DNA breakage and cytotoxicity. Eur J Biochem. 1989 Jun 15;182(2):209–212. doi: 10.1111/j.1432-1033.1989.tb14819.x. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Rao G., Halliwell B., Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch Biochem Biophys. 1991 Mar;285(2):317–324. doi: 10.1016/0003-9861(91)90366-q. [DOI] [PubMed] [Google Scholar]
- Dypbukt J. M., Thor H., Nicotera P. Intracellular Ca2+ chelators prevent DNA damage and protect hepatoma 1C1C7 cells from quinone-induced cell killing. Free Radic Res Commun. 1990;8(4-6):347–354. doi: 10.3109/10715769009053368. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Gutteridge J. M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem J. 1987 May 1;243(3):709–714. doi: 10.1042/bj2430709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Superoxide-dependent formation of hydroxyl radicals from ferric-complexes and hydrogen peroxide: an evaluation of fourteen iron chelators. Free Radic Res Commun. 1990;9(2):119–125. doi: 10.3109/10715769009148579. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Marklund S. L., Westman N. G., Lundgren E., Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982 May;42(5):1955–1961. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Hori K., Terazawa K., Kawakishi S. Comparison of the cytotoxicity of different hydroperoxides to V79 cells. Free Radic Res Commun. 1991;14(3):173–178. doi: 10.3109/10715769109088946. [DOI] [PubMed] [Google Scholar]
- Olive P. L. DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen. 1988;11(4):487–495. doi: 10.1002/em.2850110409. [DOI] [PubMed] [Google Scholar]
- Rush J. D., Koppenol W. H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986 May 25;261(15):6730–6733. [PubMed] [Google Scholar]
- Rush J. D., Maskos Z., Koppenol W. H. Distinction between hydroxyl radical and ferryl species. Methods Enzymol. 1990;186:148–156. doi: 10.1016/0076-6879(90)86104-4. [DOI] [PubMed] [Google Scholar]
- Sandström B. E., Carlsson J., Marklund S. L. Variations among cultured cells in glutathione peroxidase activity in response to selenite supplementation. Biochim Biophys Acta. 1987 Jul 6;929(2):148–153. doi: 10.1016/0167-4889(87)90170-4. [DOI] [PubMed] [Google Scholar]
- Sandström B. E., Grankvist K., Marklund S. L. Selenite-induced increase in glutathione peroxidase activity protects human cells from hydrogen peroxide-induced DNA damage, but not from damage inflicted by ionizing radiation. Int J Radiat Biol. 1989 Nov;56(5):837–841. doi: 10.1080/09553008914552121. [DOI] [PubMed] [Google Scholar]
- Sandström B. E., Granström M., Marklund S. L. New roles for quin2: powerful transition-metal ion chelator that inhibits copper-, but potentiates iron-driven, Fenton-type reactions. Free Radic Biol Med. 1994 Feb;16(2):177–185. doi: 10.1016/0891-5849(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Sandström B. E., Marklund S. L. Effects of variation in glutathione peroxidase activity on DNA damage and cell survival in human cells exposed to hydrogen peroxide and t-butyl hydroperoxide. Biochem J. 1990 Oct 1;271(1):17–23. doi: 10.1042/bj2710017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Tachon P. Ferric and cupric ions requirement for DNA single-strand breakage by H2O2. Free Radic Res Commun. 1989;7(1):1–10. doi: 10.3109/10715768909088155. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- Weaver J., Pollack S. Low-Mr iron isolated from guinea pig reticulocytes as AMP-Fe and ATP-Fe complexes. Biochem J. 1989 Aug 1;261(3):787–792. doi: 10.1042/bj2610787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K., Tanigawa Y., Burzio L., Koide S. S. Evidence for adenosine diphosphate ribosylation of Ca2+, Mg2+-dependent endonuclease. Proc Natl Acad Sci U S A. 1975 Jan;72(1):289–293. doi: 10.1073/pnas.72.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]


